Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(21):6413-6420. doi:10.7150/jca.46978 This issue Cite
Research Paper
Genetic polymorphisms in folate-metabolizing genes associated with gastric cancer prognosis in northwest China subjects
1. Department of General Surgery, Tangdu Hospital, The Air Force Military Medical University, Xi'an, 710038, China.
2. Department of Microbiology, The Air Force Military Medical University, Xi'an, 710032, China.
*These authors contributed equally to this work.
Received 2020-4-13; Accepted 2020-8-3; Published 2020-9-13
Abstract
Influence of folate metabolism has long been studied in cancer and copies evidences have suggested that the key genes involved were correlated with GC risk and prognosis. However, their genetically association and contribution for GC prognosis are still elusive. To evaluate the effect of folate metabolism related genes polymorphisms on the prognosis of gastric cancer (GC), the genotype of seven single nucleotide polymorphisms (SNPs) of three genes were selected and genotyped in a cohort of 664 GC patients, including genes of Methylenetetrahydrofolate reductase (MTHFR), Methionine synthase reductase (MTRR), and Methionine synthase (MTR). Kaplan-Meier Curve, long-rank tests and multivariate Cox proportional hazard model were used for prognosis analysis. The results demonstrated that TT or CT/TT genotypes of SNP rs1532268 in MTRR gene coding region are significantly associated with a poorer overall survival (OS) when compared with CC genotype (HR=2.340, 95% CI: 1.240-4.414, p=0.009; or HR=1.502, 95% CI: 1.083-2.085, p=0.015, respectively). Furthermore, comparing to that of the CC genotype, the detrimental effect of rs1532268 TT genotype was also evident in the special subgroups of GC patients, especially in patients with BMI<24 and H. pylori infection. Moreover, significant association between increased relapse and TT genotype of rs1532268 was also observed in patients who are females, BMI<24 and without chemotherapy. In addition, the joint analysis demonstrated that integration of rs1532268 genotypes and BMI, H. pylori infection status, clinical stage and tumor site may significantly improve the predictive abilities for predicting OS of GC patients. In conclusion, it suggested that the MTRR rs1532268 polymorphism is significantly associated with clinical outcomes of GC patients, especially in those with lower BMI (BMI<24) or positive H. pylori infection status, which warrants further validation. And the polymorphism of MTRR rs1532268 may be a potential prognostic factor for GC patients.
Keywords: folate metabolism, MTRR, MTHFR, MTR, polymorphism, gastric cancer prognosis
Introduction
Gastric cancer is the third deadliest cancer in the world and the absolute number of cases is increasing every year due to aging and growing of high-risk populations [1]. Geographically, about 43% of total global cases are concentrated in China [2] and its mortality remains the third among all human cancers in China [3]. The development of gastric cancer (GC) represents a complex interaction of host factors with infections agents and environmental factors [4]. Despite recent important developments in our understanding of the pathophysiology of GC, patients diagnosed with this disease still have a poor prognosis, with a 5-year survival rate <25% [5].
There are still reduced therapeutic options for GC patients and the survival and prognosis of GC patients still depend on the stage of the tumor at the time of diagnosis. Recently, genomic analyses of gastric tumors have emphasized their molecular heterogeneity [6]. The distinction of gastric cancer molecular subtypes may be a key to identify novel therapeutic targets, to guide early diagnosis strategies, predict patient outcome, and response to therapy [7, 8].
It has been suggested that dysfunctions of folate-mediated one-carbon metabolism (FOCM) could contribute to carcinogenesis, which is a key pathway essential for the processes of DNA synthesis, methylation and repair [9, 10]. Furthermore, it may highlight therapeutic targets for gastrointestinal cancer [11]. The enzymes, including Methylenetetrahydrofolate reductase (MTHFR), Methionine synthase (MTR) and Methionine synthase reductase (MTRR) are crucial components in FOCM, to catalyze S-adenosylmethionine (SAM) synthesis from folate uptake. MTHFR is crucial rate-limiting step for FOCM, carrying out an irreversible conversion of 5,10-methylene-tetrahydrofolate (5,10-MTHF) to 5-methyl-tetrahydrofolate (5-MTHF) [12]. MTR catalyzes the re-methylation of homocysteine to methionine, which is a precursor of SAM [13]. And MTRR is a flavor-protein maintains the activity of MTR [14]. The genetic variants of these genes may influence enzyme activity and folate status, which may modulate gastric cancer development and progress [9, 12, 15].
Accumulating evidences have supported that the functional polymorphisms of the genes of MTHFR, MTR and MTRR may affect the risk of GC [16-24], but the results are variable. Recently, the effects of genomic polymorphisms in FOCM related genes on survival of gastric cancer patients were studied [25, 26], however, it still need more evidences to unveil the detailed association and enable better prognosis. Herein, the effects of seven SNPs of these three folate metabolizing genes on the clinical outcomes of 664 Chinese people were assessed. Additionally, we performed analyses stratified by BMI status to address the possibility that the lifestyle factor may modify the effect on the genetic polymorphism of GC clinical outcomes, besides age, sex, tumor diameter, H. pylori infection status, clinical stage and chemotherapy.
Materials and Methods
Study subjects
681 pathologically confirmed incident GC cases were enrolled from the Tangdu hospital (between November, 2007 and October, 2012) and Xijing Hospital (between October, 2006 to May, 2009) in Shaanxi province. All GC cases received surgical resection and had no previous history of other cancers or any preoperative anticancer treatment or blood transfusion within 3 months before surgery. There were no age, sex, or disease stage restrictions for case recruitment. Socio-demographic and clinical data were collected during recruitment. Clinical staging of GC tumors was done according to the WHO standard. Finally, 664 patients with resected gastric adenocarcinoma were included in the present study for prognostic analysis. The present study protocol was approved by the Institutional Review Board of Air Force Military Medical University. The procedures were performed according to the approved guidelines and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from each participant included in the study.
Demographic and clinical data
Demographic and clinical data were collected through in-person interviews at the initial visit or follow up in the clinics, medical records, or consultation with treating physicians, including age, sex, ethnicity, residential region, time of diagnosis, time of surgery and/or adjuvant chemotherapy (ACT), time of relapse and/or death, tumor stage, and treatment protocol. Clinical stage 0 and I were sorted as early stage group, while clinical stage II and III were considered as middle stage group, clinical IV were considered as late stage group. Cases were followed for survival status and chemotherapy data every 6 months. The latest follow-up data in this analysis was obtained in October 2014. Overall survival (OS) was defined as the time from surgery to GC-specific death. Relapse-free survival (RFS) was defined as the time from surgery to the date of the first recurrence or distant metastasis of GC. Patients alive at the last follow-up were censored.
Genotyping
Peripheral blood samples from GC patients were drawn in to coded sodium citrate anticoagulant tubes and were centrifuged within 30 min by the investigators. The E.Z.N.A. Blood DNA Midi Kit (Omega Bio-Tek, Norcross, GA, USA) was used for genomic DNA extraction. All the genomic DNA was aliquoted and stored at -80 °C for future analysis.
The candidate functional SNPs of the folate metabolism related genes MTHFR, MTR and MTRR were performed according to a set of web-based SNP selection tools (https://manticore.niehs.nih.gov/snpinfo/snpfunc.html)[27]. Finally, seven functional SNPs were selected, including MTHFR rs2274976 (C>T), rs1801133 (G>A); MTR rs1805087 (A>G), rs2853522 (A>C); MTRR rs1801394 (A>G), rs1532268 (C>T), rs162036 (A>G). Genotyping of seven candidate SNPs was performed using Agena MassARRAY RS1000 system according to the standard protocol (Applied Biosystems, Foster City, CA, USA). Internal quality controls were used to ensure genotyping accuracy.
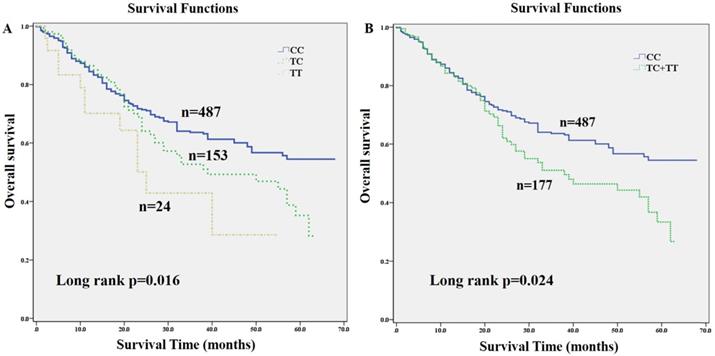
Kaplan-Meier estimates of overall survival (OS) for gastric cancer (GC) patients stratified by genetic variants of MTRR gene. OS of GC patients stratified by SNP rs1532268. (A) Overall survival of MTRR rs1532268 co-dominant genotypes in GC patients; (B) overall survival of MTRR rs1532268 dominant genotypes in GC patients.
Statistical analysis
Statistics analyses were carried out using the IBM SPSS Statistics 20.0 software (IBM). Kaplan-Meier curves and log-rank tests were also used to evaluate effect of the individual SNPs on OS and RFS. Cox proportional hazard regression model was applied to assess the effect of individual SNP and patients' characteristics on OS or RFS. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated with adjustment for age, gender, BMI, H. pylori infection status, clinical stage, tumor diameter, and chemotherapy status. All statistical tests were two-sided, with p < 0.05 as the boundary value.
Results
Association of polymorphisms and clinical outcome
The clinical characteristics of 664 GC patients were summarized in Table 1. At latest follow-up, 287 patients developed relapse and 226 died. Significant OS and RFS of patients were observed among subgroup of tumor size, clinical stage and chemotherapy condition by univariate Cox regression analysis (p <0.05), respectively (Table 1).
The association of three folate metabolizing genes SNP genotypes with GC clinical outcome were assessed using multivariate Cox regression analysis with adjustment for age, gender, tumor diameter, BMI status, H. pylori infection status, clinical stage and chemotherapy (Table 2). The results showed that SNP rs1532268 polymorphism was significantly associated with OS of GC patients. Compared to patients with CC genotype, patients with TT genotype or combined TT/CT genotypes had significantly higher death risk (HR=2.34, 95% CI: 1.240-4.414, p=0.009; HR=1.502, 95% CI: 1.083-2.085, p=0.015), respectively. In addition, Kaplan-Meier curves analysis also provided a strong association with OS. The median OS time was 47 months in patients with the CC genotype, 39 months in patients with CT genotype, 25 months in patients with TT genotype. Patients carrying TT or combined TT/CT genotypes of rs1532268 had worse OS than did those with CC genotype (p=0.016; p=0.024) (Fig. 1), respectively, which also indicated that the rs1532268 polymorphism played prognostic role in GC. However, negative results were obtained for the other SNPs involved in this study using multivariate Cox regression analysis.
Stratified analysis on association of MTRR rs1532268 polymorphisms with clinical outcome by host variables
Stratified analyses were conducted to evaluate the associations between genotypes of MTRR rs1532268 and clinical outcome by age, gender, clinical stage, H. pylori infection status, BMI, tumor diameter, tumor site and ACT (Table 3). The significant detrimental effects conferred by rs1532268 TT genotype was more prominent in special subgroups. Compared with CC genotype, the significant increased death risk associated with TT genotype of rs1532268 was observed in patients in age <60 years (HR=3.064, 95% CI: 1.400-6.704), females (HR=6.975, 95% CI: 1.475-32.981), with positive H. pylori infection status (HR=3.169, 95% CI: 1.497-6.712), negative ACT status (HR=4.249, 95% CI: 1.252-14.422), middle stage GC (clinical stage II/III) (HR=2.245, 95% CI: 1.118-4.509), and BMI<24 (HR=3.217, 95% CI: 1.672-6.190). Moreover, TC and TT genotypes of rs1532268 showed association with increased death risk in patients with non-cardia GC (HR=1.616, 95% CI: 1.077-2.516; HR=2.367, 95% CI: 1.063-5.270), respectively, when compared with CC genotype. Furthermore, the significant increased relapse risk associated with TT genotype of rs1532268 was also observed in female patients (HR=4.827, 95% CI: 1.083-21.523), and patients with BMI<24 (HR=2.265, 95% CI: 1.221-4.200), negative ACT status (HR=4.674, 95% CI: 1.390-15.719), cardia GC (HR=3.170, 95% CI: 1.042-9.638), when compared with CC genotype.
Joint effect between rs1532268 genotypes and H. pylori infection, BMI status, clinical stage, tumor site and chemotherapy on OS
A joint analysis was performed to assess the potential modulating effect of rs1532268 polymorphism on the clinical characteristics associated with OS of GC patients. Comparing with patients carrying rs1532268 CC genotypes and without H. pylori infection, those with TT genotype and H. pylori infection had a significantly increase death risk (HR=2.361, 95% CI: 1.086-5.134). The similar results were also observed in patients with rs1532268 TT genotype and BMI <24 (HR=2.691, 95% CI: 1.414-5.122). Moreover, compared with patients carrying CC genotype and non-cardia type, patients with TC genotype and non-cardia type (HR=1.583, 95% CI: 1.041-2.406), TT genotype and non-cardia type (HR=2.233, 95% CI: 1.014-4.917), CC genotype and cardia type (HR=2.12, 95% CI: 1.402-3.207) showed increased death risk. In addition, compared with patients with CC genotype and in early stage, patients with CC genotype and in middle stage (HR=3.951, 95% CI: 1.864-8.371), CC genotype and in late stage (HR=16.234, 95% CI: 7.033-37.476), TC genotype and in middle stage (HR=6.034, 95% CI: 2.706-13.452), TC genotype and in late stage (HR=14.791, 95% CI: 5.057-43.262), TT genotype and in middle stage (HR=8.835, 95% CI: 3.307-23.605), TT genotype and in late stage (HR=33.751, 95% CI: 6.861-166.040) also showed increased death risk. However, negative results were observed in the integration of rs1532268 genotypes and chemotherapy status. In conclusion, these results provide the potential additional predictive abilities of rs1532268 polymorphism and the clinical characters in predicting GC OS.
Discussion
More attention has been paid on the folate metabolism for its important role in cancer [10]. MTHFR, MTR and MTRR genes play key roles in folate metabolism pathway and were most examined in cancer risk and prognosis. In present study, the effects of polymorphisms in these genes on prognosis of GC patients were investigated. The results demonstrated that TT or CT/TT genotypes of SNP rs1532268 in MTRR coding region are significantly associated with a poorer OS in a set of 664 GC patients when compared with CC genotypes. Furthermore, comparing to that of the CC genotype, the detrimental effect of rs1532268 TT genotype was also evident in the subgroups of GC patients. The rs1532268 TT genotype was associated with increased death risk of GC patients in age <60 years, females, BMI<24, middle stage GC (clinical stage II/III), with H. pylori infection and without chemotherapy. Moreover, significant association between increased relapse and TT genotype of rs1532268 was also observed in patients who are females, BMI<24 and without chemotherapy.
Copies evidences proved that MTRR polymorphisms were associated with risk and prognosis of GC. But most reports focus on the MTRR rs1801394 polymorphisms, which displayed a protective effect on GC among Chinese population [25, 26]. Our knowledge on the association of cancer and MTRR rs1532268 polymorphism is very limited. It has been reported that MTRR rs1532268 polymorphism was associated with increased risk of prostate cancer [28], while other studies did not reveal any obviously significant differences of MTRR rs1532268 polymorphisms among other cancers. Our finding indicated that patients with MTRR rs1532268 CT/TT genotypes played harmful role on GC prognosis.
MTRR is a flavoprotein that maintains the activity of MTR [14], which catalyzed the remethylation of homocysteine to produce methionine, functioning as a precursor for the universal methyl group donor S-adenosylmethionine. The polymorphism of MTRR rs1532268, causing a serine to leucine switch in protein sequence, may impact MTRR enzymatic activity. Definitely positive relationship between polymorphisms of rs1532268 and gastrointestinal stomal tumor has been noted (https://www.snpedia.com/index.php/Special:FormEdit/ClinVar_Disease/Gastrointestinal_stromal_tumor), and we found that MTRR rs1532268 CT/TT genotype showed a comparatively worse OS of GC patients in this study, especially the TT genotype was associated with increased death risk in middle stage GC patients. The reason may be that the MTRR TT/CT genotype might reduce the affinity of MTRR for MTR and less efficient reactivation [29], leading to increased homocysteine [30]. Therefore, the less remethylation of homocysteine may generate less methionine for DNA methylation. It is proposed that the reduced methylation on promoters of tumor genes would strength the GC cell growth and invasiveness.
Distribution of patients' characteristics and prognosis analysis
| Variables | OS | RFS | |||||
|---|---|---|---|---|---|---|---|
| Total/Death | HR (95%CI) | pa | Total/Relapse | HR (95%CI) | pa | ||
| Age | <60 | 375/122 | 1.000 | 375/159 | 1.000 | ||
| ≥60 | 289/104 | 1.185 (0.912-1.539) | 0.204 | 289/128 | 1.064 (0.843-1.343) | 0.601 | |
| Gender | male | 512/168 | 1.000 | 512/213 | 1.000 | ||
| female | 152/58 | 1.178 (0.874-1.588) | 0.283 | 152/74 | 1.214 (0.932-1.581) | 0.151 | |
| BMIb | <24 | 416/135 | 1.000 | 416/170 | 1.000 | ||
| ≥24 | 163/46 | 0.858 (0.614-1.199) | 0.370 | 163/60 | 0.881 (0.656-1.182) | 0.398 | |
| H. pylorib | No | 187/55 | 1.000 | 187/81 | 1.000 | ||
| Yes | 392/126 | 1.002 (0.728-1.38) | 0.989 | 392/149 | 0.851 (0.648-1.117) | 0.245 | |
| Tumor diameterb | <5 | 377/93 | 1.000 | 377/128 | 1.000 | ||
| ≥5 | 273/128 | 2.071 (1.586-2.705) | <0.001 | 273/153 | 1.968 (1.556-2.489) | <0.001 | |
| Clinical stagesb | 0 | 14/2 | 1.000 | 14/2 | 1.000 | ||
| Ⅰ | 126/9 | 0.478 (0.103-2.215) | 0.345 | 126/13 | 0.669 (0.151-2.965) | 0.597 | |
| Ⅱ | 316/102 | 2.659 (0.655-10.789) | 0.171 | 316/129 | 3.418 (0.845-13.819) | 0.085 | |
| Ⅲ | 148/70 | 5.986 (1.464-24.47) | 0.013 | 148/95 | 8.789 (2.162-35.734) | 0.002 | |
| Ⅳ | 55/41 | 12.394 (2.986-51.445) | <0.001 | 55/45 | 14.937 (3.61-61.802) | <0.001 | |
| ACTb | No | 233/58 | 1.000 | 233/63 | 1.000 | ||
| Yes | 430/168 | 1.515 (1.124-2.043) | 0.006 | 430/224 | 2.029 (1.534-2.684) | <0.001 | |
Note: HR: hazard ratio; CI: confidence interval; ACT, adjuvant chemotherapy;
a: univariate analysis by COX proportional hazard regression model;
b: Patient numbers may not add up to 100% of available subjects because of missing clinical data.
Genotypes of MTHFR, MTR, MTRR genes polymorphism with clinical outcome of gastric cancer patients
| SNP ID | Genotype | Total/Events | OS | Total/Events | RFS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pc | MSTb | HR (95%CI) | pa | pc | MST | HR (95%CI) | pa | ||||
| rs2274976 | CC | 588/202 | 57 | 1.000 | 588/255 | 34 | 1.000 | ||||
| TC | 73/22 | 0.001 | 47b | 0.614 (0.352-1.072) | 0.086 | 73/30 | 0.004 | 51 | 0.641 (0.393-1.045) | 0.074 | |
| TT | 3/2 | 12 | 4.882 (0.67-35.589) | 0.118 | 3/2 | 4 | 4.014 (0.543-29.688) | 0.173 | |||
| Dominant | 76/24 | 0.528 | 46 b | 0.655 (0.382-1.123) | 0.124 | 76/32 | 0.844 | 51 | 0.672 (0.417-1.083) | 0.103 | |
| rs1801133 | AA | 189/62 | 57 | 1.000 | 189/78 | 34 | 1.000 | ||||
| GA | 318/96 | 0.018 | 47 b | 1.015 (0.715-1.441) | 0.934 | 318/128 | 0.036 | 55 | 1.019 (0.748-1.389) | 0.904 | |
| GG | 154/67 | 32 | 1.189 (0.791-1.786) | 0.405 | 154/79 | 22 | 1.112 (0.77-1.604) | 0.572 | |||
| Dominant | 472/163 | 0.7 | 62 | 1.07 (0.773-1.48) | 0.683 | 472/207 | 0.619 | 38 | 1.048 (0.785-1.399) | 0.751 | |
| rs1805087 | AA | 562/194 | 57 | 1.000 | 562/240 | 37 | 1.000 | ||||
| GA | 98/29 | 0.221 | 48 b | 0.763 (0.485-1.201) | 0.242 | 98/43 | 0.028 | 32 | 1.029 (0.712-1.487) | 0.881 | |
| GG | 4/3 | 19 | 0.848 (0.194-3.703) | 0.826 | 4/4 | 5 | 3.516 (0.807-15.325) | 0.094 | |||
| Dominant | 102/32 | 0.334 | 47 b | 0.769 (0.498-1.189) | 0.238 | 102/47 | 0.945 | 28 | 1.075 (0.75-1.539) | 0.695 | |
| rs2853522 | CC | 218/82 | 49 | 1.000 | 218/102 | 26 | 1.000 | ||||
| AC | 322/106 | 0.455 | 62 | 0.914 (0.658-1.270) | 0.593 | 322/141 | 0.321 | 38 | 0.961 (0.719-1.283) | 0.787 | |
| AA | 123/38 | 57 | 0.784 (0.499-1.232) | 0.291 | 123/44 | 40b | 0.7 (0.461-1.063) | 0.094 | |||
| Dominant | 445/144 | 0.218 | 62 | 0.879 (0.643-1.201) | 0.418 | 445/185 | 0.280 | 42 | 0.89 (0.675-1.174) | 0.410 | |
| rs1532268 | CC | 487/156 | 47b | 1.000 | 487/208 | 46 | 1.000 | ||||
| TC | 153/58 | 0.016 | 39 | 1.386 (0.975-1.97) | 0.069 | 153/66 | 0.257 | 29 | 1.084 (0.788-1.49) | 0.621 | |
| TT | 24/12 | 25 | 2.340 (1.240-4.414) | 0.009 | 24/13 | 16 | 1.692 (0.927-3.088) | 0.087 | |||
| Dominant | 177/70 | 0.024 | 38 | 1.502 (1.083-2.085) | 0.015 | 177/79 | 0.435 | 26 | 1.158 (0.86-1.559) | 0.333 | |
| rs162036 | AA | 466/164 | 57 | 1.000 | 466/209 | 31 | 1.000 | ||||
| GA | 181/58 | 0.654 | 45 b | 0.929 (0.666-1.296) | 0.666 | 181/75 | 0.171 | 42 | 0.944 (0.706-1.263) | 0.700 | |
| GG | 16/4 | 56 | 0.682 (0.167-2.786) | 0.594 | 16/3 | 46 b | 0.24 (0.033-1.722) | 0.156 | |||
| Dominant | 197/62 | 0.522 | 45 b | 0.916 (0.66-1.271) | 0.600 | 197/78 | 0.339 | 40 b | 0.903 (0.677-1.206) | 0.491 | |
| rs1801394 | AA | 358/122 | 0.997 | 57 | 1.000 | 258/155 | 35 | 1.000 | |||
| GA | 260/90 | 59 | 1.014 (0.744-1.383) | 0.930 | 260/118 | 0.512 | 33 | 1.261 (0.962-1.654) | 0.093 | ||
| GG | 45/14 | 50 | 0.854 (0.434-1.679) | 0.647 | 45/14 | 40 b | 0.763 (0.395-1.474) | 0.421 | |||
| Dominant | 305/104 | 0.946 | 57 | 0.994 (0.735-1.345) | 0.971 | 305/132 | 0.908 | 37 | 1.195 (0.917-1.559) | 0.188 | |
a: adjusted by age, gender, BMI, H. pylori infection status, clinical stage, tumor diameter, and chemotherapy status;
b:Mean survival time was provided when MST could not be calculated;
c: Log-rank p;
HR: hazard ratio; CI: confidence interval; MST, median survival time.
Stratified analysis of the MTRR rs1532268 polymorphism with gastric cancer OS and RFS
| Genotype | Total/ Event | OS | Total/ Event | RFS | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | pa | HR (95%CI) | pa | |||||
| Age | <60 | CC | 277/83 | 1.000 | 277/115 | 1.000 | ||
| TC | 83/31 | 1.559 (0.958-2.538) | 0.074 | 83/36 | 1.223 (0.796-1.878) | 0.359 | ||
| TT | 15/8 | 3.064 (1.4-6.704) | 0.005 | 15/8 | 1.741 (0.816-3.715) | 0.152 | ||
| ≥60 | CC | 210/73 | 1.000 | 210/93 | 1.000 | |||
| TC | 70/27 | 1.396 (0.825-2.362) | 0.214 | 70/30 | 1.018 (0.623-1.662) | 0.944 | ||
| TT | 9/4 | 1.752 (0.533-5.759) | 0.355 | 9/5 | 1.782 (0.633-5.018) | 0.274 | ||
| Gender | female | CC | 115/40 | 1.000 | 115/57 | 1.000 | ||
| TC | 34/15 | 2.185 (0.992-4.814) | 0.052 | 34/14 | 1.33 (0.635-2.787) | 0.450 | ||
| TT | 3/3 | 6.975 (1.475-32.981) | 0.014 | 3/3 | 4.827 (1.083-21.523) | 0.039 | ||
| male | CC | 372/116 | 1.000 | 372/151 | 1.000 | |||
| TC | 119/43 | 1.239 (0.822-1.867) | 0.307 | 119/52 | 1.09 (0.758-1.567) | 0.642 | ||
| TT | 21/9 | 1.898 (0.942-3.824) | 0.073 | 21/10 | 1.47 (0.761-2.841) | 0.252 | ||
| H. pylori | no | CC | 139/39 | 1.000 | 139/61 | 1.000 | ||
| TC | 41/13 | 1.31 (0.659-2.605) | 0.442 | 41/17 | 1.088 (0.611-1.938) | 0.774 | ||
| TT | 7/3 | 1.328 (0.395-4.464) | 0.646 | 7/3 | 1.245 (0.374-4.142) | 0.721 | ||
| Yes | CC | 284/85 | 1.000 | 284/104 | 1.000 | |||
| TC | 92/33 | 1.383 (0.913-2.094) | 0.126 | 92/36 | 1.088 (0.739-1.602) | 0.670 | ||
| TT | 16/8 | 3.169 (1.497-6.712) | 0.003 | 16/9 | 2.229 (1.104-4.499) | 0.025 | ||
| BMI | BMI≥24 | CC | 120/34 | 1.000 | 120/45 | 1.000 | ||
| TC | 40/12 | 1.343 (0.665-2.71) | 0.411 | 40/15 | 1.183 (0.638-2.193) | 0.594 | ||
| TT | 3/0 | NA | 0.972 | 3/0 | NA | 0.976 | ||
| BMI<24 | CC | 303/90 | 1.000 | 303/120 | 1.000 | |||
| TC | 93/34 | 1.491 (0.983-2.261) | 0.060 | 93/38 | 1.139 (0.779-1.666) | 0.503 | ||
| TT | 20/11 | 3.217 (1.672-6.190) | <0.001 | 20/12 | 2.265 (1.221-4.200) | 0.009 | ||
| Tumor diameter | < 5 cm | CC | 278/61 | 1.000 | 278/90 | 1.000 | ||
| TC | 84/26 | 1.573 (0.934-2.65) | 0.088 | 84/31 | 1.136 (0.72-1.793) | 0.583 | ||
| TT | 15/6 | 2.417 (1.012-5.773) | 0.047 | 15/7 | 1.722 (0.778-3.813) | 0.180 | ||
| ≥5 cm | CC | 201/92 | 1.000 | 201/114 | 1.000 | |||
| TC | 63/30 | 1.258 (0.770-2.054) | 0.359 | 63/33 | 1.092 (0.688-1.732) | 0.709 | ||
| TT | 9/6 | 2.818 (1.085-7.321) | 0.033 | 9/6 | 2.013 (0.789-5.133) | 0.143 | ||
| Clinical stage | early | CC | 108/10 | 1.000 | 108/14 | 1.000 | ||
| TC | 28/1 | 0.491 (0.055-1.372) | 0.524 | 28/1 | 0.37 (0.047-2.944) | 0.347 | ||
| TT | 4/0 | NA | 0.991 | 4/0 | NA | 0.986 | ||
| middle | CC | 335/113 | 1.000 | 335/158 | 1.000 | |||
| TC | 112/49 | 1.48 (0.999-2.193) | 0.050 | 112/55 | 1.084 (0.762-1.543) | 0.652 | ||
| TT | 17/10 | 2.245 (1.118-4.509) | 0.023 | 17/11 | 1.702 (0.885-3.274) | 0.111 | ||
| late | CC | 42/32 | 1.000 | 42/35 | 1.000 | |||
| TC | 10/7 | 0.814 (0.309-2.146) | 0.678 | 10/8 | 1.08 (0.453-2.575) | 0.863 | ||
| TT | 3/2 | 2.710 (0.522-14.078) | 0.236 | 3/2 | 2.363 (0.475-11.758) | 0.294 | ||
| ACT | negative | CC | 186/44 | 1.000 | 186/51 | 1.000 | ||
| TC | 41/11 | 1.984 (0.929-4.234) | 0.077 | 41/9 | 1.205 (0.530-2.737) | 0.656 | ||
| TT | 6/3 | 4.249 (1.252-14.422) | 0.020 | 6/3 | 4.674 (1.390-15.719) | 0.013 | ||
| positive | CC | 301/112 | 1.000 | 301/157 | 1.000 | |||
| TC | 111/47 | 1.267 (0.852-1.885) | 0.243 | 111/57 | 1.072 (0.758-1.517) | 0.693 | ||
| TT | 18/9 | 2 (0.948-4.221) | 0.069 | 18/10 | 1.344 (0.673-2.685) | 0.403 | ||
| Tumor site | Cardia | CC | 87/35 | 1.000 | 87/43 | 1.000 | ||
| TC | 34/12 | 1.02 (0.507-2.050) | 0.956 | 34/15 | 0.797 (0.428-1.483) | 0.474 | ||
| TT | 4/3 | 2.187 (0.589-8.12) | 0.242 | 4/4 | 3.170 (1.042-9.638) | 0.042 | ||
| Non-cardia | CC | 312/80 | 1.000 | 312/112 | 1.000 | |||
| TC | 95/33 | 1.646 (1.077-2.516) | 0.021 | 95/37 | 1.268 (0.862-1.865) | 0.228 | ||
| TT | 18/7 | 2.367 (1.063-5.270) | 0.035 | 18/7 | 1.361 (0.621-2.982) | 0.441 | ||
a: adjusted by age, gender, BMI, H. pylori infection status, clinical stage, tumor diameter, and chemotherapy status;
NA: the corresponding value could not be calculated;
HR: hazard ratio; CI, confidence interval; ACT, adjuvant chemotherapy.
Furthermore, the current study demonstrated that the SNP rs1532268 affect OS and RFS of GC patients more prominent in specific subgroup patients. Obesity is a major health issue and a risk factor for cancer prognosis [31]. BMI ≥24 was used to designate over-weight in Chinese, and rs1532268 TT genotype had poor prognosis in patients with low BMI (BMI<24), while the effect of BMI on gastric cancer is still inconsistent [32-34]. H. pylori infection is another important factor in GC risk [35], significant relationship between rs1532268 polymorphism and clinical outcomes was also observed in the patients with H. pylori infection. And for females it also showed worse OS and RFS, who was considered as low gastric cancer risk. In addition, integration of rs1532268 genotypes and clinical characters may improve the predictive abilities for predicting OS of GC patients. Therefore, the genetic factor rs1532268 polymorphism showed its interactions among patients with different HP infection status and life style factors and its promising role in modulating tumor progression.
Joint effect of rs1532268 genotypes and HP infection, BMI status, clinical stage, tumor site and chemotherapy on OS
| Variables | HR (95%CI) | pa |
|---|---|---|
| Genotype with/without H. pylori | ||
| CC+ without H. pylori infection | 1.000 | |
| CC+ with H. pylori infection | 0.869 (0.589-1.283) | 0.480 |
| TC+ without H. pylori infection | 1.316 (0.684-2.531) | 0.411 |
| TC+ with H. pylori infection | 1.178 (0.732-1.894) | 0.500 |
| TT+ without H. pylori infection | 1.331 (0.408-4.346) | 0.635 |
| TT+ with H. pylori infection | 2.361 (1.086-5.134) | 0.030 |
| Genotype + BMI | ||
| CC+BMI<24 | 1.000 | |
| CC+BMI≥24 | 1.097 (0.725-1.658) | 0.662 |
| TC+BMI<24 | 1.414 (0.937-2.135) | 0.099 |
| TC+BMI≥24 | 1.280 (0.692-2.365) | 0.431 |
| TT+BMI<24 | 2.691 (1.414-5.122) | 0.003 |
| TT+BMI≥24 | NA | 0.959 |
| Genotype stage | ||
| CC+ early stage | 1.000 | |
| CC+ middle stage | 3.951 (1.864-8.371) | <0.001 |
| CC+ late stage | 16.234 (7.033-37.476) | <0.001 |
| TC+ early stage | 0.594 (0.074-4.760) | 0.624 |
| TC+ middle stage | 6.034 (2.706-13.452) | <0.001 |
| TC+ late stage | 14.791 (5.057-43.262) | <0.001 |
| TT+ early stage | NA | 0.959 |
| TT+ middle stage | 8.835 (3.307-23.605) | <0.001 |
| TT+ late stage | 33.751 (6.861-166.040) | <0.001 |
| Genotype with/without chemotherapy | ||
| CC+ without chemotherapy | 1.000 | |
| CC+ with chemotherapy | 0.884 (0.597-1.307) | 0.535 |
| TC+ without chemotherapy | 1.843 (0.889-3.821) | 0.100 |
| TC+ with chemotherapy | 1.083 (0.683-1.718) | 0.735 |
| TT+ without chemotherapy | 2.967 (0.909-9.686) | 0.072 |
| TT + with chemotherapy | 1.67 (0.774-3.607) | 0.191 |
| Genotype with/without cardia | ||
| CC+ Non-cardia | 1.000 | |
| CC+ cardia | 2.120 (1.402-3.207) | <0.001 |
| TC+ Non-cardia | 1.583 (1.041-2.406) | 0.032 |
| TC+ cardia | 1.822 (0.971-3.420) | 0.062 |
| TT+ Non-cardia | 2.233 (1.014-4.917) | 0.046 |
| TT+ cardia | 3.100 (0.958-10.033) | 0.059 |
a: adjusted by age, gender, BMI, H. pylori infection status, clinical stage, tumor diameter, and chemotherapy status.
NA: the corresponding value could not be calculated.
Overall, our data strongly suggest that rs1532268 of MTRR involved in folate metabolism pathway had a significant effect on the clinical outcome of GC patients in a Chinese population, especially for patients with lower BMI or positive HP infection status. The present study has potential clinical significance in helping to refine therapeutic decisions in treatment of GC. Moreover, since our study was restricted to Han Chinese, we cannot rule out the generalizability issue. Future studies in larger populations and other ethnics are warranted.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 81572916, 81502424). The funding body has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Camargo MC, Figueiredo C, Machado JC. Review: Gastric malignancies: Basic aspects. Helicobacter. 2019;24(Suppl 1):e12642
2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
4. de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219-40
5. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-917
6. Oh SC, Sohn BH, Cheong JH, Kim SB, Lee JE, Park KC. et al. Clinical and genomic landscape of gastric cancer with a mesenchymal phenotype. Nat Commun. 2018;9:1777
7. Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J. et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476-85 85 e1-11
8. Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS. et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-56
9. Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000;130:129-32
10. Newman AC, Maddocks ODK. One-carbon metabolism in cancer. Br J Cancer. 2017;116:1499-504
11. Konno M, Asai A, Kawamoto K, Nishida N, Satoh T, Doki Y. et al. The one-carbon metabolism pathway highlights therapeutic targets for gastrointestinal cancer (Review). Int J Oncol. 2017;50:1057-63
12. Kim YI. Methylenetetrahydrofolate reductase polymorphisms, folate, and cancer risk: a paradigm of gene-nutrient interactions in carcinogenesis. Nutr Rev. 2000;58:205-9
13. Gos M Jr, Szpecht-Potocka A. Genetic basis of neural tube defects. II. Genes correlated with folate and methionine metabolism. J Appl Genet. 2002;43:511-24
14. Moyers S, Bailey LB. Fetal malformations and folate metabolism: review of recent evidence. Nutr Rev. 2001;59:215-24
15. Kim W, Woo HD, Lee J, Choi IJ, Kim YW, Sung J. et al. Dietary folate, one-carbon metabolism-related genes, and gastric cancer risk in Korea. Mol Nutr Food Res. 2016;60:337-45
16. Shen H, Xu Y, Zheng Y, Qian Y, Yu R, Qin Y. et al. Polymorphisms of 5,10-methylenetetrahydrofolate reductase and risk of gastric cancer in a Chinese population: a case-control study. Int J Cancer. 2001;95:332-6
17. Miao X, Xing D, Tan W, Qi J, Lu W, Lin D. Susceptibility to gastric cardia adenocarcinoma and genetic polymorphisms in methylenetetrahydrofolate reductase in an at-risk Chinese population. Cancer Epidemiol Biomarkers Prev. 2002;11:1454-8
18. Stolzenberg-Solomon RZ, Qiao YL, Abnet CC, Ratnasinghe DL, Dawsey SM, Dong ZW. et al. Esophageal and gastric cardia cancer risk and folate- and vitamin B(12)-related polymorphisms in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2003;12:1222-6
19. Kim JK, Kim S, Han JH, Kim HJ, Chong SY, Hong SP. et al. Polymorphisms of 5,10-methylenetetrahydrofolate reductase and risk of stomach cancer in a Korean population. Anticancer Res. 2005;25:2249-52
20. Shen H, Newmann AS, Hu Z, Zhang Z, Xu Y, Wang L. et al. Methylenetetrahydrofolate reductase polymorphisms/haplotypes and risk of gastric cancer: a case-control analysis in China. Oncol Rep. 2005;13:355-60
21. Wang L, Ke Q, Chen W, Wang J, Tan Y, Zhou Y. et al. Polymorphisms of MTHFD, plasma homocysteine levels, and risk of gastric cancer in a high-risk Chinese population. Clin Cancer Res. 2007;13:2526-32
22. Cui LH, Shin MH, Kweon SS, Kim HN, Song HR, Piao JM. et al. Methylenetetrahydrofolate reductase C677T polymorphism in patients with gastric and colorectal cancer in a Korean population. BMC Cancer. 2010;10:236
23. De Re V, Cannizzaro R, Canzonieri V, Cecchin E, Caggiari L, De Mattia E. et al. MTHFR polymorphisms in gastric cancer and in first-degree relatives of patients with gastric cancer. Tumour Biol. 2010;31:23-32
24. Lin J, Zeng RM, Li RN, Cao WH. Aberrant DNA methylation of the P16, MGMT, and hMLH1 genes in combination with the methylenetetrahydrofolate reductase C677T genetic polymorphism and folate intake in gastric cancer. Genet Mol Res. 2014;13:2060-8
25. Zhao T, Gu D, Xu Z, Huo X, Shen L, Wang C. et al. Polymorphism in one-carbon metabolism pathway affects survival of gastric cancer patients: Large and comprehensive study. Oncotarget. 2015;6:9564-76
26. Zhao T, Xu Z, Gu D, Wu P, Huo X, Wei X. et al. The effects of genomic polymorphisms in one-carbon metabolism pathways on survival of gastric cancer patients received fluorouracil-based adjuvant therapy. Sci Rep. 2016;6:28019
27. Bao G, Qu F, He L, Zhao H, Wang N, Ji G. et al. Prognostic Significance of Tag SNP rs1045411 in HMGB1 of the Aggressive Gastric Cancer in a Chinese Population. PLoS One. 2016;11:e0154378
28. Basir A. Methionine Synthase Reductase-A66G and -C524T Single Nucleotide Polymorphisms and Prostate Cancer: A Case-Control Trial. Asian Pac J Cancer Prev. 2019;20:1445-51
29. Olteanu H, Munson T, Banerjee R. Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry. 2002;41:13378-85
30. Garcia-Minguillan CJ, Fernandez-Ballart JD, Ceruelo S, Rios L, Bueno O, Berrocal-Zaragoza MI. et al. Riboflavin status modifies the effects of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) polymorphisms on homocysteine. Genes Nutr. 2014;9:435
31. Sahakyan MA, Shahbazyan SS, Martirosyan A, Gabrielyan A, Petrosyan H, Sahakyan AM. Gastrectomy for Gastric Cancer in Patients with BMI >/= 30 kg/m(2). Am Surg. 2020;86:158-63
32. Voglino C, Di Mare G, Ferrara F, De Franco L, Roviello F, Marrelli D. Clinical and Oncological Value of Preoperative BMI in Gastric Cancer Patients: A Single Center Experience. Gastroenterol Res Pract. 2015;2015:810134
33. Wada T, Kunisaki C, Ono HA, Makino H, Akiyama H, Endo I. Implications of BMI for the Prognosis of Gastric Cancer among the Japanese Population. Dig Surg. 2015;32:480-6
34. Chen S, Nie RC, OuYang LY, Li YF, Xiang J, Zhou ZW. et al. Body mass index (BMI) may be a prognostic factor for gastric cancer with peritoneal dissemination. World J Surg Oncol. 2017;15:52
35. Martinez-Campos C, Torres-Poveda K, Camorlinga-Ponce M, Flores-Luna L, Maldonado-Bernal C, Madrid-Marina V. et al. Polymorphisms in IL-10 and TGF-beta gene promoter are associated with lower risk to gastric cancer in a Mexican population. BMC Cancer. 2019;19:453
Author contact
![]() Corresponding authors: Department of General Surgery, Tangdu Hospital, The Air Force Military Medical University, 569 Xinsi Street, Xi'an 710038, China. Tel.: + 86 29 84774573; Fax: + 86 29 83226349. E-mail addresses: guoqiangedu.cn (G. Bao), wangheedu.cn (X. He).
Corresponding authors: Department of General Surgery, Tangdu Hospital, The Air Force Military Medical University, 569 Xinsi Street, Xi'an 710038, China. Tel.: + 86 29 84774573; Fax: + 86 29 83226349. E-mail addresses: guoqiangedu.cn (G. Bao), wangheedu.cn (X. He).

 Global reach, higher impact
Global reach, higher impact