Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(13):3745-3750. doi:10.7150/jca.44512 This issue Cite
Research Paper
Delayed post-diuretic 18F-FDG PET/CT for preoperative evaluation of renal pelvic cancer
Department of Nuclear Medicine, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
*These authors contributed equally to this work.
Received 2020-2-3; Accepted 2020-3-28; Published 2020-4-6
Abstract
Background: Application of 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in urological oncology was relatively slowly due to the urinary elimination of 18F-FDG. We investigated whether delayed post-diuretic 18F-FDG PET/CT could be used for diagnosing renal pelvic cancer.
Methods: 51 patients were included who underwent delayed post-diuretic 18F-FDG PET/CT for detecting renal pelvic space-occupying lesions. The comparations of delayed PET/CT parameters and clinical characteristics between renal pelvic cancer and benign polyp were investigated.
Results: Among the 51 patients, 47 were found to have renal pelvic urothelial carcinoma, and 4 had benign polyp. ROC analysis identified the lesion maximum standardized uptake value (SUVmax) of 6.2 as the optimal cut-off value to distinguish from renal pelvic urothelial carcinoma to benign polyp. With the SUVmax cut-off of 6.2, the sensitivity, and specificity for predicting of renal pelvic urothelial carcinoma were 91.5% (43/47), and 100% (4/4). We also found a significant difference in tumor size between the positive (SUVmax > 6.2) and negative (SUVmax ≤ 6.2) PET groups in renal pelvic cancers. In patients with tumor size < 1.1 cm, the probability of being in the negative PET group was 75%. In such patients, a substantial proportion of renal pelvic cancer demonstrated negative SUVmax similar to that in patients with benign polyp.
Conclusion: Delayed 18F-FDG PET/CT could be used for differentiating renal pelvic cancer from benign polyp. In patients with small tumor size, renal pelvic cancer may present low 18F-FDG uptake, mimicking the metabolic phenotypes of patients with benign polyp.
Keywords: PET/CT, renal pelvic cancer, SUVmax
Introduction
Renal pelvic cancer is relatively uncommon urological malignancies [1, 2]. Urothelial carcinoma is the most common histologic type of renal pelvic cancer [3, 4]. Magnetic resonance imaging and computed tomography are routinely used for diagnosis of renal pelvic cancer [5, 6]. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has been widely used in many malignant tumors [7, 8]. However, application of 18F-FDG PET/CT specifically in urological oncology is relatively slowly due to the urinary elimination of 18F-FDG [9, 10]. It is encouraging to note that 18F-FDG PET/CT is used in the evaluation of bladder cancer through delayed post-diuretic imaging and has exhibited high sensitivity and accuracy in several studies [11-14]. However, few studies have examined the role of 18F-FDG PET/CT in the diagnosis of renal pelvic cancer. So far, whether delayed post-diuretic 18F-FDG PET/CT could be used for evaluation of renal pelvic cancer and the accuracy remains unclear.
The purpose of our study was to assess the value of delayed post-diuretic 18F-FDG PET/CT in the diagnosis of renal pelvic cancer and to distinguish between renal pelvic cancer and benign polyp. In addition, we also assessed the clinicopathologic features which were correlated with 18F-FDG uptake and identify the patients with renal pelvic carcinoma who may demonstrate negative 18F-FDG PET/CT results.
Methods
Patients
We retrospectively reviewed 51 patients with renal pelvic space-occupying lesions who were examined by delayed post-diuretic 18F-FDG PET/CT at the Shanghai Jiaotong University-affiliated Ren Ji Hospital from January 2012 to July 2019. Among the 51 patients, 41 patients were treated with nephroureterectomy; the remaining 10 patients were treated with ureteroscopy and biopsy. The pathological results were acquires using the specimen obtained from nephroureterectomy (n=41), or ureteroscopy (n=5) and biopsy (n=5). RenJi Hospital institutional review board approved this study. The informed consent was waived because it was a retrospective study.
Delayed post-diuretic 18F-FDG PET/CT imaging
After fasting for at least 6 h, the patients received an intravenous 3.7 MBq/kg injection of 18F-FDG. 18F-FDG PET/CT scanning was carried out using a whole-body scanner (Biograph mCT; Siemens) (early PET/CT imaging). After early PET/CT imaging, delayed post-diuretic PET/CT imaging was carried out after 120 min of early PET/CT imaging. Patients received 20 mg of furosemide by the oral route and an oral intake of more than 500 mL water. Patients were required to void frequently to reduce the urine physiological uptake of the radiotracer 18F-FDG. Delayed PET/CT comprised a range of two bed positions centered at the location of the renal pelvis. Regions of interest were placed over the most intense area of 18F-FDG uptake on delayed post-diuretic PET/CT imaging to calculate the SUVmax.
Statistical analysis
The data are used as mean ± SD. The relationship between the clinicopathological characteristics and renal pelvic carcinoma and benign polyp were analysed by the chi-square test, Fisher's exact test or Mann-Whitney U test, where applicable. Receiver operating characteristic (ROC) curve analysis was performed to determine the cut-off values for differentiating renal pelvic cancer from benign polyp. P<0.05 was considered statistically significant. SPSS, version 13.0 (SPSS Inc.) was used to perform statistical analyses.
Results
Patient characteristics
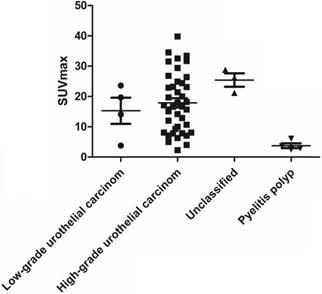
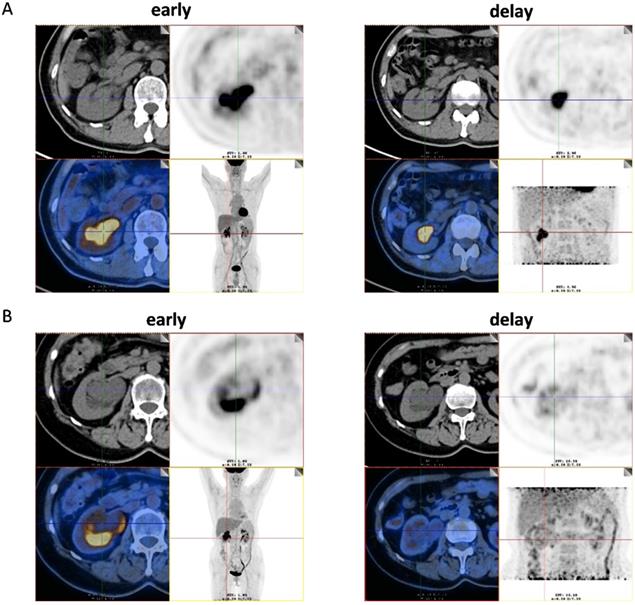
Among the 51 patients, 47 had malignant tumors: these included 40 cases of high- grade urothelial carcinoma, 4 cases of low-grade urothelial carcinoma and 3 cases of unclassified urothelial carcinoma. The remaining 4 patients had benign lesions, which were confirmed as benign polyp. Patient characteristics were shown in Table 1. The scatter plot of maximum standardized uptake value (SUVmax) for each urothelial carcinoma grade and benign polyp is shown in Figure 1. Mean SUVmax for high-grade urothelial carcinoma, low-grade urothelial carcinoma and benign polyp were 17.9 ± 9.7, 15.3 ± 8.6 and 3.8 ± 1.6, respectively. Both high-grade urothelial carcinoma (17.9 ± 9.7 vs 3.8 ± 1.6, P < 0.001) and low-grade urothelial carcinoma (15.3 ± 8.6 vs 3.8 ± 1.6, P = 0.039) showed significantly higher SUVmax compared with the benign polyp. However, there was no significant difference in SUVmax between high-grade urothelial carcinoma and low-grade urothelial carcinoma (17.9±9.7 vs 15.3 ± 8.6, P = 0.651). Representative images of a patient with renal pelvic carcinoma with high SUVmax (SUVmax=14.1) (Fig. 2A) and a patient with benign polyp with low SUVmax (SUVmax=2.9) (Fig.2B) who underwent early PET/CT imaging and delayed post-diuretic PET/CT scanning were shown in Figure 2.
The scatter plot of SUVmax for each urothelial carcinoma grade and benign polyp.
Patient characteristics (n=51)
| Histology and Grade | No. Patients (n) | Age (year) | Male: Female (n) |
|---|---|---|---|
| Renal pelvic carcinoma | |||
| High-grade urothelial carcinoma | 40 | 65.1±10.3 | 21:19 |
| Low-grade urothelial carcinoma | 4 | 70.3±9.5 | 3:1 |
| Unclassified urothelial carcinoma | 3 | 68.0±8.9 | 3:0 |
| Benign lesions | |||
| Benign polyp | 4 | 48.0±11.5 | 1:3 |
(A) Images of a 56-year-old man with renal pelvic cancer with high SUVmax (SUVmax=14.1). On the 18F-FDG PET/CT images, axial CT show that the space-occupying lesion was located in right renal pelvis. However, the 18F-FDG uptake of lesion cannot be easily detected because of urine interference in the early image (early). On the image of 18F-FDG PET/CT in the delayed phase, the ureter is distended and lesion can be easily visualized by axial CT and PET (SUVmax, 14.1) (delay) as the 18F-FDG uptake of urine was very low. The patient underwent radical resection of renal pelvic cancer, and low-grade urothelial carcinoma was confirmed by histopathology. (B) Images of a 61-year-old woman with benign polyp with low SUVmax (SUVmax=2.9). On the 18F-FDG PET/CT images, axial CT show that the space-occupying lesion was located in right renal pelvis. However, the 18F-FDG uptake of lesion cannot be easily detected because of urine interference in the early image (early). On the image of 18F-FDG PET/CT in the delayed phase, the ureter is distended and lesion can be easily visualized by axial CT and PET (SUVmax, 2.9) (delay) as the 18F-FDG uptake of urine was very low. The patient underwent pyeloureterectomy, and benign polyp was confirmed by histopathology.
The mean SUVmax of normal kidney tissues from all patients was calculated as 4.4 ± 1.2, and it was not significantly different urothelial carcinoma grades and benign polyp (Kruskal-Wallis test; P = 0.337); therefore, this value was used as the control SUVmax. The rates of positive 18F-FDG accumulation were significantly higher in patients with renal pelvic cancer than in benign polyp (95% [44/47] vs. 25% [1/4], respectively; P = 0.004) when compared with normal kidney tissues.
Differences 18F-FDG PET/CT parameters between renal pelvic carcinoma and benign polyp
Table 2 depicts patients' characteristics and delayed 18F-FDG PET/CT imaging grouped on the basis of renal pelvic carcinoma and benign polyp. No significant intergroup differences were found in terms of sex, age, primary site laterality, and lesion size. However, significant intergroup differences were found with respect to SUVmax. To elaborate, renal pelvic carcinoma had higher SUVmax than benign polyp (17.6 ± 11.5 vs. 4.5 ± 2.8; P < 0.001).
Patient characteristics according to renal pelvic carcinoma and benign polyp (n=51)
| Characteristics | Total (n=51) | Renal pelvic carcinoma (n=47) | Benign polyp (n=4) | P |
|---|---|---|---|---|
| Age (y) | ||||
| ≤60 | 16 | 13 | 3 | 0.086 |
| >60 | 35 | 34 | 1 | |
| Sex | ||||
| Male | 28 | 27 | 1 | 0.316 |
| Female | 23 | 20 | 3 | |
| Lesion laterality | ||||
| Right | 26 | 23 | 3 | 0.61 |
| Left | 25 | 24 | 1 | |
| Lesion Size | 31.3 ± 17.6 | 16.5± 10.5 | 0.117 | |
| SUVmax | 18.2 ± 9.5 | 3.8 ± 1.6 | <0.001 | |
Measurement of SUVmax cut-off value
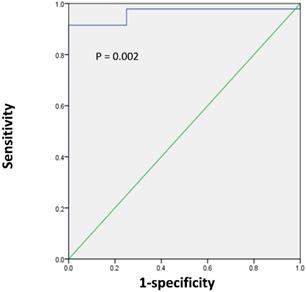
Next, we sought to determine the optimal SUVmax for differentiation between renal pelvic carcinoma and benign polyp. ROC analysis showed that the highest accuracy (92.2%) was obtained with an SUVmax cutoff of 6.2 and that the area under the curve was 0.963 ± 0.028 (Figure 3). With an SUVmax cut-off of 6.2, the sensitivity, specificity, positive predictive value, and negative predictive value for the prediction of renal pelvic carcinoma were 91.5% (43/47), 100% (4/4), 100% (43/43), and 50.0% (4/8), respectively.
In the multivariate analysis including factors with a P value of 0.15 or less, SUVmax remained the independent prognostic factor for differentiation between renal pelvic carcinoma and benign polyp [Table 3; OR, 2.76; 95% CI, 1.01-7.545; P = 0.048].
The relationships between clinicopathologic features and 18F-FDG PET/CT results in renal pelvic cancers
Of the 47 renal pelvic cancers, 38 patients were treated with radical resection of renal pelvic cancer. We further evaluated the associations between clinicopathologic features and delayed PET/CT results in these 38 patients by univariate analysis (Table 4). According to the SUVmax of tumors, the patients were divided into positive PET group (SUVmax > 6.2) and negative PET group (SUVmax ≤ 6.2). No significant intergroup differences were found inclung sex, age, tumor laterality, TNM stage, and lymph node metastasis. However, the tumor size was significantly larger in the positive PET group than the negative PET group (32.1 ± 17.4 vs. 8.7 ± 2.3; P = 0.027).
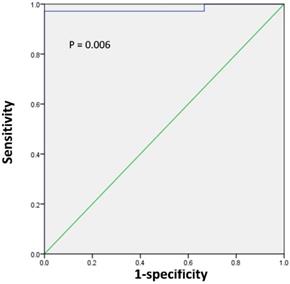
Next, we sought to determine the tumor size threshold for predicting tumor SUVmax in positive or negative PET group. ROC analysis demonstrated that the highest accuracy (97.4%) was obtained with the tumor size cutoff of 1.1 cm and that the area under the curve was 0.981 ± 0.022 (Figure 4). With the tumor size of 1.1 cm, the sensitivity, specificity, positive predictive value, and negative predictive value for predicting tumor SUVmax in positive PET group were 97.1% (34/35), 100% (3/3), 100% (34/34), and 75% (3/4), respectively.
Multivariate analysis for differentiation between renal pelvic carcinoma and benign polyp
| Factors | Odds Ratio | OR (95% CI) | P |
|---|---|---|---|
| SUVmax | 2.76 | 1.01-7.545 | 0.048 |
| lesion size | 0.894 | 0.744-1.074 | 0.231 |
| age | 162.48 | 0.217-121899.462 | 0.132 |
Patient characteristics according to positive and negative PET group (n=38)
| Characteristics | Total (n=38) | Positive | Negative | P |
|---|---|---|---|---|
| Age | ||||
| ≤60 | 10 | 9 | 1 | 0.774 |
| >60 | 28 | 26 | 2 | |
| Sex | ||||
| Male | 19 | 18 | 1 | 0.547 |
| Female | 19 | 17 | 2 | |
| Tumor laterality | ||||
| Right | 19 | 18 | 1 | 0.547 |
| Left | 19 | 17 | 2 | |
| Tumor Size (mm) | 32.1±17.4 | 8.7±2.3 | 0.027 | |
| TNM stage | ||||
| 0 | 18 | 15 | 3 | 0.097 |
| 2-4 | 20 | 20 | 0 | |
| Lymph node metastasis | ||||
| 0 | 30 | 31 | 3 | 0.536 |
| 1 | 8 | 4 | 0 |
ROC curve analysis for the differentiating renal pelvic cancer from benign polyp according to the SUVmax of lesion in 51 patients with renal pelvic space-occupying lesions. The area under the curve was 0.963 (95%CI 0.909-1.0, P = 0.002), and 6.2 was determined as the best SUVmax for predicting renal pelvic cancer. With an SUVmax cut-off of 6.2, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the prediction of renal pelvic carcinoma were 91.5% (43/47), 100% (4/4), 100% (43/43), and 50.0% (4/8), respectively.
ROC curve analysis for predicting tumor SUVmax in positive or negative PET group according to the tumor size in 38 patients with renal pelvic cancer. The area under the curve was 0.981 (95%CI 0.938-1.0, P = 0.006), and 1.1 cm was determined as the best tumor size for predicting tumor SUVmax in positive or negative PET group. With the tumor size of 1.1 cm, the sensitivity, specificity, positive predictive value, and negative predictive value for predicting tumor SUVmax in positive PET group were 97.1% (34/35), 100% (3/3), 100% (34/34), and 75% (3/4), respectively.
Discussion
18F-FDG PET/CT is used for diagnosis of many malignant tumors [15, 16]. Unfortunately, 18F-FDG is not an ideal radiotracer for use in urology due to its urinary elimination [12]. In our study, the application of delayed post-diuretic 18F-FDG PET/CT for diagnosing renal pelvic space-occupying lesions was analyzed. So far, our study was the first to demonstrate that delayed post-diuretic 18F-FDG PET/CT could be used for diagnosing renal pelvic cancer and distinguishing between renal pelvic cancer and benign polyp.
Although most of renal pelvic space-occupying lesions are renal pelvic cancer, it should also be distinguished from other renal pelvic space-occupying benign lesions [17]. Early differentiation between renal pelvic cancer and benign polyp is crucial for executing an effective treatment plan and predicting prognosis. Although magnetic resonance imaging and computed tomography have been widely used for the diagnosis of renal pelvic cancer, its accuracy for distinguishing between renal pelvic cancer and benign polyp is limited; in addition, ureteroscopy, which requires general anesthesia, is an invasive method that carries the risk of infection [18, 19]. Our study found that delayed post-diuretic 18F-FDG PET/CT could be used to differentiate renal pelvic cancer from benign polyp. The ROC analysis indicated that SUVmax could potentially be used to predict whether the renal pelvic space-occupying lesions is malignant or benign polyp. Multivariate analysis demonstrated that the SUVmax of the lesion was the significant predictor of the nature of the renal pelvic space-occupying lesions. Our study is the first to conclude that the SUVmax of the renal pelvic space-occupying lesions could be used to differentiate between renal pelvic cancer and benign polyp. With an SUVmax cut-off of 6.2, the sensitivity and specificity for the prediction of renal pelvic cancer were 91.5% and 100%, respectively. Thus, lesions with SUVmax greater than 6.2 indicate a high possibility of renal pelvic cancer.
In this study, we further analyzed the relationships between clinicopathologic features and delayed post-diuretic 18F-FDG uptake in renal pelvic cancers. Our findings suggest that tumor size was significantly correlated with the SUVmax of renal pelvic cancer; whereas other feathers such as age, sex, tumor laterality, tumor grade and TNM stage do not play an important role in determining the SUVmax of tumors. We found that when patients had tumor size < 1.1 cm, their probability of being in the negative PET group was 75%. In such patients, a substantial proportion of renal pelvic cancer demonstrated low 18F-FDG uptake similar to that in patients with benign polyp. Therefore, when using SUVmax to distinguish renal pelvic cancer from benign polyp, the lesion size should also be considered, especially in the negative PET results. For patients with lesion size < 1.1 cm, even if the SUVmax of renal pelvic space-occupying lesions was relatively low (SUVmax < 6.2), we cannot arbitrarily assume that it is benign polyp.
Our study was its limitation because it was a retrospective study and the sample size was relatively small. Although delayed post-diuretic 18F-FDG PET/ CT may have a good diagnostic performance, in the clinical setting it is not possible to establish a threhold for SUVmax, and delayed post-diuretic 18F-FDG PET/CT could not replace ureteroscopy, biopsy, post-operative histopathology for determining the lesion nature of renal pelvic space-occupying lesions.
Conclusions
Our results were the first to show the application of delayed post-diuretic 18F-FDG PET/CT in the renal pelvic space-occupying lesions and demonstrated that delayed 18F-FDG PET/CT could be used for differentiating renal pelvic cancer from benign polyp. These results may advance the development of noninvasive methods to predict benign and malignant disease of renal pelvic space-occupying lesions. Further larger and prospective studies that include more clinical samples are needed to confirm the value and efficacy of delayed 18F-FDG PET/CT in the renal pelvic space-occupying lesions.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (nos. 81701724, 81771858, 81602415, 81830052, 81601536, 81601520, 81771861), Innovative research team of high-level local universities in Shanghai and Cooperative research project of collaborative innovation center of translational medicine (nos. TM201806).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kirkali Z, Tuzel E. Transitional cell carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol. 2003;47:155-169
2. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66
3. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Gontero P, Van Rhijn BWG, Mostafid AH. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol. 2018;73:111-122
4. Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594-601
5. Zeikus E, Sura G, Hindman N, Fielding JR. Tumors of Renal Collecting Systems, Renal Pelvis, and Ureters: Role of MR Imaging and MR Urography Versus Computed Tomography Urography. Magn Reson Imaging Clin N Am. 2019;27:15-32
6. Li F, Bai M, Wu Y, He Y, Gu J, Xing J, Du L. Comparative Diagnostic Performance of Contrast-Enhanced ultrasound versus Baseline Ultrasound for Renal Pelvis Lesions. Ultrasound Med Biol. 2015;41:3109-3119
7. Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med. 2009;50:1820-1827
8. Plathow C, Weber WA. Tumor cell metabolism imaging. J Nucl Med. 2008;49(Suppl 2):43S-63S
9. Kitajima K, Yamamoto S, Fukushima K, Minamimoto R, Kamai T, Jadvar H. Update on advances in molecular PET in urological oncology. Jpn J Radiol. 2016;34:470-485
10. Mafeld S, Vasdev N, Patel A, Ali T, Lane T, Boustead G, Thorpe AC, Adshead JM, Haslam P. Evolving role of positron emission tomography (PET) in urological malignancy. BJU Int. 2015;116:538-545
11. Anjos DA, Etchebehere EC, Ramos CD, Santos AO, Albertotti C, Camargo EE. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med. 2007;48:764-770
12. Razik A, Das CJ, Sharma S. PET-CT and PET-MR in urological cancers other than prostate cancer: An update on state of the art. Indian J Urol. 2018;34:20-27
13. Huo J, Chu Y, Chamie K, Smaldone MC, Boorjian SA, Baillargeon JG, Kuo YF, Kerr P, O'Malley P, Orihuela E. Increased Utilization of Positron Emission Tomography/Computed Tomography (PET/CT) Imaging and Its Economic Impact for Patients Diagnosed With Bladder Cancer. Clin Genitourin Cancer. 2017;16:e99-111
14. Chen R, Zhou X, Liu J, Huang G. Relationship between the expression of PD-1/PD-L1 and (18)F-FDG uptake in bladder cancer. Eur J Nucl Med Mol Imaging. 2019;46:848-854
15. Zhou X, Chen R, Xie W, Ni Y, Liu J, Huang G. Relationship between 18F-FDG accumulation and lactate dehydrogenase A expression in lung adenocarcinomas. J Nucl Med. 2014;55:1766-1771
16. Chen R, Wang Y, Zhou X, Huang G, Liu J. Preoperative PET/CT (18)F-FDG Standardized Uptake by Lymph Nodes as a Significant Prognostic Factor in Patients with Colorectal Cancer. Contrast Media Mol Imaging. 2018;2018:5802109
17. Baard J, de Bruin DM, Zondervan PJ, Kamphuis G, de la Rosette J, Laguna MP. Diagnostic dilemmas in patients with upper tract urothelial carcinoma. Nat Rev Urol. 2017;14:181-191
18. Cutress ML, Stewart GD, Zakikhani P, Phipps S, Thomas BG, Tolley DA. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int. 2012;110:614-628
19. Keeley FX, Kulp DA, Bibbo M, McCue PA, Bagley DH. Diagnostic accuracy of ureteroscopic biopsy in upper tract transitional cell carcinoma. J Urol. 1997;157:33-37
20. Nayak B, Dogra PN, Naswa N, Kumar R. Diuretic 18F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. Eur J Nucl Med Mol Imaging. 2013;40:386-393
21. Fraczek M, Kamecki H, Kamecka A, Sosnowski R, Sklinda K, Czarniecki M, Krolicki L, Walecki J. Evaluation of lymph node status in patients with urothelial carcinoma-still in search of the perfect imaging modality: a systematic review. Transl Androl Urol. 2018;7:783-803
Author contact
![]() Corresponding authors: Jianjun Liu, 160 Pujian Road, Shanghai 200127, China, Tel: +86 21 68383530 , Fax: +86 21 63842916, E-mail:nuclearjcom or Qian Xia,160 Pujian Road, Shanghai 200127, China, Tel: +86 21 68383530 , Fax: +86 21 63842916, E-mail:kara_xiacom
Corresponding authors: Jianjun Liu, 160 Pujian Road, Shanghai 200127, China, Tel: +86 21 68383530 , Fax: +86 21 63842916, E-mail:nuclearjcom or Qian Xia,160 Pujian Road, Shanghai 200127, China, Tel: +86 21 68383530 , Fax: +86 21 63842916, E-mail:kara_xiacom

 Global reach, higher impact
Global reach, higher impact