Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(17):5056-5068. doi:10.7150/jca.44408 This issue Cite
Review
Radiobiology of stereotactic ablative radiotherapy (SABR): perspectives of clinical oncologists
Department of Oncology, Xiangya Hospital, Central South University, No. 87, Xiangya Road, Changsha, Hunan Province 410008, China
Received 2020-1-30; Accepted 2020-6-12; Published 2020-6-27
Abstract
Stereotactic ablative radiotherapy (SABR) is a novel radiation treatment method that delivers an intense dose of radiation to the treatment targets with high accuracy. The excellent local control and tolerance profile of SABR have made it become an important modality in cancer treatment. The radiobiology of SABR is a key factor in understanding and further optimizing the benefits of SABR. In this review, we have addressed several issues in the radiobiology of SABR from the perspective of clinical oncologists. The appropriateness of the linear-quadratic (LQ) model for SABR is controversial based on preclinical data, but it is a reliable tool from the perspective of clinical application because the biological effective dose (BED) calculated with it can represent the tumor control probability (TCP). Hypoxia is a common phenomenon in SABR in spite of the relatively small tumor size and has a negative effect on the efficacy of SABR. Preliminary studies indicate that a hypoxic radiosensitizer combined with SABR may be a feasible strategy, but so far there is not adequate evidence to support its application in routine practice. The vascular change of endothelial apoptosis and blood perfusion reduction in SABR may enhance the response of tumor cells to radiation. Combination of SABR with anti-angiogenesis therapy has shown promising efficacy and good tolerance in advanced cancers. SABR is more powerful in enhancing antitumor immunity and works better with immune checkpoint inhibitors (ICIs) than conventional fractionation radiotherapy. Combination of SABR with ICIs has become a practical option for cancer patients with metastases.
Keywords: Radiobiology, stereotactic ablative radiotherapy, SABR, oncologist
Introduction
Stereotactic ablative radiotherapy (SABR) is a novel radiation treatment method that delivers an intense dose of radiation to the treatment targets with a higher dose per fraction (> 5 Gy) and fewer fractions (1-5 fractions) compared with conventional fractionation radiotherapy [1]. SABR is also known as stereotactic radiosurgery (SRS) for treating brain lesions and as stereotactic body radiation therapy (SBRT) in the treatment of extracranial tumors [2]. Due to its excellent local control and tolerance profile, SABR currently plays a very important role in the treatment of many cancers, such as non-small cell lung cancer (NSCLC), liver cancer, pancreatic cancer, and brain tumors.
The radiobiology of SABR is a key factor in understanding and further optimizing the benefits of SABR [2, 3]. However, so far, the majority of publications on radiobiology are based on the perspectives of radiobiologists or researchers of basic science. As clinical oncologists, we are eager to improve patient survival and reduce treatment toxicity with assistance from the radiobiology of SABR. Therefore, in this review, we have addressed several issues in the radiobiology of SABR from the perspective of clinical application, including the appropriateness of linear-quadratic (LQ) model in SABR, the role of hypoxia in SABR, the role of vascular change in SABR, and the synergistic effect of SABR and immune checkpoint inhibitors.
1. Is the LQ model appropriate for SABR?
As a biologically based and practical model, the LQ formalism is the most commonly accepted tool for evaluating the relationship between the radiation dose and its biological effects [4]. The biological effective dose (BED) deduced from the LQ model has been widely used by clinical oncologists to predict tumor control probability (TCP) and normal-tissue tolerance [5]. However, the validity of the LQ model at high dose per fraction (> 5 Gy) is controversial. Some preclinical studies based on either cells or animal models showed that the LQ model failed to accurately predict a high-dose response [2, 6, 7], while other studies showed that the LQ model fitted the in vitro and in vivo survival data well up to 15-20 Gy per fraction [8, 9]. Besides, several modified models with more parameters, such as the universal survival curve (USC) model, the linear-quadratic-linear (LQ-L) model, and the Pade linear quadratic (PLQ) model, have been proposed to replace the LQ model and reported to fit the dose-response curve better at high dose per fraction in preclinical experiments [10-12].
In spite of the controversy in preclinical studies, the most important question for clinical oncologists is whether BED calculated with the LQ model can represent TCP. It is generally accepted that higher BED results in higher TCP until it reaches a plateau. Therefore, a practical method to examine the validity of the LQ model is to investigate the relationship between TCP and BED. Brown et al. analyzed 2696 patients with stage I NSCLC who were treated with 3-dimension conformal radiotherapy (3D-CRT) or SBRT and plotted TCP against the BED calculated with the LQ model. Their results demonstrated that TCP increased monotonically with BED for different SBRT regimens and the data for 3D-CRT also fell on the curve, indicating that the BED calculated with the LQ model can represent TCP for both SBRT and 3D-CRT [13]. To further confirm the validity of the LQ model in SABR, several studies compared the LQ model with other models in the fitness of TCP and BED. The LQ-L model and the USC model are the most commonly reported modified models that require additional parameters, as shown in Table 1. Mehta et al. reviewed the data of the same 2696 patients mentioned above and showed that the fitness of TCP and BED was similar for the LQ model and the USC model [14]. Guckenberger et al. compared the LQ and LQ-L formula for modeling TCP in 395 stage I NSCLC patients treated with SBRT, and they showed that the fit of the LQ-L model was not significantly better than that of the LQ model [15]. Similarly, Santiago et al. analyzed 31 studies that reported 3-year local control in 2319 patients with stage I NSCLC, and they showed that the fit of the LQ and LQ-L models did not differ substantially [16]. Moreover, Shuryak et al. analyzed the TCP data of 2965 patients with lung tumors or brain metastases who received SABR, and they showed that the LQ model provided a significantly better fit over the entire range of treatment doses than the LQ-L model and the USC model [17].
It is also worth mentioning that simplicity is an unneglectable property of a model in clinical application. As shown in Table 1, α and β are the only parameters required for the LQ model, while other modified models require extra parameters. To obtain more reliable data, clinicians generally calculate α and β with statistical methods based on TCP from clinical studies, which is very complicated and has great heterogeneity [18]. All of the modified models require additional parameters, which makes it much more difficult in clinical practice. Therefore, the LQ model is simplest of all of the models.
To summarize, the LQ model is an appropriate model for SABR as it can represent TCP and it performs better than or equivalent to the other modified models. As per George Box's well-worn aphorism: 'All models are wrong, but some are useful,' the LQ model is a very useful tool for SABR in spite of the controversy.
LQ model, USC model and LQ-L model
| Model | Parameters | BED calculation |
|---|---|---|
| LQ model [109] | α, β | BED LQ = nd (1 + 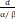 ) ) |
| USC model [14, 109] | α, β, Dq, D0, Dt | BED USC = n (1 + (1 + 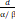 ), ),  <Dt <DtBED USC = 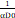 (n (n - nDq), - nDq),  ≥ Dt ≥ Dt |
| LQ-L model [15, 16] | α, β, Dt | BED LQL = n (1 + (1 + 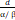 ), ),  <Dt <DtBED LQL = nDt (1 + 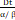 ) +n ( ) +n (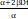 )( )(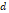 - Dt), - Dt),  ≥ Dt ≥ Dt |
n = number of treatment fractions, d = dose per fraction.
2. The role of hypoxia in SABR
Due to the oxygen enhancement effect, it is well known that tumor hypoxia correlates with treatment failure after radiotherapy of conventional fractionation regimens. With respect to SABR, because reoxygenation decreases as a result of fewer fractions, the influence of hypoxia is supposed to be more powerful theoretically. However, the high local control rate by SABR seems to attenuate the importance of oxygen. Several issues regarding hypoxia in SABR are discussed below from the perspective of clinical oncologists.
2.1 Is hypoxia a common phenomenon in SABR?
Generally, SABR is only suitable for tumors with a small size due to the dose constraints of nearby normal tissues. For example, SABR is commonly recommended for tumors smaller than 5 cm in size for early-stage NSCLC according to the NCCN guidelines (version 1.2020). Hypoxia may not be a common phenomenon in SABR because experiments on animal models have shown that smaller tumors may be less likely to become hypoxic [19, 20]. However, other studies have indicated that tumor hypoxia is independent of tumor size [21]. Hockel et al. measured the hypoxic status of 103 cervical cancer patients with a computerized polarographic electrode system, and they found that tumor oxygenation was independent of tumor size [22]. Similarly, Le et al. measured pO2 in 20 patients with resectable NSCLC (median tumor volume 10.8 ml) intraoperatively using the Eppendorf polarographic electrode and showed that tumor hypoxia existed in 19 patients and the severity of hypoxia was independent of tumor volume [23]. Moreover, with modern hypoxia imaging techniques, such as the positron emission tomography (PET), we can examine the tumor hypoxia status directly during SABR. Kelada et al. performed dynamic 18F-fluoromisonidazole PET-CT during the SBRT treatment in 6 NSCLC patients, and among them, 4 patients showed tumor hypoxia before and during the course of SBRT [24]. In addition, another study by Qian et al. also showed imageable hypoxia in 6 of 16 early-stage NSCLC patients treated with SABR by performing 18F- pentafluorinated etanidazole PET [25]. Therefore, hypoxia is a common phenomenon in SABR in spite of its relatively small tumor size.
2.2 Is hypoxia a neglectable factor in determining the efficacy of SABR?
As hypoxia is a common phenomenon in SABR, it is necessary to evaluate its influence on the efficacy of SABR. For radiotherapy with conventional fractionation, it is well accepted that hypoxia is a major factor for tumor radioresistance [26]. With respect to SABR, a study by Carlson et al. showed that hypoxia caused a more significant decrease in cell killing during SABR compared to the conventional fractionation regimen in cell lines of prostate cancer and head and neck cancer [27]. Another study in animal models also showed that tumors with lower pO2 had decreased TCP after SRS [28]. With respect to clinical evidence, Goodman et al. reviewed 682 brain metastases treated with SRS and found that 1-year freedom from progression (FFP) probabilities for homogeneously-, heterogeneously-, and ring-enhancing lesions were 90%, 76%, and 57%, respectively, which indicated that hypoxic tumor cells in the necrotic regions were associated with radioresistance [29]. Further, Qian et al. also found that imageable hypoxia was associated with worse overall survival (OS), regional failure, and distant failure in 16 early-stage NSCLC patients who were treated with SBRT [25]. In addition, Jensen et al. analyzed 162 meningioma patients who received SRS, and they found that the expression of hypoxia-inducible factor 1α (HIF-1α), which is an endogenous marker of hypoxia, was correlated with worse local control after SRS (p=0.046) [30]. Taken together, hypoxia has a negative effect on the efficacy of SABR.
2.3 Is hypoxic modification necessary in SABR?
Based on the information presented above, it is reasonable to investigate the role of hypoxic modification in SABR. As is already known, the most extensively investigated approach for hypoxic modification is hypoxic radiosensitizer. For conventional fractionation radiotherapy, hypoxic radiosensitizer combined with radiotherapy has provided promising benefits in TCP and OS in cervical cancer and head and neck cancer [26]. With respect to SABR, preliminary studies have indicated that SABR plus hypoxic modifiers may be a feasible strategy. A study by Wittenborn et al. showed that hypoxic modifiers (nimorazole, nicotinamide, carbogen breathing, and OXi4503) effectively improved the treatment outcome in a preclinical tumor model treated with stereotactic radiation schedules [31]. The RTOG study 95-02, which is a phase Ib clinical trial, showed that SRS combined with etanidazole (a hypoxic cell radiosensitizer) at a dose of 12 g/m2 was well tolerated by patients with brain tumors and brain metastases [32]. In addition, a phase I clinical trial (NCT03824327) is currently evaluating the safety and efficacy of papaverine hydrochloride (a radiosensitizer targeting mitochondrial respiration) combined with SBRT in treating early-stage NSCLC patients. Other hypoxic modification approaches, such as the increase in oxygen availability through hyperbaric oxygen, dose escalation for hypoxic tumor volume, and adoption of higher linear energy transfer radiation have less impact on general clinical practice and their combination with SABR has seldom been reported. To summarize, preliminary studies indicate that a hypoxic radiosensitizer combined with SABR may be a feasible strategy, but so far there is not adequate evidence to support its application in routine practice.
From the perspective of clinical application, a key question in further improving the efficacy of this combination strategy is identifying the appropriate patients. It has been indicated that the hypoxia status is an important factor in determining the efficacy of hypoxic radiosensitizers when combined with radiotherapy. Toustrup et al. classified tumors as “more” and “less” hypoxic according to the expressions of hypoxia responsive genes, and they found that only patients with “more” hypoxic tumors obtained survival benefits from hypoxic modification of radiotherapy [33]. Similar conclusions were reported in the subgroup analysis of the results of the IAEA-HypoX trial, which was an international multicenter randomized trial aimed at investigating the efficacy of combining nimorazole with radiotherapy in head and neck cancer [34]. In addition, Yang et al. also found that tumor hypoxia status could predict benefits from hypoxic modification for bladder cancer patients receiving radiotherapy [35]. Currently, there are several noninvasive hypoxia imaging techniques available in clinical practice, such as the 18F-fluoromisonidazole PET, the 18F-fluoroazomycin arabinoside PET, the oxygen-enhanced magnetic resonance imaging (MRI), and the blood oxygen level dependent (BOLD) MRI [36, 37]. Further, it should be noted that the status of hypoxia in tumors can change during the treatment course of SABR [24]. Therefore, monitoring the status of tumor hypoxia during the treatment course of SABR and selecting patients with hypoxic tumors as the candidates is a potential strategy for further improving the efficacy of combining SABR with hypoxia modifiers.
3. The role of vascular change in SABR
3.1 The vascular change in SABR
It is well known that the intratumor microenvironment has a great influence on the oncogenesis, invasion, and metastasis of tumor cells. As an important part of the microenvironment, tumor microvasculature plays a key role in providing tumor cells with oxygen and nutrients. Therefore, it is necessary to investigate the vascular change in SABR in order to understand the radiobiology better. Park et al. analyzed 43 representative studies on radiation-induced tumor vascular change and found that although the reported results were inconsistent, they could be generalized as follows. For conventional fractionation radiotherapy (< 3 Gy per fraction), the morphology and function of vasculature and the blood perfusion were not impaired until the end of the treatment, which was attributed to the declined demands for nutrients and oxygen as a result of radiation-induced tumor cell death. For SABR regimens (> 5 Gy per fraction), irradiation of 5-10 Gy in a single dose caused relatively mild decrease in tumor blood flow, but irradiation of higher than 10 Gy per fraction induced severe and rapid blood perfusion reduction, which was attributed to the damage of the integrity and viability of vascular endothelial cells by irradiation [38]. Similar results have been reported by several other studies later, which adopted different methods to measure the change of vasculature in animal models [39-41].
A key question regarding the SABR induced endothelial apoptosis and blood perfusion reduction is whether it affects the response of the tumor to radiotherapy. As is well known, irradiation can lead to direct cancer cell death through DNA damage. Can the vascular change cause indirect cancer cell death in SABR by depriving the supply of oxygen and nutrients? Kocher et al. developed a 3-dimensional computer simulation method to determine the factors affecting the tumor response to radiotherapy, and they showed that the therapeutic effect of SRS in brain tumors cannot be explained without the consideration of vascular effects [42]. Similarly, Monica et al. proposed that the tumor response to radiotherapy was regulated by endothelial cell apoptosis. In their study, the MCA/129 fibrosarcomas transplanted on endothelial apoptosis resistant mice displayed markedly reduced baseline microvascular endothelial apoptosis and were resistant to single-dose radiation up to 20 Gy [43]. Later, Moeller et al. suggested that endothelial apoptosis contributed more significantly to tumor cell death in single dose radiation (> 8-10 Gy) than conventional fractionation regimens (1.8-3 Gy/fraction), because the death signaling pathway in endothelium was repressed by the activation of HIF-1α during the process of hypoxia/reoxygenation [44, 45]. Therefore, endothelial apoptosis and blood perfusion reduction in SABR may enhance the response of tumor cells to radiation.
3.2 The combination of anti-angiogenic therapy and SABR
Anti-angiogenic therapy, which focuses on inhibiting neovascularization or endothelial cell function, has currently become an important strategy in cancer treatment [46, 47]. It has been reported that the normalization of vascular flow by anti-angiogenesis drugs can reverse the hypoxia and low pH in the tumor microenvironment, thus improving the radiosensitivity of cancer cells [48]. Combination of anti-angiogenic therapy with conventional fractionation radiotherapy has been reported to be a promising strategy in several clinical trials [49, 50]. With respect to SABR, many studies are investigating the safety and efficacy of its combination with different types of anti-angiogenesis drugs, as shown in Table 2.
Bevacizumab is one of the most widely used anti-angiogenesis drugs in cancer treatments. Although bevacizumab plus conventional regimen raidotherapy for the newly diagnosed glioblastoma obtained negative results in two well-known randomized controlled trials [51, 52], bevacizumab plus SRS for recurrent or progressive glioblastoma showed a promising clinical outcome with good tolerance. Morris et al. retrospectively reviewed 45 recurrent glioblastoma patients treated with SRS plus bevacizumab and showed a satisfying median progression free survival (PFS) of 5.3 months and a median OS of 13.3 months without radiation-related adverse events [53]. A phase II clinical trial (NCT02120287) with 16 recurrent or progressive glioblastoma patients who underwent SRS plus bevacizumab also achieved a promising outcome with a median OS of 11.7 months and a 6-month PFS of 56.2%. Similar results have been reported in several other restrospective studies or prospective clinical trials with a small sample size [54-56]. Prospective studies with a larger sample size and a longer follow-up time are needed to further confirm the efficacy and safety of this combination stategy. With respect to extra-cranial lesions, Mazzola et al. retrospectively reviewed 40 lung metastases of 23 colon cancer patients who underwent SBRT with or without bevacizumab, and they showed that 1-year local control rate in Bevacizumab-group was 93% versus 86% in No-Bevacizumab group and no toxicity superior or equal to grade 3 was recorded in both groups at the time of the analysis [57]. Similarly, a phase II clinical trial (NCT01569984) is investigating the combination of bevacizumab with SBRT for treating colorectal liver metastases.
Sorafenib, a multi-target inhibitor that targets the vascular endothelial growth factor receptor (VEGFR)/platelet-derived growth factor (PDGFR) pathway in tumor vasculature and the RAF/MEK/ERK pathway in tumor cells, has been widely used in the treatment of advanced liver cancer and renal cancer [58-60]. Preclinical experiments have shown that SBRT plus sorafenib improved the outcome for advanced hepatocellular carcinoma with acceptable toxicity [61, 62]. A phase I clinical trial by Brade et al. showed that the combination of SBRT with sorafenib in locally advanced liver cancer patients achieved a promising response rate (36%-50%), but with significant toxicity in patients with highly irradiated liver volume (30%-60%) [63]. In addition, a phase III clinical trial is currently comparing sorafenib plus SBRT and sorafenib alone for treating primary liver cancer (NCT01730937). Sunitinib, another multi-target inhibitor that inhibits several targets, including VEGFR1, VEGFR2, and VEGFR3, has been approved for the treatment of renal cancer, pancreatic cancer, and gastrointestinal stromal cancer. The combination of sunitinib with SABR has also been reported as a feasible strategy in several phase I/II clinical trials. A phase II study (NCT00463060) showed that SBRT plus sunitinib achieved a durable clinical response with manageable toxicity in a subset of cancer patients with oligometastases [64]. Another phase I/II trial showed that kidney and prostate cancer patients with oligometastases who received SBRT plus sunitinib achieved a significantly improved OS (hazard ratio = 0.25, p = 0.04) with manageable toxicity in most of the cases [65]. Further, a phase II trial showed that SRS plus adjuvant sunitinib exhibited an acceptable safety profile and a comparable PFS to SRS plus WBRT in patients with 1-3 brain metastases [66].
Registered clinical trials of combining SABR with anti-angiogenesis therapy
| Trial ID | Anti-angiogenesis drugs | Study design | Patients | Interventions | Primary endpoint |
|---|---|---|---|---|---|
| NCT02313272 | Bevacizumab | Phase I | Recurrent high-grade gliomas | SABR + Bevacizumab + Pembrolizumab | Safety and tolerability |
| NCT02829931 | Bevacizumab | Phase I | Recurrent high-grade gliomas | SABR + Bevacizumab + Nivolumab + Ipilimumab | Safety and tolerability |
| NCT01392209 | Bevacizumab | Phase I | Recurrent high-grade gliomas | SABR + Bevacizumab | Maximum tolerated dose of SABR |
| NCT02672995 | Bevacizumab | Phase I | Brain metastases | SABR + Bevacizumab | Maximum tolerated dose of SABR |
| NCT01569984 | Bevacizumab | Phase II | Colorectal liver metastasis | SBRT+ Bevacizumab | Tumor perfusion |
| NCT02120287 | Bevacizumab | Phase II | Recurrent or progressive glioblastoma | SRS+ Bevacizumab | OS |
| NCT01005875 | Sorafenib | Not Applicable | Unresectable hepatocellular carcinoma | SBRT + Sorafenib | Safety and tolerability |
| NCT01276210 | Sorafenib | Phase I | Brain Metastases | SBRT + Sorafenib | Maximum tolerated dose of Sorafenib |
| NCT00672178 | Sorafenib | Phase I/II | Metastatic, recurrent, or unresectable renal cell cancer | SBRT + Sorafenib | Response rate |
| NCT01730937 | Sorafenib | Phase III | Primary liver cancer | Arm A: Sorafenib Arm B: SBRT + Sorafenib | OS |
| NCT00910039 | Sunitinib | Phase II | Newly diagnosed brain metastases | SRS + Sunitinib | PFS |
| NCT00981890 | Sunitinib | Phase I | Brain Metastases | SRS + Sunitinib | Maximum tolerated dose of Sunitinib |
| NCT00463060 | Sunitinib | Phase I/II | Limited extent metastatic cancer | SABR+ Sunitinib | Dose limiting toxicity |
| NCT02019576 | Sunitinib | Phase II | Metastatic kidney cancer | SABR + Sunitinib | Local control of metastases |
| ChiCTR-ONC-13003720 | Endostar | Phase II | Advanced pancreatic cancer | SABR + Endostar + Capecitabine | PFS |
| NCT03356600 | Apatinib | Phase II | Brain metastases | SABR + Apatinib | PFS of intracranial lesions |
| NCT00822887 | Vandetanib | Phase I | Recurrent malignant gliomas | SABR + Vandetanib | Safety and tolerability |
In addition, several other studies are also investigating the combination of SABR with other anti-angiogenesis drugs, such as pazopanib [67], apatinib (NCT03356600), vandetanib (NCT00822887), and Endostar [68]. To summarize, combining SABR with anti-angiogenic therapy has showed promising efficacy and good tolerance in advanced cancers, and more prospective studies are needed to further confirm its efficacy and toxicity.
3.3 Issues to be solved in clinical application
From the perspective of clinical application, there are several practical issues remained to be solved regarding the combination of SABR with anti-angiogenic therapy, including the optimal timing of combination, the optimal dose/fractionation regimen of SABR, the optimal anti-angiogenesis agent, and the appropriate patients. Unfortunately, so far, the available data is too limited to draw definite conclusions regarding these issues. However, based on exsiting preclinical and clinical studies, we can make a hypothesis regarding the timing of combination. Preclinical studies have demonstrated that anti-angiogenesis agents cause a transient increase in blood perfusion by normalizing the abnormal tumor vasculature in the acute phase (1st to 3rd day after administration), followed by a significant reduction in blood perfusion resulted from the decreased tumor microvessel density on the 5th to 7th day [69, 70]. As a result, hypoxia in the tumor microenvironment, which is detrimental for the radiosensitivity, is alleviated in the acute phase. Therefore, the acute phase of anti-angiogenesis agents would be the optimal timing for SABR. Several clinical studies, in which SABR was performed shortly after the start of the administration of anti-angiogenesis agents, have obtained satisfying clinical outcomes [54, 63, 65]. In addition, for recurrent malignant glioma patients who received SRS and bevacizumab, the median OS was 14.4 months in a study in which SRS was performed immediately after the administration of bevacizumab [54], while the median OS was 10 months in another study in which SRS was performed before the administration of bevacizumab [56]. The results of these studies indicate that performing SABR shortly after the start of the administration of anti-angiogenesis agents is the opitmal timing, but more convincing evidence from well-designed prospective stuides are needed to further confirm it.
4. Synergistic effect of SABR and immune checkpoint inhibitors
Recently, the emergence of immune checkpoint inhibitors (ICIs) has changed the patterns of cancer treatment. A variety of preclinical and clinical studies have indicated that combining ICIs with SABR can induce a synergistic effect. A typical case of this synergistic effect was reported by Michael et al., in which SABR (28.5 Gy/3 F) reversed the acquired resistance to ipilimumab and achieved disease responses both inside and outside of the radiation field in a patient with recurrent and unresectable melanoma [71]. Several issues regarding the synergistic effect are discussed below from the perspective of clinical oncologists.
4.1 What is the mechanism for the synergistic effect?
It has been reported that radiotherapy can enhance tumor specific immunity via multiple mechanisms, including increasing the release of tumor associated antigens from irradiation induced dying cancer cells, enhancing the recruitment and activation of antigen presenting cells, promoting the priming of tumor specific T-cells, and inducing the release of cytokine and chemokines, such as type I/II interferons and complements [72, 73]. However, radiotherapy also has a negative impact on the immune system as leukocytes are highly sensitive to radiation and lymphopenia is a common phenomenon during the treatment of radiotherapy. In addition, the immune suppressive cells and inhibitory cytokines within the tumor microenvironment, such as the myeloid-derived suppressor cells, T regulatory (Treg) cells, transforming growth factor-β, and interleukin-10, can also induce local immune suppression [74]. Therefore, combination of radiotherapy with immunotherapy is a promising strategy for augmenting the antitumor immune response. As is well known, the most common type of immunotherapy in clinical practice is ICIs, such as the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) inhibitors (e.g., ipilimumab) and the programmed cell death 1/ programmed cell death ligand 1 (PD1/PD-L1) inhibitors (e.g., pembrolizumab, nivolumab, and durvalumab). A study by Dovedi et al. showed that fractionated radiotherapy caused PD-L1 upregulation on tumor cells in mouse models and combination of PD1/PD-L1 inhibitors with radiotherapy generated efficacious CD8 T-cell responses and better tumor control rate [75]. Similarly, another study by Sharabi et al. also showed that combining PD1 inhibitors with radiotherapy resulted in increased tumor antigen-specific T cell- and B cell-mediated immune responses in animal models [76]. In addition, a preclinical experiment showed that anti-CTLA4 inhibited Treg cells and increased the CD8 T-cell to Treg (CD8/Treg) ratio, while radiotherapy enhanced the diversity of the T-cell receptor (TCR) repertoire. Combination of anti-CTLA4 with radiotherapy promoted the expansion of cytotoxic T cells with an extended TCR repertoire [77].
It is noteworthy that the synergistic effect of radiotherapy with ICIs seems to be more commonly reported in SABR than conventional fractionation radiotherapy. Is SABR more powerful in enhancing antitumor immunity? On one hand, some studies have shown that a higher dose per fraction creates greater tumor immunogenicity. Schaue et al. irradiated mice bearing B16-OVA murine melanoma with a single dose of 5 Gy, 7.5 Gy, 10 Gy, or 15 Gy, and they showed that tumor-reactive T cells increased with the size of the radiation dose [78]. Moreover, an animal model experiment by Lan et al. compared the synergistic effect of anti-PDL1 with two fractionation regimens of the same BED (23 Gy/2 F vs 36 Gy/9 F) and showed that the 23 Gy/2 F group achieved better tumor control and OS than the 36 Gy/9 F group [79]. On the other hand, focused irradiation field of SABR resulted in less damage to the immune system. A study by Ladbury et al. evaluated the estimated dose of radiation to immune cells (EDRIC) in 117 patients with stage III NSCLC who received definitive radiotherapy. Their results showed that a higher EDRIC was correlated with greater risk of grade ≥3 lymphopenia (P = 0.004) and EDRIC was independently associated with OS (HR 1.17, P = 0.03), local PFS (HR 1.17, P = 0.02), and disease free survival (DFS) (HR 1.15, P = 0.04) [80]. Therefore, SABR is more powerful in enhancing antitumor immunity and works better with ICIs than conventional fractionation radiotherapy.
4.2 How is the efficacy and safety of the ICI-SABR combination in clinical practice?
As is already known, the prognosis of most advanced cancers is not satisfactory [81-83]. ICIs have obtained a promising clinical outcome in the treatment of many cancers, especially in NSCLC and melanoma [84-86]. SABR is commonly used as a palliative modality for advanced cancer with intent to achieve local control of metastatic lesions, such as brain and bone metastases. Based on the mechanism mentioned above, the ICI-SABR combination is expected to improve both local and systemic responses.
NSCLC is the most common type of cancer as well as a main cause of cancer-associated death worldwide [83, 87]. With the emergence of ICIs, the treatment of metastatic NSCLC has changed dramatically, as several well-known randomized controlled trials, such as CheckMate017 [88], CheckMate057 [89], and KEYNOTE010 [90], have obtained inspiring results. Meanwhile, SABR has also been regarded as an effective local treatment option for NSCLC with oligometastases [91]. Therefore, combining ICI with SABR is a strategy worth trying for treating advanced NSCLC. A phase 2 randomized trial (PEMBRO-RT) of 76 patients with recurrent or metastatic NSCLC showed that SBRT plus pembrolizumab achieved a higher overall response than pembrolizumab alone (36% vs 18%) without an increase in treatment-related toxicity [92]. In addition, Rodolfo et al. recently published a systemic review, which analyzed 1736 metastatic NSCLC patients in 6 phase I-II prospective studies and 12 retrospective studies from 2009 to 2019. Their results showed that SABR combined with anti-PD1, anti-PDL1 or anti-CTLA4 drugs obtained high rates of local control (71%) and distant/abscopal response (41%) with a good safety profile [93]. To further confirm the efficacy and safety of this strategy, several phase II and phase III clinical trials are ongoing, including NCT02492568, NCT03955198, and NCT03867175. In addition, SABR plus dual ICIs (anti PD1/PDL1 and anti-CTLA4) is also under investigation in a phase I clinical trial for advanced NSCLC (NCT03275597). It is worth mentioning that the ICI-SABR combination is also a promising strategy for early-stage NSCLC. It is well known that for early-stage NSCLC, SABR is a standard treatment option for patients who are medically inoperable or who refuse surgery. However, a main failure pattern of early-stage NSCLC is distant metastasis, and systemic treatment with good tolerability may be needed [94]. Therefore, the combination of SABR with ICI in early-stage NSCLC is a potential option, and several clinical trials are currently ongoing to investigate the feasibility of this strategy, as shown in Table 3.
Ongoing clinical studies of combining SABR with immune checkpoint inhibitors on ClinicalTrial.gov
| Registration ID | Study design | Patients | Interventions | Primary endpoint | |
|---|---|---|---|---|---|
| NSCLC | NCT02599454 | Phase I | Stage I NSCLC | SBRT+ Atezolizumab | Maximum tolerated dose |
| NCT03574220 | Phase I | Medically Inoperable Early Stage NSCLC | SBRT + Pembrolizumab | Percent of patients tolerant to Pembrolizumab | |
| NCT02599454 | Phase I | Stage I NSCLC | SBRT + Atezolizumab | Maximum tolerated dose | |
| NCT03050554 | Phase I/II | Early Stage NSCLC | SBRT + Avelumab | Incidence of adverse events | |
| NCT03383302 | Phase I/II | Stage I/II NSCLC | SBRT+ Nivolumab | Lung toxicity (pneumonitis) | |
| NCT03446911 | phase I/II | Stage I NSCLC (planned for surgery) | Arm A: SABR (Prior to surgery) Arm B: SABR + Pembrolizumab (Prior to surgery) | Incidence and severity of adverse events | |
| NCT02904954 | Phase II | Stage I (tumors > 2cm)/ II/ IIIA NSCLC | Arm A: Durvalumab + surgery Arm B: SBRT + Durvalumab + surgery | DFS | |
| NCT03110978 | Phase II | Stage I-IIA or Recurrent NSCLC | Arm A: SBRT + Nivolumab Arm B: SBRT | Event-free survival | |
| NCT03924869 | phase III | Stage I or IIA NSCLC | Arm A: SBRT (45-54Gy/3-5F) +Pembolizumab Arm B: SBRT (45-54Gy/3-5F) +Placebo | Event-free survival | |
| NCT03833154 | phase III | Unresected stage I/II, lymph node negative (T1-3N0M0) NSCLC. | Arm A: SBRT + Durvalumab Arm B: SBRT+ Placebo | PFS | |
| NCT03275597 | Phase I | Oligometastatic NSCLC | SBRT + Durvalumab+Tremelimumab | Incidence of adverse events | |
| NCT02492568 | Phase II | Advanced NSCLC | Arm A: Pembrolizumab Arm B: SBRT + Pembrolizumab | Overall response rate (ORR) | |
| NCT03955198 | phase II | NSCLC patients with 1 to 4 brain metastases | Arm A: SABR Arm B: SABR + Durvalumab | Time to intra-cranial progression | |
| NCT03867175 | phase III | Stage IV NSCLC | Arm A: SBRT +Pembolizumab Arm B: Pembolizumab | PFS | |
| Melanoma | NCT03354962 | phase I/II | Melanoma with extra-cranial metastases | Arm A: Nivolumab + Ipilimumab alone Arm B: SBRT with Nivolumab + Ipilimumab | Phase I: Dose Limiting Toxicities incidence. Phase II: PFS |
| NCT02858869 | Phase I | Melanoma or NSCLC with brain metastases | Arm A: Pembrolizumab+SRS (6Gy) Arm B: Pembrolizumab+SRS (9Gy) Arm C: Pembrolizumab+SRS (18-21Gy) | Incidence of adverse events | |
| NCT02716948 | Phase I | Melanoma with brain or spine metastases | SABR + Nivolumab | Incidence of serious adverse events | |
| Head and neck cancer | NCT03522584 | phase I/II | Recurrent/Metastatic squamous cell carcinomas of the head and neck | SBRT + Tremelimumab + Durvalumab, | Incidence of adverse effects |
| NCT03546582 | Phase II | Recurrent or second primary head and neck squamous cell carcinoma | Arm A: SBRT Arm B: SBRT + Pembrolizumab | PFS | |
| NCT03749460 | phase I/II | Salivary gland cancers | SBRT + Nivolumab + Ipilimumab | Incidence of adverse events | |
| NCT03539198 | Not applicable | Recurrent/Progressive locoregional or metastatic head and neck Cancer | Nivolumab+ proton SBRT | ORR | |
| NCT03618134 | Phase I/II | HPV positive oropharyngeal squamous cell caner | Arm A: SBRT + Durvalumab + TORS + neck dissection Arm B: SBRT + Durvalumab + Tremelimumab + TORS + neck dissection | Phase I: Incidence of adverse events Phase II: PFS | |
| NCT03402737 | Not Applicable | Recurrent squamous cell carcinoma of head and neck | Nivolumab + SBRT (2*6-8Gy, 3*6-8Gy, 3*6-10Gy ,3*6-12Gy) | Maximum tolerated dose of SBRT | |
| Breast cancer | NCT03464942 | Phase II | Advanced triple negative breast cancer | Arm A: SABR (20Gy * 1F) + Atezolizumab Arm B: SABR (8Gy * 3F) + Atezolizumab | PFS |
| NCT03449238 | Phase I/II | Metastatic breast cancer with at least 2 brain metastases | SRS + Pembrolizumab | Tumor response for non-irradiated brain lesions | |
| Liver cancer | NCT03817736 | Phase II | Hepatocellular carcinoma | SBRT + TACE + ICI | Number of patients amendable to curative surgical interventions |
| NCT03203304 | Phase I | Unresectable hepatocellular carcinoma | Arm A: SBRT + Nivolumab Arm B: SBRT+ Nivolumab+ Ipilimumab | Incidence of adverse events | |
| Pancreatic cancer | NCT03599362 | Phase II | Locally advanced unresectable pancreatic cancer | SBRT + Nivolumab + Cabiralizumab | Incidence of adverse events |
| NCT03716596 | Phase I | Late stage or recurrent pancreatic cancer patients | SBRT +Pembolizumab | OS | |
| NCT02311361 | Phase I/II | Unresectable pancreatic cancer | Arm A1: Durvalumab + SBRT (8Gy * 1F) Arm A2: Durvalumab + SBRT (5Gy * 5F) Arm B1: Tremelimumab+ SBRT (8Gy * 1F) Arm B2: Tremelimumab+ SBRT (5Gy * 5F) Arm C1: Tremelimumab+ Durvalumab + SBRT (8Gy * 1F) Arm C2: Tremelimumab+ Durvalumab + SBRT (5Gy * 5F) | Incidence of adverse events | |
| Genital tumor | NCT03452332 | Phase I | Recurrent or Metastatic cervical, vaginal, or vulvar cancers | SABR + Tremelimumab + Durvalumab | Incidence of adverse events |
| NCT03795207 | Phase II | Prostate cancer with oligometastatic relapse | Arm A: SBRT + Durvalumab Arm B: SBRT | PFS | |
| NCT03614949 | Phase II | Recurrent, persistent, or metastatic cervical cancer | SBRT + Atezolizumab | ORR | |
| NCT03312114 | Phase II | Persistent or recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer | SABR + Avelumab | ORR | |
| Merkel cell cancer | NCT03304639 | phase II | Advanced/metastatic Merkel cell carcinoma | Arm A: Pembrolizumab Arm B: Pembrolizumab + SBRT | PFS |
| NCT03071406 | Phase II | Metastatic Merkel cell carcinoma | Arm A: Nivolumab + Ipilimumab Arm B: Nivolumab + Ipilimumab + SBRT | ORR | |
| Soft tissue cancer | NCT03548428 | Phase II | Sarcoma with oligometastases | Arm A: SBRT + Atezolizumab Arm B: SBRT | PFS |
| NCT03399552 | Phase I/II | Malignant mesothelioma | SBRT + Avelumab | ORR |
Melanoma is another type of cancer which showed inspiring benefits from treatment with ICIs. Nowadays, ICIs have become the first-line treatment option for unresectable or metastatic melanoma. However, prognosis of advanced melanoma remains very poor, even after treatment with ICIs [86]. Therefore, the combination of ICI with SABR is under investigation to determine its feasibility. Murphy et al. analyzed 26 patients with metastatic melanoma who received ICIs (pembrolizumab, nivolumab, and/or ipilimumab) plus SRS for brain metastases and showed a favorable median survival of 26.1 months compared with historical controls without grade 4-5 toxicity [95]. Similarly, Minniti et al. retrospectively reviewed 80 melanoma patients with brain metastases who received SRS plus ipilimumab/nivolumab and showed meaningful intracranial control (6-month PFS 48-69%, 12-month PFS 17-42%) [96]. In addition, Diao et al. reviewed 91 melanoma patients treated with SRS for brain metastases and showed that patients who received ipilimumab had better OS than patients who did not received ipilimumab (median OS 15.1 months vs 7.8 months, p = 0.02) [97]. Besides, similar results have been reported in some other retrospective studies [98-100]. Therefore, ICI-SABR is a promising strategy for metastatic melanoma and several phase I/II clinical trials are underway to further confirm the safety and efficacy of this combination (NCT03354962, NCT02858869, and NCT02716948).
In addition, clinical trials of SABR plus ICIs are also under investigation for several other cancers, including head and neck cancer, breast cancer, liver cancer, pancreatic cancer, genital cancer, Merkel cell cancer, and soft tissue cancer, as listed in Table 3. To summarize, SABR plus ICIs has become a practical option for patients with metastases, especially for those with NSCLC and melanoma.
4.3 Issues to be solved in clinical application
From the perspective of clinical application, there are several practical issues remained to be solved regarding the ICI-SABR combination, especially the optimal sequence of combination and the optimal dose/fractionation regimen of SABR.
With respect to the sequence of combination, there are three modes under investigation: SABR followed by ICI, ICI followed by SABR, and concurrent SABR and ICI. As mentioned above, SABR can enhance anti-tumor immunity via multiple mechanisms, while ICI can enhance the efficacy of SABR by overcoming radiation-induced immunosuppression. Theoretically, concurrent SABR and ICI would be the best mode to obtain the synergistic effect, and this hypothesis is supported by several studies. Pinnamaneni et al. reviewed the survival outcomes of metastatic lung cancer patients who received nivolumab and SABR, and they found that patients receiving SABR during nivolumab treatment had significantly better OS than patients receiving SABR followed by nivolumab [101]. In addition, a randomized controlled study compared the efficacy of SABR followed by ipilimumab and SABR alone in 799 patients with metastatic prostate cancer, and no survival differences were observed between the two groups, indicating that SABR followed by ipilimumab was not an effective combination strategy [102]. Further, Chen et al. analyzed 260 cancer patients who had brain metastases treated with SRS, and they found that concurrent SRS and ICI was associated with a better OS compared with non-concurrent SRS and ICI (HR 2.40, p = 0.006) [99].Similar results have been reported in several other retrospective studies [103, 104]. It is worth mentioning that a major concern about the strategy of concurrent SABR and ICI is the safety issue, as radiotherapy and ICI may result in overlapping toxicities, such as the pneumonitis. However, evidence from retrospective studies and preliminary results of prospective studies has indicated that concurrent radiotherapy and ICI is tolerable. A phase 2 clinical study showed that concurrent radiotherapy with nivolumab was safe and tolerable regarding the 6-month rate of pneumonitis grade ≥ 3 for NSCLC [105]. A meta-analysis of 17 clinical studies showed that that concurrent SRS and ICI did not increase the overall incidence of radionecrosis than the non-concurrent group [104]. Taken together, concurrent SABR and ICI is the optimal sequence of combination, but more prospective stuides are needed to further confirm its efficacy and safety.
With respect to the optimal dose/fractionation regimen for the ICI-SABR combination, there is not enough data available to draw a definite conclusion, but the BED seems to be a potential reference for selecting appropriate regimens. A meta-analysis of the abscopal effect in preclinical models indicated that a SABR regimen with higher BED was more likely to trigger the abscopal effect, and a BED of 60 Gy resulted in a probability of 50% in generating abscopal effects [106]. In addition, Foster et al. analyzed 44,498 patients with stage IV NSCLC from the National Cancer Database, and their results showed that for patients receiving SABR and immunotherapy, a SABR regimen with BED higher than 60Gy was associated with better OS [107]. Further, Bang et al. retrospectively reviewed 133 patients with metastatic NSCLC, melanoma, or renal cell cancer who received ICI treatment and hypofractionation radiotherapy, and they found a significant association between increased BED and immune-related adverse events (p = 0.01) [108]. Therefore, taking BED as a reference to identify the appropriate dose/fractionation regimens with good efficacy and tolerability may be a feasible strategy.
5. Conclusions
To summarize, the appropriateness of the LQ model for SABR is controversial based on preclinical data, but it is a reliable tool from the perspective of clinical application because the BED calculated with it can represent the TCP. Hypoxia is a common phenomenon in SABR in spite of its relatively small tumor size and has a negative effect on the efficacy of SABR. Preliminary studies indicate that a hypoxic radiosensitizer combined with SABR may be a feasible strategy, but so far there is not adequate evidence to support its application in routine practice. The vascular change of endothelial apoptosis and blood perfusion reduction in SABR may enhance the response of tumor cells to radiation. Combination of SABR with anti-angiogenesis therapy has showed promising efficacy and good tolerance in advanced cancers. SABR is more powerful in enhancing antitumor immunity and works better with ICIs than conventional fractionation radiotherapy. Combination of SABR with ICIs has become a practical option for cancer patients with metastases.
Abbreviations
BED: biological effective dose; CTLA-4: cytotoxic T lymphocyte-associated antigen 4; DFS: disease free survival; EDRIC: estimated dose of radiation to immune cells; FFP: freedom from progression; HIF-1α: hypoxia-inducible factor 1α; ICI: immune checkpoint inhibitor; LQ: linear quadratic; LQ-L: linear-quadratic-linear; NSCLC: non-small cell lung cancer; ORR: overall response rate; OS: overall survival; PD1: programmed cell death 1; PD-L1: programmed cell death ligand 1; PDGFR: platelet-derived growth factor; PFS: progression free survival; PLQ: Pade linear quadratic; SABR: stereotactic ablative radiotherapy; SBRT: stereotactic body radiation therapy; SRS: stereotactic radiosurgery; TCP: tumor control probability; TCR: T-cell receptor; USC: universal survival curve; VEGFR: vascular endothelial growth factor receptor; 3D-CRT: 3-dimension conformal radiotherapy.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81974466). The authors would like to thank Letpub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chang JY. Stereotactic ablative radiotherapy: aim for a cure of cancer. Annals of translational medicine. 2015;3:12
2. Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? International journal of radiation oncology, biology, physics. 2014;88:254-62
3. Nahum AE. The radiobiology of hypofractionation. Clinical oncology (Royal College of Radiologists (Great Britain)). 2015;27:260-9
4. Brenner DJ, Hlatky LR, Hahnfeldt PJ, Huang Y, Sachs RK. The linear-quadratic model and most other common radiobiological models result in similar predictions of time-dose relationships. Radiation research. 1998;150:83-91
5. Fowler JF. 21 years of biologically effective dose. The British journal of radiology. 2010;83:554-68
6. Sheu T, Molkentine J, Transtrum MK, Buchholz TA, Withers HR, Thames HD. et al. Use of the LQ model with large fraction sizes results in underestimation of isoeffect doses. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013;109:21-5
7. Wang JZ, Huang Z, Lo SS, Yuh WT, Mayr NA. A generalized linear-quadratic model for radiosurgery, stereotactic body radiation therapy, and high-dose rate brachytherapy. Science translational medicine. 2010;2:39-48
8. Otsuka S, Shibamoto Y, Iwata H, Murata R, Sugie C, Ito M. et al. Compatibility of the linear-quadratic formalism and biologically effective dose concept to high-dose-per-fraction irradiation in a murine tumor. International journal of radiation oncology, biology, physics. 2011;81:1538-43
9. Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Seminars in radiation oncology. 2008;18:234-9
10. Guerrero M, Carlone M. Mechanistic formulation of a lineal-quadratic-linear (LQL) model: split-dose experiments and exponentially decaying sources. Medical physics. 2010;37:4173-81
11. Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. International journal of radiation oncology, biology, physics. 2008;70:847-52
12. Andisheh B, Edgren M, Belkic D, Mavroidis P, Brahme A, Lind BK. A comparative analysis of radiobiological models for cell surviving fractions at high doses. Technology in cancer research & treatment. 2013;12:183-92
13. Brown JM, Brenner DJ, Carlson DJ. Dose escalation, not "new biology," can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. International journal of radiation oncology, biology, physics. 2013;85:1159-60
14. Mehta N, King CR, Agazaryan N, Steinberg M, Hua A, Lee P. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: A pooled analysis of biological equivalent dose and local control. Pract Radiat Oncol. 2012;2:288-95
15. Guckenberger M, Klement RJ, Allgauer M, Appold S, Dieckmann K, Ernst I. et al. Applicability of the linear-quadratic formalism for modeling local tumor control probability in high dose per fraction stereotactic body radiotherapy for early stage non-small cell lung cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013;109:13-20
16. Santiago A, Barczyk S, Jelen U, Engenhart-Cabillic R, Wittig A. Challenges in radiobiological modeling: can we decide between LQ and LQ-L models based on reviewed clinical NSCLC treatment outcome data? Radiation oncology (London, England). 2016;11:67
17. Shuryak I, Carlson DJ, Brown JM, Brenner DJ. High-dose and fractionation effects in stereotactic radiation therapy: Analysis of tumor control data from 2965 patients. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015;115:327-34
18. van Leeuwen CM, Oei AL, Crezee J, Bel A, Franken NAP, Stalpers LJA. et al. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiation oncology (London, England). 2018;13:96
19. Zhao D, Constantinescu A, Hahn EW, Mason RP. Tumor oxygen dynamics with respect to growth and respiratory challenge: investigation of the Dunning prostate R3327-HI tumor. Radiation research. 2001;156:510-20
20. Milross CG, Tucker SL, Mason KA, Hunter NR, Peters LJ, Milas L. The effect of tumor size on necrosis and polarographically measured pO2. Acta oncologica (Stockholm, Sweden). 1997;36:183-9
21. Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxidants & redox signaling. 2014;21:1516-54
22. Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer research. 1996;56:4509-15
23. Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R. et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:1507-14
24. Kelada OJ, Decker RH, Nath SK, Johung KL, Zheng MQ, Huang Y. et al. High Single Doses of Radiation May Induce Elevated Levels of Hypoxia in Early-Stage Non-Small Cell Lung Cancer Tumors. International journal of radiation oncology, biology, physics. 2018;102:174-83
25. Qian Y, Von Eyben R, Liu Y, Chin FT, Miao Z, Apte S. et al. (18)F-EF5 PET-based Imageable Hypoxia Predicts Local Recurrence in Tumors Treated With Highly Conformal Radiation Therapy. International journal of radiation oncology, biology, physics. 2018;102:1183-92
26. Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. Journal of radiation research. 2016;57(Suppl 1):i90-i8
27. Carlson DJ, Keall PJ, Loo BW Jr, Chen ZJ, Brown JM. Hypofractionation results in reduced tumor cell kill compared to conventional fractionation for tumors with regions of hypoxia. International journal of radiation oncology, biology, physics. 2011;79:1188-95
28. Kirkpatrick JP, Cardenas-Navia LI, Dewhirst MW. Predicting the effect of temporal variations in PO2 on tumor radiosensitivity. International journal of radiation oncology, biology, physics. 2004;59:822-33
29. Goodman KA, Sneed PK, McDermott MW, Shiau CY, Lamborn KR, Chang S. et al. Relationship between pattern of enhancement and local control of brain metastases after radiosurgery. International journal of radiation oncology, biology, physics. 2001;50:139-46
30. Jensen RL, Minshew L, Shrieve AF, Hu N, Shrieve DC. Stereotactic radiosurgery and radiotherapy for meningiomas: biomarker predictors of patient outcome and response to therapy. Journal of radiosurgery and SBRT. 2012;2:41-50
31. Wittenborn TR, Horsman MR. Targeting tumour hypoxia to improve outcome of stereotactic radiotherapy. Acta oncologica (Stockholm, Sweden). 2015;54:1385-92
32. Drzymala RE, Wasserman TH, Won M, Shaw E, Cmelak AJ, Loeffler J. et al. A phase I-B trial of the radiosensitizer: etanidazole (SR-2508) with radiosurgery for the treatment of recurrent previously irradiated primary brain tumors or brain metastases (RTOG Study 95-02). Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2008;87:89-92
33. Toustrup K, Sorensen BS, Lassen P, Wiuf C, Alsner J, Overgaard J. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;102:122-9
34. Hassan Metwally MA, Ali R, Kuddu M, Shouman T, Strojan P, Iqbal K. et al. IAEA-HypoX. A randomized multicenter study of the hypoxic radiosensitizer nimorazole concomitant with accelerated radiotherapy in head and neck squamous cell carcinoma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015;116:15-20
35. Yang L, Taylor J, Eustace A, Irlam JJ, Denley H, Hoskin PJ. et al. A Gene Signature for Selecting Benefit from Hypoxia Modification of Radiotherapy for High-Risk Bladder Cancer Patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23:4761-8
36. O'Connor JPB, Robinson SP, Waterton JC. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. The British journal of radiology. 2019;92:20180642
37. Stieb S, Eleftheriou A, Warnock G, Guckenberger M, Riesterer O. Longitudinal PET imaging of tumor hypoxia during the course of radiotherapy. European journal of nuclear medicine and molecular imaging. 2018;45:2201-17
38. Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiation research. 2012;177:311-27
39. El Kaffas A, Giles A, Czarnota GJ. Dose-dependent response of tumor vasculature to radiation therapy in combination with Sunitinib depicted by three-dimensional high-frequency power Doppler ultrasound. Angiogenesis. 2013;16:443-54
40. Song CW, Lee YJ, Griffin RJ, Park I, Koonce NA, Hui S. et al. Indirect Tumor Cell Death After High-Dose Hypofractionated Irradiation: Implications for Stereotactic Body Radiation Therapy and Stereotactic Radiation Surgery. International journal of radiation oncology, biology, physics. 2015;93:166-72
41. Maeda A, Chen Y, Bu J, Mujcic H, Wouters BG, DaCosta RS. In vivo Imaging Reveals Significant Tumor Vascular Dysfunction and Increased Tumor Hypoxia-Inducible Factor-1alpha Expression Induced by High Single-Dose Irradiation in a Pancreatic Tumor Model. International journal of radiation oncology, biology, physics. 2017;97:184-94
42. Kocher M, Treuer H, Voges J, Hoevels M, Sturm V, Muller RP. Computer simulation of cytotoxic and vascular effects of radiosurgery in solid and necrotic brain metastases. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2000;54:149-56
43. Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A. et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science (New York, NY). 2003;300:1155-9
44. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer cell. 2005;8:89-91
45. Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY. et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer cell. 2005;8:99-110
46. Lupo G, Caporarello N, Olivieri M, Cristaldi M, Motta C, Bramanti V. et al. Anti-angiogenic Therapy in Cancer: Downsides and New Pivots for Precision Medicine. Frontiers in pharmacology. 2016;7:519
47. Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y. et al. Role of tumor microenvironment in tumorigenesis. Journal of Cancer. 2017;8:761-73
48. Zeng J, Baik C, Bhatia S, Mayr N, Rengan R. Combination of stereotactic ablative body radiation with targeted therapies. The Lancet Oncology. 2014;15:e426-34
49. Bao Y, Peng F, Zhou QC, Yu ZH, Li JC, Cheng ZB. et al. Phase II trial of recombinant human endostatin in combination with concurrent chemoradiotherapy in patients with stage III non-small-cell lung cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015;114:161-6
50. Kang M, Wang F, Liao X, Zhou P, Wang R. Intensity-modulated radiotherapy combined with endostar has similar efficacy but weaker acute adverse reactions than IMRT combined with chemotherapy in the treatment of locally advanced nasopharyngeal carcinoma. Medicine. 2018;97:e11118
51. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA. et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370:699-708
52. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R. et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370:709-22
53. Morris SL, Zhu P, Rao M, Martir M, Zhu JJ, Hsu S. et al. Gamma Knife Stereotactic Radiosurgery in Combination with Bevacizumab for Recurrent Glioblastoma. World neurosurgery. 2019;127:e523-e33
54. Cabrera AR, Cuneo KC, Desjardins A, Sampson JH, McSherry F, Herndon JE 2nd. et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. International journal of radiation oncology, biology, physics. 2013;86:873-9
55. Abbassy M, Missios S, Barnett GH, Brewer C, Peereboom DM, Ahluwalia M. et al. Phase I Trial of Radiosurgery Dose Escalation Plus Bevacizumab in Patients With Recurrent/Progressive Glioblastoma. Neurosurgery. 2018;83:385-92
56. Cuneo KC, Vredenburgh JJ, Sampson JH, Reardon DA, Desjardins A, Peters KB. et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. International journal of radiation oncology, biology, physics. 2012;82:2018-24
57. Mazzola R, Levra NG, Ricchetti F, Fersino S, Fiorentino A, Aiello D. et al. Increased Efficacy of Stereotactic Ablative Radiation Therapy in Combination with Bevacizumab in Lung Oligopersistent/Oligoprogressive Metastases from Colon Cancer. International Journal of Radiation Oncology • Biology • Physics. 2017;99:E437
58. Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods in enzymology. 2006;407:597-612
59. Wang HT, Xia M. A meta-analysis of efficacy and safety of sorafenib versus other targeted agents for metastatic renal cell carcinoma. Medicine. 2019;98:e13779
60. Pinter M, Peck-Radosavljevic M. Review article: systemic treatment of hepatocellular carcinoma. Alimentary pharmacology & therapeutics. 2018;48:598-609
61. Hsieh CH, Chen YJ, Tsai TH, Wang LY. Liver Stereotactic Body Radiation Therapy Modulates Systemic Pharmacokinetics of Sorafenib Through p-Glycoprotein. International Journal of Radiation Oncology • Biology • Physics. 2018;102:e55
62. Li Q, Hu Y, Xi M, He L, Zhao L, Liu M. Sorafenib modulates the radio sensitivity of hepatocellular carcinoma cells in vitro in a schedule-dependent manner. BMC cancer. 2012;12:485
63. Brade AM, Ng S, Brierley J, Kim J, Dinniwell R, Ringash J. et al. Phase 1 Trial of Sorafenib and Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. International journal of radiation oncology, biology, physics. 2016;94:580-7
64. Tong CC, Ko EC, Sung MW, Cesaretti JA, Stock RG, Packer SH. et al. Phase II trial of concurrent sunitinib and image-guided radiotherapy for oligometastases. PloS one. 2012;7:e36979
65. Kao J, Chen CT, Tong CC, Packer SH, Schwartz M, Chen SH. et al. Concurrent sunitinib and stereotactic body radiotherapy for patients with oligometastases: final report of a prospective clinical trial. Targeted oncology. 2014;9:145-53
66. Ahluwalia MS, Chao ST, Parsons MW, Suh JH, Wang D, Mikkelsen T. et al. Phase II trial of sunitinib as adjuvant therapy after stereotactic radiosurgery in patients with 1-3 newly diagnosed brain metastases. Journal of neuro-oncology. 2015;124:485-91
67. De Wolf K, Rottey S, Vermaelen K, Decaestecker K, Sundahl N, De Lobel L. et al. Combined high dose radiation and pazopanib in metastatic renal cell carcinoma: a phase I dose escalation trial. Radiation oncology (London, England). 2017;12:157
68. Shan J, Qing Y, Li M, Sui J, Zhang S, Feng Y. et al. A phase II clinical trial of capecitabine combined with recombinant human endostatin and stereotactic radiotherapy as first-line treatment in patients with locally advanced pancreatic cancer. Journal of Clinical Oncology. 2015;33:e15253
69. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer cell. 2014;26:605-22
70. Sun X, Deng L, Lu Y. Challenges and opportunities of using stereotactic body radiotherapy with anti-angiogenesis agents in tumor therapy. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2018;30:147-56
71. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. The New England journal of medicine. 2012;366:925-31
72. Walshaw RC, Honeychurch J, Illidge TM. Stereotactic ablative radiotherapy and immunotherapy combinations: turning the future into systemic therapy? The British journal of radiology. 2016;89:20160472
73. Xing D, Siva S, Hanna GG. The Abscopal Effect of Stereotactic Radiotherapy and Immunotherapy: Fool's Gold or El Dorado? Clinical oncology (Royal College of Radiologists (Great Britain)). 2019;31:432-43
74. Ishihara D, Pop L, Takeshima T, Iyengar P, Hannan R. Rationale and evidence to combine radiation therapy and immunotherapy for cancer treatment. Cancer immunology, immunotherapy: CII. 2017;66:281-98
75. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer research. 2014;74:5458-68
76. Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E. et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer immunology research. 2015;3:345-55
77. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373-7
78. Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. International journal of radiation oncology, biology, physics. 2012;83:1306-10
79. Lan J, Li R, Yin LM, Deng L, Gui J, Chen BQ. et al. Targeting Myeloid-derived Suppressor Cells and Programmed Death Ligand 1 Confers Therapeutic Advantage of Ablative Hypofractionated Radiation Therapy Compared With Conventional Fractionated Radiation Therapy. International journal of radiation oncology, biology, physics. 2018;101:74-87
80. Ladbury CJ, Rusthoven CG, Camidge DR, Kavanagh BD, Nath SK. Impact of Radiation Dose to the Host Immune System on Tumor Control and Survival for Stage III Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. International journal of radiation oncology, biology, physics. 2019;105:346-55
81. Zhao Q, Zheng B, Meng S, Xu Y, Guo J, Chen LJ. et al. Increased expression of SLC46A3 to oppose the progression of hepatocellular carcinoma and its effect on sorafenib therapy. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;114:108864
82. Liu S, Qi L, Han W, Wan X, Jiang S, Li Y. et al. Overexpression of wip1 is associated with biologic behavior in human clear cell renal cell carcinoma. PloS one. 2014;9:e110218
83. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394-424
84. Chen Y, Zhou Y, Tang L, Peng X, Jiang H, Wang G. et al. Immune-Checkpoint Inhibitors as the First Line Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Journal of Cancer. 2019;10:6261-8
85. Xu Z, Yan Y, Wang X, Zeng S, Gong Z. Lung Immune Prognostic Index for Outcome Prediction to Immunotherapy in Patients With NSCLC. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2019;14:e207-e8
86. Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D. Immunotherapy in melanoma: Recent advances and future directions. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2017;43:604-11
87. Kuang X, Xiao J, Dai L-X, Zhang L-H, He B-X. Correlative analysis of gene mutation and clinical features in patients with non-small cell lung cancer. Translational Cancer Research. 2019;8:736-51
88. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:123-35
89. Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ. et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Annals of oncology: official journal of the European Society for Medical Oncology. 2018;29:959-65
90. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England). 2016;387:1540-50
91. Song A, Lu B. Utility of stereotactic ablative radiotherapy/stereotactic body radiation therapy in the setting of oligometastatic non-small cell lung cancer. Journal of thoracic disease. 2018;10:657-60
92. Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV. et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncology. 2019;5:1276-82
93. Chicas-Sett R, Morales-Orue I, Castilla-Martinez J, Zafra-Martin J, Kannemann A, Blanco J. et al. Stereotactic Ablative Radiotherapy Combined with Immune Checkpoint Inhibitors Reboots the Immune Response Assisted by Immunotherapy in Metastatic Lung Cancer: A Systematic Review. International journal of molecular sciences. 2019;20:E2173
94. Vansteenkiste J, Wauters E, Reymen B, Ackermann CJ, Peters S, De Ruysscher D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol. 2019;30:1244-53
95. Murphy B, Walker J, Bassale S, Monaco D, Jaboin J, Ciporen J. et al. Concurrent Radiosurgery and Immune Checkpoint Inhibition: Improving Regional Intracranial Control for Patients With Metastatic Melanoma. American journal of clinical oncology. 2019;42:253-7
96. Minniti G, Anzellini D, Reverberi C, Cappellini GCA, Marchetti L, Bianciardi F. et al. Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. Journal for immunotherapy of cancer. 2019;7:102
97. Diao K, Bian SX, Routman DM, Yu C, Ye JC, Wagle NA. et al. Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. Journal of neuro-oncology. 2018;139:421-9
98. Amaral T, Tampouri I, Eigentler T, Keim U, Klumpp B, Heinrich V. et al. Immunotherapy plus surgery/radiosurgery is associated with favorable survival in patients with melanoma brain metastasis. Immunotherapy. 2019;11:297-309
99. Chen L, Douglass J, Kleinberg L, Ye X, Marciscano AE, Forde PM. et al. Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma. International journal of radiation oncology, biology, physics. 2018;100:916-25
100. Lanier CM, Hughes R, Ahmed T, LeCompte M, Masters AH, Petty WJ. et al. Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries. Neuro-oncology practice. 2019;6:402-9
101. Pinnamaneni R, Hegde AM, Cherukuri SD, Arastu HH, Bowling M, Leinweber C. et al. Sequence of stereotactic ablative radiotherapy and immune checkpoint blockade in the treatment of metastatic lung cancer. Journal of Clinical Oncology. 2017;35:e20665
102. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology. 2014;15:700-12
103. Samstein R, Rimner A, Barker CA, Yamada Y. Combined Immune Checkpoint Blockade and Radiation Therapy: Timing and Dose Fractionation Associated with Greatest Survival Duration Among Over 750 Treated Patients. International Journal of Radiation Oncology • Biology • Physics. 2017;99:S129-S30
104. Lehrer EJ, Peterson J, Brown PD, Sheehan JP, Quinones-Hinojosa A, Zaorsky NG. et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2019;130:104-12
105. Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A. et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung cancer (Amsterdam, Netherlands). 2019;133:83-7
106. Marconi R, Strolin S, Bossi G, Strigari L. A meta-analysis of the abscopal effect in preclinical models: Is the biologically effective dose a relevant physical trigger? PloS one. 2017;12:e0171559
107. Foster CC, Sher DJ, Rusthoven CG, Verma V, Spiotto MT, Weichselbaum RR. et al. Overall survival according to immunotherapy and radiation treatment for metastatic non-small-cell lung cancer: a National Cancer Database analysis. Radiation oncology (London, England). 2019;14:18
108. Bang A, Wilhite TJ, Pike LRG, Cagney DN, Aizer AA, Taylor A. et al. Multicenter Evaluation of the Tolerability of Combined Treatment With PD-1 and CTLA-4 Immune Checkpoint Inhibitors and Palliative Radiation Therapy. International journal of radiation oncology, biology, physics. 2017;98:344-51
109. Macia IGM. Radiobiology of stereotactic body radiation therapy (SBRT). Reports of practical oncology and radiotherapy: journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology. 2017;22:86-95
Author contact
![]() Corresponding author: Liangfang Shen; slf658312edu.cn
Corresponding author: Liangfang Shen; slf658312edu.cn

 Global reach, higher impact
Global reach, higher impact