Impact Factor
ISSN: 1837-9664
J Cancer 2020; 11(24):7320-7328. doi:10.7150/jca.47314 This issue Cite
Research Paper
Sodium to globulin ratio as a prognostic factor for patients with advanced gastric cancer
1. Medical Oncology Department of Gastrointestinal Cancer, Liaoning Cancer Hospital & Institute, Cancer Hospital of China Medical University, No.44 Xiaoheyan Road, Dadong District, Shenyang 110042, Liaoning Province, China.
2. Department of Medical Oncology, Shenyang Fifth People Hospital, Tiexi District, Shenyang 110020, Liaoning Province, China.
3. Department of Medical Oncology, Liaohua Hospital, Hongwei District, Liaoyang 111003, Liaoning Province, China.
4. Department of Hepatobiliary Surgery, Liaoning Cancer Hospital & Institute, Cancer Hospital of China Medical University, No.44 Xiaoheyan Road, Dadong District, Shenyang 110042, Liaoning Province, China.
5. Department of Gastrointestinal Surgery, The Second Hospital Affiliated to Harbin Medical University, No. 246, Xuefu Road, Nangang District, Harbin 150086, Heilongjiang Province, China.
Received 2020-4-22; Accepted 2020-10-12; Published 2020-10-23
Abstract
Background: Electrolyte disturbance and systemic inflammation contributes to poor prognosis of cancer patients. Levels of serum sodium and globulin can reflect electrolyte homeostasis and inflammatory state, respectively, therefore have potential as prognostic factors for cancer patients. In this study, we hypothesized that sodium to globulin ratio (SGR) could have superior accuracy in predicting cancer patient survival, than sodium and globulin alone. We therefore sought to investigate its efficacy in prognosis of patients with advanced gastric cancer (GC) receiving first-line chemotherapy.
Methods: A total of 265 patients, with advanced GC, were recruited in this retrospective study from January 2014 to January 2019. We first determined SGR cut-off values using the receiver operating characteristic (ROC) analysis, then analyzed the relationship between pretreatment SGR and clinicopathological features and the effect of chemotherapy. Finally, we evaluated progression-free survival (PFS) and overall survival (OS) rates of the entire and subgroup populations using univariate and multivariate logistic regressions.
Results: SGR recorded a cut-off value of 5.54, and had a significantly higher area under the curve (AUC) value (0.619, p = 0.001) than fibrinogen (0.575, p = 0.034) and albumin (0.610, p = 0.002) alone. Organ metastasis, and peritoneal invasion ratios, as well as neutrophil and CA72-4 levels varied significantly between the low-SGR (SGR≤ 5.54) and high SGR (SGR> 5.54) groups (all p < 0.05). Specifically, patients in the low-SGR group exhibited significantly lower disease control rates (83.4%) than those in the high-SGR group (97.2%) (p < 0.001). Results from multivariate analysis indicated that high-SGR was an independent risk factor for PFS (Hazard ratio [HR]: 0.539, p < 0.001) and OS (HR: 0.574, p < 0.001). Moreover, patients in the low-SGR group exhibited significantly worse PFS (134 vs. 221 days, p < 0.001) and OS (311 vs. 420 days, p < 0.001) than those in the high-SGR group. Furthermore, subgroup analysis revealed that SGR was still a powerful prognostic indicator in GC patients with good prognosis or normal biochemical indexes, including no peritoneal infiltration, normal neutrophil counts, and normal serum sodium and globulin levels (all p < 0.001).
Conclusions: Overall, our findings indicate that SGR is a novel and promising prognostic factor for GC patients. It has superior accuracy, to sodium and globulin alone, hence it is a powerful tool for evaluating effects of treatment, PFS, and OS in patients with advanced GC, who receive first-line chemotherapy.
Keywords: sodium to globulin ratio, gastric cancer, first-line chemotherapy, prognosis, progression-free survival, overall survival
Introduction
Gastric cancer (GC), one of the most common malignancies, has been associated with high mortality rates [1]. Technological advancement in gastric cancer screening has significantly improved detection of early GC patients, resulting in a high (90%) five-year survival rate of early-stage GC patients, following curative treatment. However, for many patients with advanced stage GC, lack of or mild cancer symptoms at diagnosis, implies they cannot undergo curative resection. Despite numerous research efforts being directed towards development of various therapeutic strategies, the median survival rates for patients with advanced-stage GC remains at only 13 months[2]. To improve survival rates in this group of patients, it is imperative to identify novel and effective prognostic factors.
Previous studies have implicated various pathophysiological factors, such as electrolyte disturbance and systemic inflammation in poor outcomes of cancer patients [3-5]. In addition, serum sodium and globulin concentrations have been employed as biochemical indicators for routine laboratory tests. In fact, previous studies have shown that these indicators alone can objectively reflect electrolyte disturbance and inflammatory status of patients, thereby effectively predicting therapeutic response and prognosis of various tumors [6-9]. On the other hand, the ratio of sodium to globulin (SGR) may result in superior efficacy in predicting prognosis of cancer patients, than sodium and globulin alone. However, only a handful of studies have reported the use of SGR as a prognostic factor in cancer.
In this study, we evaluated the effect of SGR before first-line chemotherapy on treatment response, progression-free survival (PFS), and overall survival (OS) of patients with advanced GC.
Materials and Methods
Patients
A total of 265 advanced GC patients, who received first-line chemotherapy upon admission to the Cancer Hospital of China Medical University, were enrolled in the study between January 2014 to January 2019. Patients were only included if they met the following criteria: (1) gastric cancer was confirmed pathologically; (2) metastasis was confirmed in organs using computed tomography (CT), magnetic resonance imaging (MRI) scans, or other imaging methods; (3) patients' TNM staging indicated that all cases were stage III-IV; (4) subjects completed no less than two cycles of first-line chemotherapy after the diagnosis of the disease; (5) patients had no acute infection and received nutritional support; and (6) a majority of the patients were treated with a first-line cytotoxic drug: oxaliplatin, cisplatin, fluorouracil, and docetaxel. Ethical approval was obtained from the Cancer Hospital of China Medical University Ethics Committee, prior to conducting the study.
Definitions
A blood sample was collected from each patient, one week prior to cancer treatment. Standard reference ranges for serum sodium, globulin, potassium, albumin, blood urea nitrogen (BUN), creatinine (CREA), hemoglobin, neutrophil counts, thrombocyte counts, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) and carbohydrate antigen 72-4 (CA72-4) were 135-145 mmol/L, 20-35 g/L, 3.5-5.3 mmol/L, 35-55 g/L, 3.2-6.0 mmol/L, 59-105 µmoI/L, 115-155 g/L, 1.8-6.3×109/L, 100-300×109/L, 0-5 ng/ml, 0-37 U/ml, and 0-6 U/ml, respectively. In this study, we define the ratio of sodium (mmol/L) to globulin (g/L) as SGR.
We used the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) to estimate the chemotherapy response, every 2-3 cycles, and followed the patients until death or the end of the follow-up period; January 2020. The primary study endpoints were PFS and OS, whereas secondary endpoint was the disease control rate (DCR). PFS was defined as the interval between first-line chemotherapy and disease progression or the last follow-up time without progression, whereas OS was defined as the time between date of first-line chemotherapy and mortality or the end of follow-up. On the other hand, DCR was defined as the rate of complete response (CR), partial response (PR), and stable disease (SD).
Statistical analyses
Statistical analyses were performed using GraphPad Prism (GraphPad Software, www.graphpad.com) and SPSS (SPSS Inc., Chicago, IL, USA) software. We used the receiver operating characteristic (ROC) curve to calculate the cut-off values for SGR, sodium, and globulin. Additionally, we performed chi-square and Mann-Whitney U-tests to determine statistically significant differences between low- and high-SGR groups. Analysis of OS and PFS was performed using univariate and multivariate analyses. Furthermore, the Kaplan-Meier method and Log-rank test were used to plot survival curves and for comparisons, respectively. Data followed by p-value less than 0.05 were considered statistically significant.
Results
Optimal cut-off values and patient stratification
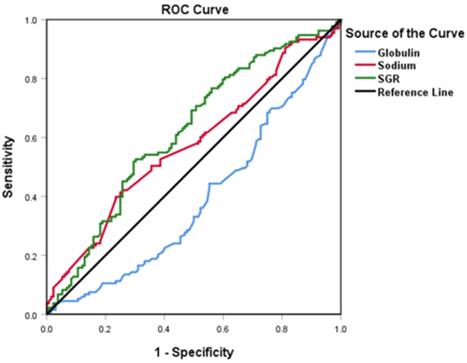
We used ROC curves to determine the optimal cut-off values for SGR, sodium, and globulin, (test) and OS (state) variables. Summarily, SGR, sodium, and globulin had optimal values of 5.54, 142.92, and 28.65, respectively. Moreover, we found AUC values of 0.619 (p = 0.001), 0.575 (p = 0.034) and 0.610 (p = 0.002) for SGR, sodium and globulin, respectively. The higher AUC in SGR indicated better prognostic accuracy in this variable, relative to the others. Consequently, participants with SGR > 5.54 were placed into the high-group, whereas those with a lower value stratified into the low-group (Figure 1).
Correlation between clinicopathological features and SGR
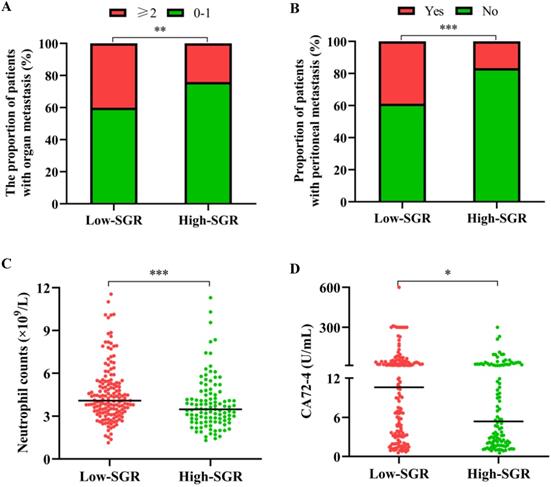
The median age of participants in our study cohort was 60 years, with 66.0% of all enrolled patients male. At the time of GC diagnosis, 33.6% of the patients had manifested metastases in more than one organ, 29.8% had developed peritoneal carcinomatosis, whereas 77.4% were found with stage IV gastric cancer. Analysis of the relationship between SGR levels and different patients' clinicopathological features revealed that those with low-SGR were more likely to have metastasis in no less than 2 organs (p = 0.007), peritoneal invasion (p < 0.001), higher neutrophil levels (p < 0.001) and CA72-4 (p = 0.014) (Table 1 and Figure 2).
Profiles of globulin, sodium, and sodium to globulin ratio (SGR) in patients with advanced gastric cancer using ROC curves.
Relationship between the pretreatment SGR and clinicopathological features
| Total | Low-SGR | High-SGR | p-value | |
|---|---|---|---|---|
| Total (n) | 265 | 157 | 108 | |
| Age (years, median) | 60.0 (52.5-65) | 60 (53.0-64.0) | 60 (52.0-66.8) | 0.435 |
| Sex (n ) | ||||
| Male | 175 (66.0%) | 106 (67.5%) | 69 (63.9%) | 0.540 |
| Female | 90 (34.0%) | 51 (32.5%) | 39 (36.1%) | |
| Body Mass Index (kg/m², median) | 21.6 (19.6-23.6) | 22.0 (19.8-23.9) | 21.4 (19.4-23.2) | 0.152 |
| ECOG (n) | ||||
| 0-1 | 225 (84.9%) | 134 (85.4%) | 91 (84.3%) | 0.807 |
| ≥2 | 40 (15.1%) | 23 (14.6%) | 17 (15.7%) | |
| Histological type (n) | ||||
| Well, Moderately | 75 (28.3%) | 39 (24.8%) | 36 (33.3%) | 0.132 |
| Poorly, Mucinous | 190 (71.7) | 118 (75.2%) | 72 (66.7%) | |
| The number of organs affected by metastasis (n) | ||||
| 0-1 | 176 (66.4%) | 94 (59.9%) | 82 (75.9%) | 0.007 |
| ≥2 | 89 (33.6%) | 63 (40.1%) | 26 (24.1%) | |
| Peritoneal metastasis (n) | ||||
| YES | 79 (29.8%) | 61 (38.9%) | 18 (16.7%) | < 0.001 |
| NO | 186 (70.2%) | 96 (61.1%) | 90 (83.3%) | |
| TNM stage (n) | ||||
| III | 60 (22.6%) | 30 (19.1%) | 30 (27.8%) | 0.098 |
| IV | 205 (77.4%) | 127 (80.9%) | 78 (72.2%) | |
| Blood parameters and index (median) | ||||
| Albumin (g/L) | 40.0 (37.0-42.3) | 40.1 (37.0-42.2) | 39.9 (36.0-43.0) | 0.631 |
| BUN (mmol/L) | 5.2 (4.2-6.5) | 5.3 (4.2-6.5) | 5.2 (4.3-6.8) | 0.804 |
| CREA (µmoI/L) | 61.5 (50.1-72.2) | 61.5 (48.2-73.0) | 61.4 (52.7-71.0) | 0.656 |
| Potassium (mmol/L) | 4.2 (3.9-4.4) | 4.2 (3.9-4.4) | 4.2 (3.9-4.5) | 0.758 |
| Neutrophil counts (×109/L) | 3.8 (3.0-5.1) | 4.1 (3.3-5.3) | 3.5 (2.6-4.5) | < 0.001 |
| Thrombocyte counts (×109/L) | 257.0 (196.0-332.5) | 266.0 (198.5-341.5) | 250 (192.3-313.5) | 0.145 |
| Hemoglobin (g/L) | 122.0 (105.0-139.0) | 123.0 (106.0-140.0) | 121.0 (102.0-136.8) | 0.161 |
| CEA (ng/mL) | 3.8 (1.6-13.0) | 4.1 (1.9-11.1) | 3.3 (1.2-15.7) | 0.396 |
| CA19-9 (U/mL) | 20.4 (8.1-122.4) | 21.4 (8.6-154.0) | 16.0 (7.6-75.1) | 0.235 |
| CA72-4 (U/mL) | 8.5 (2.4-24.1) | 10.6 (3.2-37.8) | 5.4 (2.1-20.2) | 0.014 |
SGR: sodium to globulin ratio; ECOG: Eastern Cooperative Oncology Group; TNM: Tumor-Node-Metastasis; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; CA 72-4: carbohydrate antigen 72-4.
Relationship between the pretreatment sodium to globulin ratio (SGR) and (A) the number of metastatic organs, (B) peritoneal metastasis, (C) neutrophil count, and (D) carbohydrate antigen (CA) 72-4 level. * p < 0.05, ** p < 0.01, *** p < 0.001.
Relationship between SGR and response to first-line chemotherapy
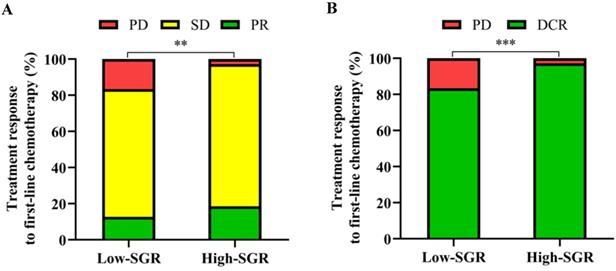
We found no patients with CR after first-line chemotherapy. However, patients in the high-SGR group exhibited higher proportions of SD and PR and lower proportions of PD (p = 0.001) relative to those in the low-SGR group. During first-line treatment, DCR rates of 97.2 and 83.4% (p < 0.001) were recorded in the high- and low-SGR groups, respectively (Figure 3).
Prognostic factors predict patient survival
Results from univariate analyses revealed that SGR (p < 0.001), number of metastatic organs (p = 0.004), peritoneal metastasis (p < 0.001), CA72-4 (p = 0.006), and neutrophil counts (p = 0.012) were prognostic factors for PFS in the studied patients. On the other hand, multivariate analysis showed that SGR > 5.54 (Hazard ratio [HR]: 0.539, p < 0.001), no peritoneal metastasis (HR: 0.701, p = 0.031), and normal neutrophil counts (HR: 0.673, p = 0.042) had independent positive effects on PFS (Table 2). With regards to OS, results from univariate analyses indicated that SGR (p < 0.001), histological type (p = 0.016), number of metastatic organs (p = 0.012), peritoneal metastasis (p < 0.001), and neutrophil counts (p = 0.042) were prognostic factors. On the other hand, multivariate analyses revealed that SGR > 5.54 (HR: 0.574, p < 0.001), no peritoneal metastasis (HR: 0.655, p = 0.008) were independent prognostic factors for superior OS (Table 3).
Relationship between pretreatment sodium to globulin ratio (SGR) and (A) progressive disease (PD), stable disease (SD), partial response (PR) and (B) disease control rate (DCR). ** p < 0.01, *** p < 0.001.
Correlations between PFS and SGR and other clinicopathological factors
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Age (≥ 60 years) | 0.965 | 0.757-1.231 | 0.774 | |||
| Sex (male) | 1.067 | 0.825-1.379 | 0.623 | |||
| Body Mass Index (<18.5 kg/m²) | 0.868 | 0.607-1.242 | 0.439 | |||
| ECOG (≥ 2) | 1.032 | 0.736-1.446 | 0.856 | |||
| Histological type (Poorly, Mucinous) | 1.283 | 0.980-1.679 | 0.070 | 1.214 | 0.924-1.596 | 0.164 |
| The number of organs affected by metastasis (≥2) | 1.462 | 1.131-1.890 | 0.004 | 1.320 | 0.984-1.770 | 0.064 |
| Peritoneal metastasis (NO) | 0.519 | 0.397-0.679 | < 0.001 | 0.701 | 0.508-0.967 | 0.031 |
| TNM stage (IV) | 1.237 | 0.925-1.655 | 0.151 | 0.892 | 0.643-1.237 | 0.494 |
| CEA (> 5 ng/mL) | 1.193 | 0.931-1.529 | 0.162 | 1.195 | 0.914-1.562 | 0.192 |
| CA19-9 (> 37 U/mL) | 1.060 | 0.826-1.361 | 0.648 | |||
| CA72-4 (> 6 U/mL) | 1.420 | 1.108-1.821 | 0.006 | 1.177 | 0.902-1.535 | 0.231 |
| Normal neutrophil counts (≤ 6.3×109/L) | 0.622 | 0.430-0.899 | 0.012 | 0.673 | 0.460-0.985 | 0.042 |
| Hemoglobin (< 115 g/L) | 0.881 | 0.685-1.132 | 0.322 | |||
| SGR > 5.54 | 0.471 | 0.365-0.607 | < 0.001 | 0.539 | 0.411-0.706 | < 0.001 |
SGR: sodium to globulin ratio; ECOG: Eastern Cooperative Oncology Group; TNM: Tumor-Node-Metastasis; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; CA 72-4: carbohydrate antigen 72-4; CI: confidence interval.
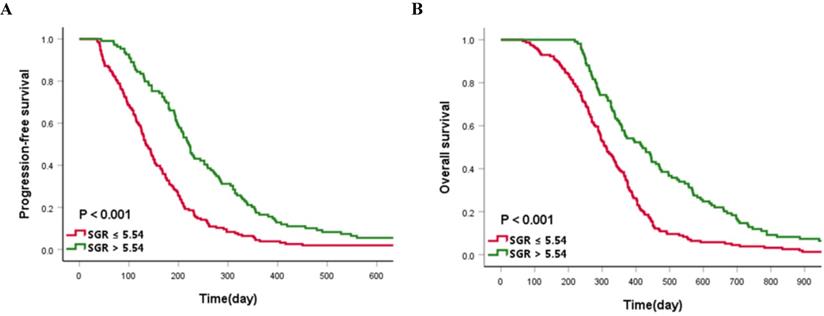
The median PFS and OS of the cases enrolled in this study were 173 and 345 days, respectively. Patients in the high-SGR group had a significantly longer median PFS (221 vs. 134 days, p < 0.001) and OS (420 vs. 311 days, p < 0.001) than those in the low-SGR group, respectively (Figure 4).
Subgroup analysis
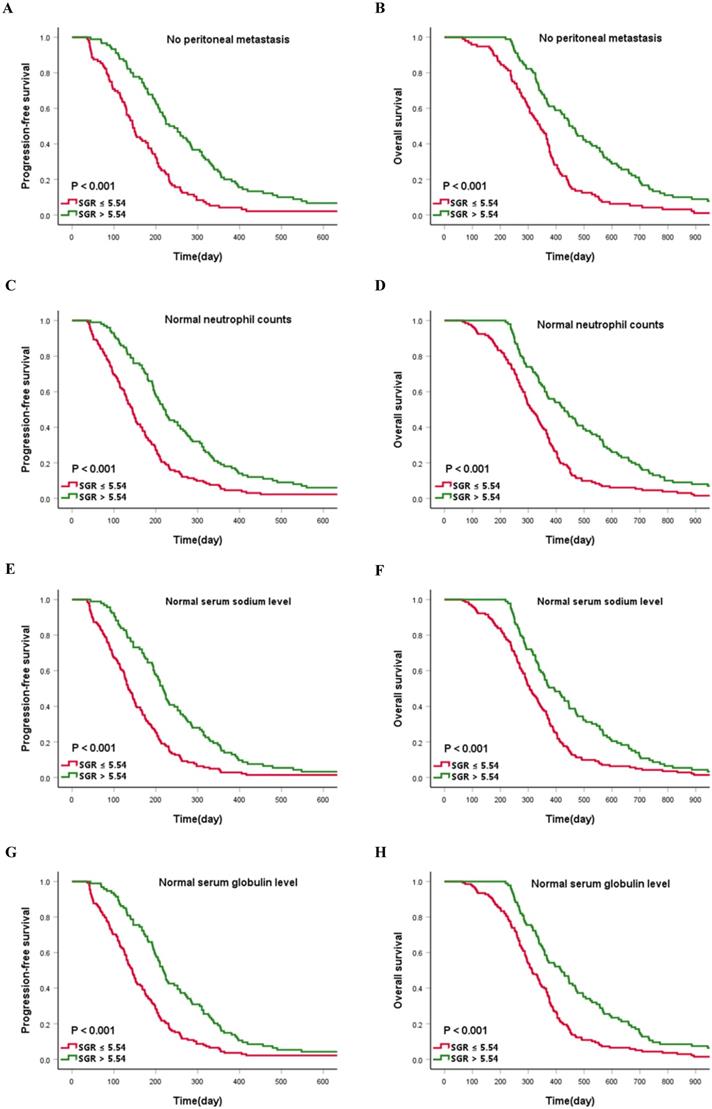
Preliminary analysis indicated that no peritoneal infiltration and normal neutrophil counts (≤ 6.3×109/L) were a protection factor for patient survival. Consequently, we assessed whether SGR validates prognosis determined by no peritoneal infiltration and normal neutrophil counts. Interestingly, patients in the high-SGR group, across no peritoneal metastasis and normal neutrophil count subgroups, still have prolonged PFS and OS relative to those in the low-SGR group (all p < 0.001). We hypothesized that clinicians may inadvertently ignore potentially critical patients with normal biochemical indexes. Consequently, we investigated whether SGR is a reliable prognostic indicator for patients with normal serum sodium (135-145 mmol/L) and globulin (20-35 g/L) levels. Summarily, patients in the high-SGR group, with normal serum sodium and globulin levels, still exhibited favorable prognosis compared with those in the low-SGR group (all p < 0.001) (Figure 5).
Correlations between OS and SGR and other clinicopathological factors
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Age (≥ 60 years) | 0.788 | 0.617-1.006 | 0.056 | 0.933 | 0.720-1.210 | 0.601 |
| Sex (male) | 1.063 | 0.823-1.373 | 0.640 | |||
| Body Mass Index (<18.5 kg/m²) | 1.106 | 0.774-1.580 | 0.581 | |||
| ECOG (≥ 2) | 1.096 | 0.782-1.535 | 0.595 | |||
| Histological type (Poorly, Mucinous) | 1.390 | 1.063-1.819 | 0.016 | 1.272 | 0.969-1.670 | 0.083 |
| The number of organs affected by metastasis (≥2) | 1.395 | 1.076-1.810 | 0.012 | 1.231 | 0.932-1.627 | 0.143 |
| Peritoneal metastasis (NO) | 0.560 | 0.428-0.733 | <0.001 | 0.655 | 0.481-0.894 | 0.008 |
| TNM stage (IV) | 1.177 | 0.881-1.572 | 0.270 | |||
| CEA (> 5 ng/mL) | 1.193 | 0.932-1.526 | 0.161 | 1.285 | 0.986-1.674 | 0.063 |
| CA19-9 (> 37 U/mL) | 1.134 | 0.883-1.455 | 0.324 | |||
| CA72-4 (> 6 U/mL) | 1.243 | 0.974-1.587 | 0.081 | 0.987 | 0.755-1.290 | 0.922 |
| Normal neutrophil counts (≤ 6.3×109/L) | 0.681 | 0.471-0.986 | 0.042 | 0.772 | 0.522-1.139 | 0.192 |
| Hemoglobin (< 115 g/L) | 0.857 | 0.667-1.101 | 0.228 | |||
| SGR > 5.54 | 0.485 | 0.375-0.628 | <0.001 | 0.574 | 0.437-0.756 | <0.001 |
SGR: sodium to globulin ratio; ECOG: Eastern Cooperative Oncology Group; TNM: Tumor-Node-Metastasis; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen 19-9; CA 72-4: carbohydrate antigen 72-4; CI: confidence interval.
Kaplan-Meier curves of (A) progression-free survival and (B) overall survival according to the cut-off value of sodium to globulin ratio (SGR).
Discussion
Numerous research efforts on prognostic cancer markers have generated a series of indicators for evaluating survival of patients with different tumors, such as gastric cancer [10-13]. Serum sodium and globulin concentration alone are cheap and readily available routine blood examination strategies that effectively reflect outcomes and survival rates of patients with diverse malignancies, including gastric cancer [8, 9, 14-17]. A combination of sodium and globulin, commonly termed sodium to globulin ratio, exhibits superior prognostic accuracy and precision relative to that of sodium and globulin alone. However, nothing is known regarding the relationship between SGR parameters and clinical outcomes. The current study, therefore, sought to validate SGR as a prognostic factor for cancer patients. To the best of our knowledge, this is the first study reporting that high-SGR GC patients, receiving first-line chemotherapy, have better DCR rates than low-SGR counterparts. This affirms the importance of SGR as an independent prognostic factor for PFS and OS in patients with advanced GC. In addition, our results revealed SGR was still a superior prognostic factor, owing to excellent prognosis, based on normal neutrophil counts, serum sodium and globulin level, and without peritoneal metastasis, in patients with advanced GC.
Electrolyte disturbance represents one of the complications for patients with malignancies. Previous studies have implicated sodium reduction in occurrence of this complication, which is associated with poor prognosis in cancer patients [3]. In addition, studies have shown that nearly 47% of patients with different malignant diseases exhibit a decrease in serum sodium levels, which is closely related to poor prognosis [6]. Functionally, abnormal functioning of several pumps or ion channels across various types of cancer could lead to disease development and progression [18]. For example, studies have reported an association between signal transduction of serum and glucocorticoid-induced protein kinase 1 (SGK1) with occurrence and progression of malignancies, including gastric cancer [19]. Similarly, a reduction in sodium levels was found to affect maintenance (or enhancement) of SGK1 signaling, indirectly maintaining the tumor-promoting activity of SGK1 [20]. In addition, several studies have demonstrated that Voltage-gated Na (+) channels (VGSC) implicated in the excitability and action potential conduction were involved in the progression of lung, prostate, breast and gastric cancers [21]. In addition, Carrie et al. [22] reported upregulation of VGSC in aggressive tumor cell lines, with its activation found to mediate the invasive potential of cancer cells. Recently, sodium was implicated in immune response processes. For example, Sodium-related receptor potential channels reportedly triggered T cell activation and innate response [23]. Previous studies have also shown that sodium impedes differentiation of macrophages into M2 subtypes with immunosuppressive characteristics, while promoting formation of anti-tumoral M1 phenotypic macrophages. Specifically, a decrease in sodium concentration resulted in production of more M2 macrophages and formation of an immunosuppressive microenvironment, which contributed to chemoresistance [24-28]. These findings indicate that chemotherapy may generate poor outcomes in GC patients with hyponatremia.
Kaplan-Meier curves describing survival for the cut-off value of sodium to globulin ratio (SGR) stratified by (A and B) no peritoneal metastasis, (C and D) normal neutrophil counts, (E and F) normal serum sodium level, and (G and H) normal serum globulin level.
Abundance of inflammatory cells and their products in the tumor microenvironment has been described as the driving factor for formation and progression of tumors [29]. Similarly, an increase hepatocyte-derived globulin levels has been reported following inflammatory response [30]. Functionally, globulin contains most of the pro-inflammatory proteins, including alpha1-globulin, C-reactive protein (CRP), and complement 3 (C3). Several reports have shown that an abnormal increase in these biomarkers has profound negative effects on cancer patients. For example, Qu et al. [31] reported that higher serum α1-globulin was associated with higher pathological stage and poor tumor status. Consequently, serum α1-globulin was found to be an independent prognostic factor for short recurrence survival and overall survival in non-small cell lung cancer. Similarly, Allin et al. [32] showed that people with high CRP levels, across a general population, had a 1.3-fold higher risk of any type of cancer as well as a 2-fold higher risk of lung cancer. Among cancer patients, those with high CRP levels had 80% risk of premature death, whereas those with elevated CRP levels with invasive breast cancer had a 1.7-fold risk of tumor-related death. In addition, Shimura et al. [33] demonstrated that serum CRP levels, before chemotherapy, might be a potential prognostic factor for metastatic GC, whereas Boire et al. [34] reported upregulation of C3 in leptomeningeal metastatic models, a common fatal condition, and further proved that this was essential for the spread of cancer cells in the leptomeningeal space. The aberrant expression of C3 from cancer cells indicates the relapse of the leptomeninges, with blocking of C3 signal found to effectively inhibit leptomeningeal metastasis. A recent study on GC has shown that C3 deposited in the tumor microenvironment can independently activate the JAK2/STAT3 pathway, and promote tumor progression, indicating its potential as a prognostic factor for patients with gastric cancer [35].
Despite the potential for sodium and globulin alone as prognostic markers for patients with malignancies, these variables are easily influenced by fluid retention and body fluid loss. To circumvent this issue, we evaluated SGR and found it to be a more powerful prognostic factor than sodium and globulin alone owing to higher AUC and lower p values in the ROC curve. This possibly reduces the adverse effects of other pathophysiological situations thereby improving its prognostic accuracy.
To our knowledge, this is the first study implicating SGR as a prognostic marker for advanced GC patients. Our findings not only indicate the benefits of first-line chemotherapy, but also the survival of patients. Notably, SGR may still be an effective prognostic predictor for patients with relatively good clinical outcomes. Taken together, these findings are expected to improve prognosis of GC patients and elucidate the actual disease mechanisms. Moreover, our results are expected to guide clinicians during patient identification as well as development of appropriate treatment therapies.
The study had some limitations. Firstly, this was a retrospective study. A randomized controlled approach may be appropriately convincing. Secondly, only sodium and globulin, but not other indicators that contribute to electrolyte disturbance and systemic inflammation, were detected in this study. It is therefore not clear whether these are better prognostic factors. In future, longer follow-up times and detection indexes are needed to supplement these results. Thirdly, although we have described possible mechanisms of action that enable sodium and globulin to be used as prognostic factors in GC cancer patients, biological mechanisms underlying the prognostic role of electrolyte disorders and systemic inflammatory factors remain unknown. Further studies are therefore required on that front.
Conclusion
SGR, before first-line chemotherapy, is a cheap, universally available, and easily applicable marker that can effectively predict sensitivity to chemotherapy. This factor is also an independent and reliable prognostic factor for PFS and OS in patients with advanced GC.
Acknowledgements
This study was supported by the National Key R and D Program of China (Grant #2018YFC1311600), the Scientific research foundation for the introduction of talents, Liaoning Cancer Hospital & Institute (No. Z1702), the Science and Technology Planning Project of Liaoning Province of China (No. 201800449), the Science and Technology Planning Project of Shenyang (No.191124088), and the National Natural Science Foundation of China (No. 81372532).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424
2. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England). 2010;376:687-97
3. Rosner MH, Dalkin AC. Electrolyte disorders associated with cancer. Advances in chronic kidney disease. 2014;21:7-17
4. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England). 2001;357:539-45
5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-44
6. Doshi SM, Shah P, Lei X, Lahoti A, Salahudeen AK. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012;59:222-8
7. Clyne M. Kidney cancer: Low serum sodium linked to poor outcomes for patients with mRCC treated with targeted therapy. Nature reviews Urology. 2013;10:679
8. Chen X, Yao J, Liu L, Zheng W, Hu X, Zhu Y. et al. Serum Alpha1-Globulin as a Novel Prognostic Factor in Metastatic Renal Cell Carcinoma Treated with Tyrosine Kinase Inhibitors. Targeted oncology. 2019;14:187-95
9. Niwa N, Matsumoto K, Ide H. The clinical implication of gamma globulin levels in patients with nonmuscle-invasive bladder cancer. Urologic oncology. 2019;37:291.e1-e7
10. Sun DW, An L, Lv GY. Albumin-fibrinogen ratio and fibrinogen-prealbumin ratio as promising prognostic markers for cancers: an updated meta-analysis. World journal of surgical oncology. 2020;18:9
11. Mori K, Janisch F, Mostafaei H, Lysenko I, Kimura S, Egawa S. et al. Prognostic value of preoperative blood-based biomarkers in upper tract urothelial carcinoma treated with nephroureterectomy: A systematic review and meta-analysis. Urologic oncology. 2020;38:315-33
12. Zhang L, Zhang J, Wang Y, Dong Q, Piao H, Wang Q. et al. Potential prognostic factors for predicting the chemotherapeutic outcomes and prognosis of patients with metastatic colorectal cancer. Journal of clinical laboratory analysis. 2019;33:e22958
13. Hara K, Aoyama T, Yamada T, Nakazono M, Nagasawa S, Shimoda Y. et al. The Prognostic Value of the Perioperative Systemic Inflammation Score for Patients With Advanced Gastric Cancer. Anticancer research. 2020;40:1503-12
14. Berardi R, Rinaldi S, Belfiori G, Stefano P, Crippa S, Torniai M. et al. Prognostic role of hyponatremia in pancreatic cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2019;30(Suppl 4):iv7
15. Fuca G, Mariani L, Lo Vullo S, Galli G, Berardi R, Di Nicola M. et al. Weighing the prognostic role of hyponatremia in hospitalized patients with metastatic solid tumors: the HYPNOSIS study. Sci Rep. 2019;9:12993
16. Xu J, Chen X, Wang X, Zhu C, Hu Y, Yang X. et al. Preoperative Hyponatremia And Hypocalcemia Predict Poor Prognosis In Elderly Gastric Cancer Patients. Cancer management and research. 2019;11:8765-80
17. Chen J, Zhou Y, Xu Y, Zhu HY, Shi YQ. Low pretreatment serum globulin may predict favorable prognosis for gastric cancer patients. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:3905-11
18. Litan A, Langhans SA. Cancer as a channelopathy: ion channels and pumps in tumor development and progression. Frontiers in cellular neuroscience. 2015;9:86
19. Yao Y, Jiang Q, Jiang L, Wu J, Zhang Q, Wang J. et al. Lnc-SGK1 induced by Helicobacter pylori infection and highsalt diet promote Th2 and Th17 differentiation in human gastric cancer by SGK1/Jun B signaling. Oncotarget. 2016;7:20549-60
20. Bruhn MA, Pearson RB, Hannan RD, Sheppard KE. Second AKT: the rise of SGK in cancer signalling. Growth factors (Chur, Switzerland). 2010;28:394-408
21. Xia J, Huang N, Huang H, Sun L, Dong S, Su J. et al. Voltage-gated sodium channel Nav 1.7 promotes gastric cancer progression through MACC1-mediated upregulation of NHE1. International journal of cancer. 2016;139:2553-69
22. House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T. et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer research. 2010;70:6957-67
23. Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annual review of immunology. 2015;33:291-353
24. Hucke S, Eschborn M, Liebmann M, Herold M, Freise N, Engbers A. et al. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. Journal of autoimmunity. 2016;67:90-101
25. Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W. et al. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. The Journal of clinical investigation. 2015;125:4223-38
26. Zhang WC, Zheng XJ, Du LJ, Sun JY, Shen ZX, Shi C. et al. High salt primes a specific activation state of macrophages, M(Na). Cell research. 2015;25:893-910
27. Cassetta L, Kitamura T. Macrophage targeting: opening new possibilities for cancer immunotherapy. Immunology. 2018;155:285-93
28. Wang D, Yang L, Yu W, Wu Q, Lian J, Li F. et al. Colorectal cancer cell-derived CCL20 recruits regulatory T cells to promote chemoresistance via FOXO1/CEBPB/NF-κB signaling. Journal for immunotherapy of cancer. 2019;7:215
29. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nature reviews Cancer. 2013;13:759-71
30. Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. The New England journal of medicine. 2012;367:2015-25
31. Qu X, Pang Z, Yi W, Wang Y, Wang K, Liu Q. et al. High percentage of α1-globulin in serum protein is associated with unfavorable prognosis in non-small cell lung cancer. Medical oncology (Northwood, London, England). 2014;31:238
32. Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Critical reviews in clinical laboratory sciences. 2011;48:155-70
33. Shimura T, Kitagawa M, Yamada T, Ebi M, Mizoshita T, Tanida S. et al. C-reactive protein is a potential prognostic factor for metastatic gastric cancer. Anticancer research. 2012;32:491-6
34. Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massague J. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell. 2017;168:1101-13.e13
35. Yuan K, Ye J, Liu Z, Ren Y, He W, Xu J. et al. Complement C3 overexpression activates JAK2/STAT3 pathway and correlates with gastric cancer progression. Journal of experimental & clinical cancer research: CR. 2020;39:9
Author contact
![]() Corresponding author: Jingdong Zhang, E-mail: jdzhangcom.
Corresponding author: Jingdong Zhang, E-mail: jdzhangcom.

 Global reach, higher impact
Global reach, higher impact