Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(6):1669-1677. doi:10.7150/jca.49673 This issue Cite
Research Paper
Histologic Lymph Nodes Regression after Preoperative Chemotherapy as Prognostic Factor in Non-metastatic Advanced Gastric Adenocarcinoma
1. Clinic of Gastroenterology, Nephrourology, and Surgery, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
2. National Centre of Pathology, Affiliate of Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania.
3. Department of Medical Oncology, National Cancer Institute, Vilnius, Lithuania.
4. Vilnius University, Faculty of Medicine, Vilnius, Lithuania.
5. Department of Pathology, Forensic Medicine and Pharmacology, Institute of Biomedical Sciences, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
* undergraduate at the time of submission.
Received 2020-6-18; Accepted 2020-11-12; Published 2021-1-16
Abstract
Background: The study aims to evaluate the lymph node (LN) response to preoperative chemotherapy and its impact on long-term outcomes in advanced gastric cancer (AGC).
Methods: Histological specimens retrieved at gastrectomy from patients who received preoperative chemotherapy were evaluated. LN regression was graded by the adapted tumor regression grading system proposed by Becker. Patients were classified as node-negative (lnNEG) in the case of all negative LN without evidence of previous tumor involvement. Patients with LN metastasis were classified as nodal responders (lnR) in case of a regression score 1a-2 was detected in the LN. Nodal non-responders (lnNR) had a regression score of 3 in all of the metastatic nodes. Survival was compared using Kaplan-Meier and Cox regression analysis.
Results: Among 87 patients included in the final analysis 29.9 % were lnNEG, 21.8 % were lnR and 48.3 % were lnNR. Kaplan-Meier curves showed a survival benefit for lnR over lnNR (p=0.03), while the survival of lnR and lnNEG patients was similar. Cox regression confirmed nodal response to be associated with decreased odds for death in univariate (HR: 0.33; 95 % CI 0.11-0.96, p=0.04) and multivariable (HR 0.37; 95 CI% 0.14-0.99, p=0.04) analysis.
Conclusions: Histologic regression of LN metastasis after preoperative chemotherapy predicts the increased survival of patients with non-metastatic resectable AGC.
Keywords: gastric cancer, preoperative chemotherapy, histological regression, nodal regression.
Introduction
Perioperative chemotherapy is the standard for resectable non-metastatic advanced gastric cancer (AGC) after large scale randomized control trials demonstrated an advantage over the surgery-first approach [1,2]. The justification for preoperative chemotherapy is the reduction of the primary tumor size, increased rates of R0 resection, and the treatment of occult micrometastasis which all translates to increased survival [3]. Although preoperative chemotherapy has been widely introduced into clinical practice guidelines, the discussion, whether current regimens are truly effective, is still ongoing [3,4]. Significant histologic tumor regression following chemotherapy where fibrosis becomes predominant over tumor cells is observed in only about 17-50 % of patients [5-7]. The histologic tumor regression grading (TRG) system for gastric adenocarcinoma was proposed by Becker et al. [8] and it is based on an estimation of the percentage of vital tumor tissue in relation to the macroscopically identifiable tumor bed [8]. TRG and postoperative lymph node (LN) status are the two major prognostic factors for AGC patients' survival [5-7,9]. TRG system by Becker as well as others evaluate histologic regression within the primary tumor, but not in LN metastases [10]. Current evidence suggests histologic nodal regression after preoperative cytotoxic treatment results in improved survival of patients with rectal and esophageal cancers [11-14]. However, there is a lack of data on pathologic LN regression after preoperative chemotherapy and its impact on long-term outcomes in AGC, which is typically accompanied by LN metastasis.
Therefore, this study was designed to evaluate histologic LN regression in AGC after preoperative chemotherapy and its impact on survival.
Materials and Methods
Ethics
Vilnius regional biomedical research ethics committee approval was obtained before this study was conducted. All study-related procedures were performed in accordance with the Declaration of Helsinki of 1975, as revised in 1983.
Patients
The cohort study was conducted at two major gastrointestinal cancer treatment centers of Lithuania: National Cancer Institute, Vilnius, Lithuania, and Vilnius University hospital Santara Clinics, Vilnius, Lithuania. All patients who underwent preoperative chemotherapy between 2014 January and 2018 December followed by surgery for advanced gastric or gastroesophageal junction adenocarcinoma were included in the study. Patients with distant metastasis revealed during gastrectomy or those with R1/2 resection were excluded from further enrolment. The primary aim of the study was to evaluate the rate of histologic regression of LN metastasis after preoperative chemotherapy and its impact on overall survival (OS). The secondary aims included the nodal response impact on disease-free survival (DFS), the rate of primary tumor regression, and its association with nodal regression.
Diagnosis and treatment
The diagnosis was confirmed by esophagogastroduodenoscopy with biopsy in all patients. The staging consisted of the chest and abdominal CT and diagnostic laparoscopy with peritoneal lavage. All patients were discussed in a multidisciplinary team meeting and those with the non-metastatic ≥cT2N0 disease were considered for perioperative chemotherapy. Patients eligible according to physical status and comorbidities underwent preoperative chemotherapy where the exact regimen for the exact patient was selected by a medical oncologist. After preoperative chemotherapy was completed patients underwent a CT scan and were scheduled for elective surgery. The extent of surgery depended on tumor localization and all patients underwent open surgery. Subtotal gastrectomy was performed when a sufficient proximal resection margin could be ensured; otherwise open total gastrectomy was performed. The standard lymphadenectomy was a D2 lymph node dissection performed as described in the 4th version of Japanese gastric cancer treatment guidelines [15]. Patients were scheduled to continue perioperative chemotherapy after they recovered from surgery.
Pathological evaluation
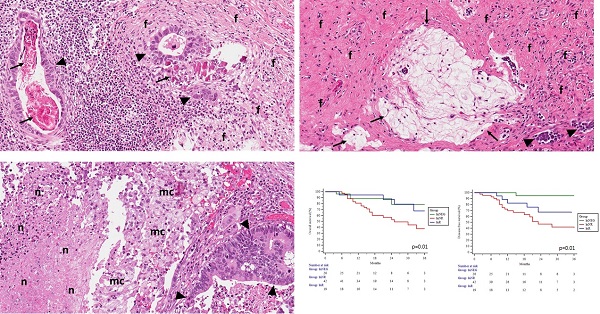
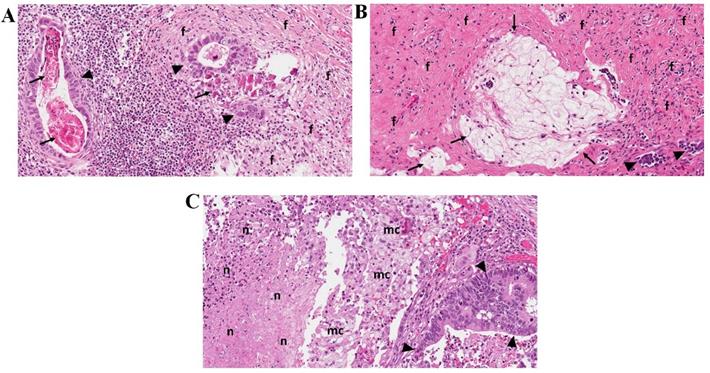
The pathological evaluation was performed at the National Center of Pathology, Vilnius, Lithuania. Final tumor histology was provided ypTNM and staged according to the American Joint Committee on Cancer Staging, 8th edition. The histological type of tumors was classified according to the WHO Classification of Tumors of the Digestive Tract (2010) and Lauren classification of gastric carcinoma. Regional lymph nodes were macroscopically identified in surgical specimens. All lymph nodes were longitudinally sectioned through the hilus and embedded into paraffin blocks. All slides were stained with hematoxylin-eosin, additional immunostaining was performed if necessary. For the study, all slides were recalled from the institutional archive. They were reviewed by the senior pathologist trainee and experienced gastrointestinal pathologists to evaluate histologic regression grade after preoperative chemotherapy in the primary tumor and metastatic lymph nodes. Regression in the tumor was graded as described by Becker et al. [8]. For nodal regression, we adapted the same grading system. Histological signs of regression in the primary tumor and metastatic lymph nodes were similar and included: areas of fibrosis, necrosis, calcifications, acellular mucin pools, cholesterol deposits, and histiocytic reaction with hemosiderin-laden and foamy macrophages (Figure 1). Regression was graded: Grade 1, complete (0% residual tumor; Grade 1a) or subtotal tumor regression (<10% residual tumor per tumor bed; Grade 1b); Grade 2, partial tumor regression (10-50% residual tumor per tumor bed), and Grade 3, minimal or no tumor regression (>50% residual tumor per tumor bed). Lymph nodes without metastasis or signs of nodal regression were classified as negative nodes.
Representative pictures of lymph nodes presenting signs of histological regression (Haematoxylin-eosin staining; original magnification 20x). A - Lymph node with residual carcinoma (▲), foci of fibrosis (f) and calcifications (↑); B - Lymph node with few residual carcinoma aggregates (▲), fibrosis (f), and acellular mucin pools (↑); C - Lymph node with residual carcinoma (▲), foamy macrophages (mc), and areas of necrosis (n).
For the purpose of the study, patients were grouped according to the regression scores recorded in the lymph nodes. Patients who had all negative nodes were allocated to the node-negative (lnNEG) group. Patients with a regression score of 1a-2 detected in at least some of the retrieved metastatic nodes were categorized as nodal responders (lnR). Non-responders (lnNR) had a score of 3 in all metastatic LN.
Follow-up
Esophagogastroduodenoscopy and CT were performed twice a year for the first two years and then annually. If patients underwent follow up visits outside of the original study institutions, data was still obtained directly from the patient or their physicians by phone interview. The date of death was obtained from Lithuania's Cancer register - a nationwide and population-based cancer registry, which covers all territory of Lithuania. The last follow-up data on death and recurrence were collected on the 1st of November, 2019.
Statistical analysis
All statistical analyses were conducted using the statistical program SPSS 24.0 (SPSS, Chicago, IL, USA). Continuous variables are presented as the mean ± standard deviation or median with interquartile range and were compared across groups using the one-way ANOVA or Kruskal-Wallis test as appropriate. Categorical variables are shown as proportions and were compared using the χ2 test or Fisher exact test, as appropriate.
Overall and disease-free survival rates were analyzed by the Kaplan-Meier method and were compared by the log-rank test. Overall survival was defined as the time from the first cycle of preoperative chemotherapy to death. Disease-free survival was defined as the time from the first cycle of chemotherapy to the locoregional or distant recurrence of the disease or death. To identify the prognostic significance of variables for long-term outcomes univariate Cox regression was performed and the results were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Those variables which resulted significantly in the univariate setting were inserted into a multivariable model and were adjusted for patients' age and comorbidities. In all statistical analyses, two-tailed tests were used and a p-value of <0.05 was considered to be significant.
Results
Patients and chemotherapy
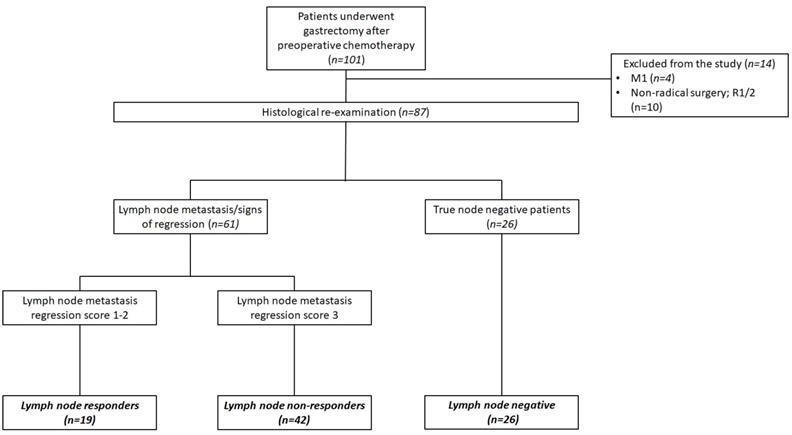
Among 101 patients identified in the database 14 (10.8 %) were excluded because of metastatic disease revealed on gastrectomy or non-radical surgery. Eighty-seven patients were included in the final analysis. After histological re-examination 26 (29.9 %) were categorized as lnNEG patients while 61 (70.1 %) had LN metastasis or signs of complete histological regression. Of 61 node-positive patients, 19 (21.8 %) were nodal responders (lnR) and 42 (48.3 %) were non-responders (lnNR) (Figure 2). The baseline clinicopathological characteristics of these patients are shown in table 1.
The vast majority (83/87; 95.4 %) of patients successfully underwent a full preoperative chemotherapy protocol. In contrast, significantly lower proportion of these received chemotherapy postoperatively (64/87; 72.4 %, p=0.01). The regimens of chemotherapy were not different between the study groups (Table 2).
Histologic regression
The median number of retrieved lymph nodes was 30 (23; 39) and 2782 lymph nodes were examined in total. Twenty-six patients in the lnNEG group had 737 nodes without metastasis or signs of regression. Within the lnNR group, 1426 lymph nodes were examined, and 342 (23.9 %) of them were metastatic, although none had a significant regression (regression score 3). Nineteen patients from the lnR group presented 619 lymph nodes of which 116 (18.7 %) were metastatic. Nodal regression by score 1a-2 was observed in 58 (50.0 %) nodes. Nine (47.3 %) of 19 lnR patients had a regression in all the metastatic lymph nodes including 3 (15.7 %) patients with a complete regression (score 1a) in all the metastatic LN and downstaging to ypN0. Ten (52.6 %) patients had a significant regression only in some of the metastatic nodes (Table 3).
Baseline clinicopathologic characteristics of the study patients.
| Positive nodes | Negative nodes | ||||
|---|---|---|---|---|---|
| lnNR (n=42) | lnR (n=19) | lnNEG (n=26) | p value | ||
| Age (years) | 58.0±10.3 | 59.4±9.1 | 59.7±12.0 | 0.79 | |
| Sex | Male | 27 (64.3 %) | 15 (78.9 %) | 14 (53.8 %) | 0.22 |
| Female | 15 (35.7 %) | 4 (21.1 %) | 12 (46.2 %) | ||
| Tumor invasion (ypT) | 1-2 | 9 (21.4 %) | 5 (26.3 %) | 14 (53.8 %) | 0.01 |
| 3-4 | 33 (78.6 %) | 14 (73.7 %) | 12 (46.2 %) | ||
| TRG in primary tumor site | 1a-2 | 9 (21.4 %) | 8 (42.1 %) | 10 (38.5 %) | 0.168 |
| 3 | 33 (78.6 %) | 11 (57.9 %) | 16 (61.5 %) | ||
| Lymph node metastasis (ypN) | 0 | 0 (0 %) | 3 (15.8 %) | 26 (100 %) | 0.01 |
| 1 | 12 (28.6 %) | 7 (36.8 %) | 0 (0 %) | ||
| 2 | 12 (28.6 %) | 5 (26.3 %) | 0 (0 %) | ||
| 3 | 18 (42.8 %) | 4 (21.1 %) | 0 (0 %) | ||
| Tumor differentiation | G1 | 0 (0 %) | 0 (0 %) | 1 (3.8 %) | 0.01 |
| G2 | 3 (7.1 %) | 5 (26.3 %) | 10 (38.5 %) | ||
| G3 | 39 (92.9 %) | 14 (73.7 %) | 15 (57.7 %) | ||
| Lauren | Intestinal/Mix | 15 (39.5 %) | 10 (66.7 %) | 13 (59.1 %) | 0.13 |
| Diffuse | 23 (60.5 %) | 5 (33.3 %) | 9 (40.9 %) | ||
| Signet ring cells component | Negative | 27 (64.3 %) | 17 (89.5 %) | 22 (84.6 %) | 0.04 |
| Positive | 15 (35.7 %) | 2 (10.5 %) | 4 (15.4 %) | ||
| Tumor localization | Upper third | 10 (23.8 %) | 4 (21.0 %) | 7 (26.9 %) | 0.38 |
| Middle third | 24 (57.2 %) | 9 (47.4 %) | 9 (34.6 %) | ||
| Lower third | 8 (19.0 %) | 6 (31.6 %) | 10 (38.5 %) | ||
| Lymphovascular invasion | No | 16 (39.0 %) | 7 (38.9 %) | 23 (88.5 %) | 0.01 |
| Yes | 25 (61.0 %) | 11 (61.1 %) | 3 (11.5 %) | ||
| Surgery | Total gastrectomy | 31 (73.8 %) | 9 (47.4 %) | 13 (50 %) | 0.05 |
| Subtotal gastrectomy | 11 (26.2 %) | 10 (52.6 %) | 13 (50 %) | ||
| CCI | 1-3 | 22 (52.4 %) | 11 (57.9 %) | 9 (34.6 %) | 0.23 |
| ≥4 | 20 (57.6 %) | 8 (42.1 %) | 17 (65.4 %) | ||
CCI: Charlson Comorbidity Index; lnNR: lymph node non-responders; lnR: lymph node responders; lnNEG: lymph node-negative; TRG: tumor regression grade (by Becker).
Flowchart of the study patients.
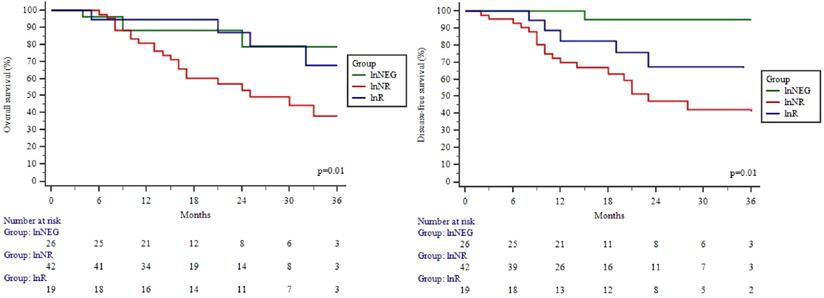
Overall and disease-free survival of the study patients by Kaplan-Meier analysis. Overall (A) and disease-free survival (B) of patients in different study groups. lnNEG: lymph nodes-negative; lnR: lymph nodes responder; lnNR: lymph nodes non-responder.
Preoperative chemotherapy regimens in different study groups.
| Chemotherapy regimen | Positive nodes | Negative nodes | ||
|---|---|---|---|---|
| lnNR (n=42) | lnR (n=19) | lnNEG (n=26) | p value | |
| CF | 25 (59.5 %) | 10 (52.6 %) | 18 (69.2 %) | 0.60 |
| ECX/EOX | 9 (21.5 %) | 6 (31.6 %) | 3 (11.5 %) | |
| FLOT | 8 (19.0 %) | 3 (15.8 %) | 5 (19.3 %) | |
lnNR: lymph node non-responders; lnR: lymph node responders; lnNEG: lymph node-negative; CF: cisplatin/5-fluorouracil doublet; ECX: epirubicin, cisplatin, capecitabine; EOX: epirubicin, oxaliplatin, capecitabine; FLOT: fluorouracil, folinic acid, oxaliplatin and docetaxel.
Significant histological regression of the primary tumor by TRG1a-2 was observed in 27 (31.0 %) patients. Although, regression in a primary tumor was not associated with a nodal regression (p=0.168) as shown in table 1. Interestingly, even 11 (57.9 %) of lnR did not show a significant regression in the primary tumor. Overall, the preoperative chemotherapy effect by tumor or/and nodal regression score of 1a-2 was observed in only 38 (43.7 %) of 87 patients.
Survival
The overall and disease-free 3-year survival rate for the study cohort was 54.3 % and 51.3 % respectively. Significant differences were observed between the OS and DFS curves in different study groups (Figure 3A; 3B). The highest OS and DFS were in the lnNEG group, while lowest in the lnNR group. The differences between these groups were significant in terms of OS (p=0.01) and DFS (p=0.01). OS of nodal responders (lnR) was similar as patients without nodal metastasis (lnNEG; p=0.97) and significantly (p=0.03) higher compared to nodal non-responders (lnNR). Although, the difference between lnR and lnNR failed to be significant in terms of DFS (p=0.29). Univariate Cox regression showed lower odds for death in patients with a lnR (HR (95 % CI): 0.33 (0.113-0.967) (Table 4) and a significant benefit of lymph node response was confirmed by a subsequent multivariable analysis (Table 5).
Recurrence of disease was observed in 27 (31.0 %) patients. Peritoneal dissemination included 17 (19.5 %) cases, nodal recurrence 7 (8 %) cases and distant metastasis - 3 (3.4 %) cases. Nodal recurrence rate in the lnNR group (11.9 %) was notably higher compared to lnR (5.3 %) or lnNEG (3.8 %) groups, although differences failed to be significant.
Discussion
This study investigated the histologic regression of LN metastasis after preoperative chemotherapy for AGC. The results of the study demonstrated the nodal response to chemotherapy as a valuable prognostication tool to predict the survival of AGC patients.
The prognosis of resectable AGC remains unsatisfactory, although, it is very different between patients with or without LN metastasis [16-18]. The node-positive patients account for the majority of AGC cases and their prognosis is significantly impaired [18-20]. However, our study nicely demonstrated a better prognosis for those who achieved a significant histologic nodal regression after preoperative chemotherapy. The OS of lnR was significantly better compared to lnNR as showed by Kaplan-Meier and Cox regression analysis. Further, the OS of lnR was not different from true node-negative patients, despite the fact, that 50.0 % of nodal responders had significant histological regression in not all the metastatic nodes. We failed to show the same impact on the DFS, although, the tendency was clearly similar, and the relatively small sample size might be responsible for the lack of significance.
Lymph node regression score in nodal responders' group.
| No. | Gender, age | Chemotherapy regimen | Surgery | No. of retrieved LN | No. of metastatic LN's | No. of lymph nodes with regression grade score | Tumor regression grade | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 2 | 3 | |||||||
| 1. | F, 55 | CF | Gastrectomy | 23 | 2 | 1 | 1 | TRG1a | ||
| 2. | M, 80 | EOX | Subtotal gastrectomy | 27 | 13 | 1 | 1 | 11 | TRG3 | |
| 3. | M, 57 | CF | Gastrectomy | 29 | 5 | 4 | 1 | TRG1b | ||
| 4. | M, 70 | CF | Subtotal gastrectomy | 25 | 2 | 1 | 1 | TRG3 | ||
| 5. | M, 53 | EOX | Subtotal gastrectomy | 34 | 2 | 1 | 1 | TRG2 | ||
| 6. | M, 49 | FLOT | Gastrectomy | 31 | 1 | 1 | TRG2 | |||
| 7. | F, 63 | CF | Subtotal gastrectomy | 25 | 7 | 1 | 1 | 5 | TRG3 | |
| 8. | M, 63 | CF | Gastrectomy | 40 | 4 | 1 | 3 | TRG3 | ||
| 9. | M, 72 | CF | Gastrectomy | 26 | 1 | 1 | TRG3 | |||
| 10. | M, 59 | CF | Subtotal gastrectomy | 36 | 20 | 3 | 17 | TRG3 | ||
| 11. | M, 55 | ECX | Subtotal gastrectomy | 26 | 5 | 2 | 1 | 2 | TRG3 | |
| 12. | M, 68 | FLOT | Subtotal gastrectomy | 34 | 1 | 1 | TRG1b | |||
| 13. | M, 62 | FLOT | Subtotal gastrectomy | 34 | 8 | 4 | 1 | 3 | TRG3 | |
| 14. | M, 57 | CF | Subtotal gastrectomy | 56 | 8 | 2 | 6 | TRG2 | ||
| 15. | F, 65 | EOX | Gastrectomy | 38 | 9 | 9 | TRG2 | |||
| 16. | M, 58 | CF | Gastrectomy | 54 | 13 | 13 | TRG3 | |||
| 17. | M, 57 | ECX | Subtotal gastrectomy | 18 | 6 | 1 | 2 | 3 | TRG3 | |
| 18. | F, 41 | EOX | Gastrectomy | 44 | 1 | 1 | TRG2 | |||
| 19. | M, 46 | CF | Gastrectomy | 19 | 8 | 1 | 7 | TRG3 | ||
| In total: | 36 | 7 | 15 | 58 | ||||||
LN; lymph nodes; M: male; F: female; CF: cisplatin/5-fluorouracil doublet; ECX: epirubicin, cisplatin, capecitabine; EOX: epirubicin, oxaliplatin, capecitabine; FLOT: fluorouracil, folinic acid, oxaliplatin, and docetaxel; TRG: tumor regression grade (by Becker).
Univariate Cox regression analysis for overall and disease-free survival.
| Death | Recurrence of disease | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Age (years) | 1.02 (0.98-1.06) | 0.22 | 1.00 (0.96-1.03) | 0.93 | |
| Lymph node response | lnNR | 1.00 (reference) | 1.00 (reference) | ||
| lnR | 0.33 (0.11-0.96) | 0.04 | 0.63 (0.26-1.51) | 0.63 | |
| lnNEG | 0.30 (0.10-0.88) | 0.02 | 0.06 (0.01-0.51) | 0.01 | |
| Sex | Male | 1.00 (reference) | 1.00 (reference) | ||
| Female | 1.09 (0.51-2.31) | 0.81 | 0.65 (0.28-1.48) | 0.30 | |
| Tumor invasion (ypT) | 1-2 | 1.00 (reference) | 1.00 (reference) | ||
| 3-4 | 1.42 (0.63-3.22) | 0.39 | 3.46 (1.19-9.99) | 0.02 | |
| TRG in primary tumor site | 1a-2 | 1.00 (reference) | 1.00 (reference) | ||
| 3 | 2.06 (0.84-5.09) | 0.11 | 1.70 (0.72-4.01) | 0.22 | |
| Lymph node metastasis (ypN) | N0 | 1.00 (reference) | 1.00 (reference) | ||
| N+ | 2.28 (0.87-6.00) | 0.09 | 7.12 (1.69-30.05) | 0.01 | |
| Tumor differentiation | G1-2 | 1.00 (reference) | 1.00 (reference) | ||
| G3 | 3.29 (0.99-10.92) | 0.05 | 2.24 (0.77-6.49) | 0.13 | |
| Lauren | Intestinal/Mix | 1.00 (reference) | 1.00 (reference) | ||
| Diffuse | 1.59 (0.74-3.40) | 0.23 | 1.84 (0.80-4.21) | 0.14 | |
| Signet ring cells component | Negative | 1.00 (reference) | 1.00 (reference) | ||
| Positive | 1.97 (0.91-4.26) | 0.08 | 2.07 (0.94-4.55) | 0.06 | |
| Tumor localization | Upper/middle third | 1.00 (reference) | 1.00 (reference) | ||
| Lower third | 0.22 (0.08-0.68) | 0.01 | 0.69 (0.32-1.48) | 0.35 | |
| Lymphovasular invasion | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.56 (0.73-3.30) | 0.24 | 2.84 (1.28-6.31) | 0.01 | |
| CCI | 1-3 | 1.00 (reference) | 1.00 (reference) | ||
| ≥4 | 1.04 (0.50-2.16) | 0.91 | 0.57 (0.26-1.23) | 0.15 | |
lnNR: lymph node non-responders; lnR: lymph node responders; TRG: tumor regression grade; LV: lymphovascular invasion; CCI: Charlson Comorbidity Index.
The present study showed that only 31.1 % of node-positive patients are nodal responders and only 43.7 % of patients show a significant regression in LN or/and tumor. A similarly low rate of 29.4 % of nodal response has been documented in the previous study comparing histological regression after preoperative chemotherapy or chemoradiotherapy [21]. This calls into question the effectiveness of preoperative chemotherapy, despite it being rapidly introduced into clinical practice guidelines after MAGIC [1] and FNCLCC-FFCD [2] trials. Moreover, there is still insufficient evidence if preoperative chemotherapy is beneficial for patients who received an appropriate D2 lymphadenectomy [3,4,22]. Although, our study does not provide evidence against the concept because we could not exclude the potential benefit of preoperative chemotherapy on micrometastasis and an increased rate of R0 resection [23]. Another potential benefit of chemotherapy preoperatively is the high rate of treatment compliance. Our results confirmed it by showing successful completion of preoperative chemotherapy in >90 % of patients compared to 72.4 % of patients receiving chemotherapy postoperatively. Such results are consistent with previous reports documenting compliance of about 70 % for AGC patients receiving chemotherapy in the adjuvant setting [24,25]. Therefore, it is clear, that preoperatively chemotherapy can be successfully utilized for a higher proportion of patients compared to postoperatively. On the other hand, nearly 15 % of patients receiving preoperative chemotherapy show the risk of disease progression at the time of preoperative treatment [26]. Therefore, the ideal clinical model would let clinicians identify those non-responders before the start of the treatment. A series of studies investigated novel biomarkers to predict the response to preoperative chemotherapy [27-33]. However, they are still not validated and widely used. Moreover, all of the current studies correlated biomarkers with a regression only in a primary tumor site [27-33]. Since our study demonstrated the importance of histological nodal regression, which is not always associated with a response in a primary tumor, current biomarkers may lack the accuracy to predict the real regression of the disease. Therefore, further studies investigating biomarkers for response prediction should test if novel tools can predict the nodal response too.
Several different chemotherapy regimens have been used in our study, without significant differences in nodal response. Although, due to the relatively small sample size this data should be interpreted cautiously. A recent randomized control trial demonstrated FLOT as the new gold standard for perioperative chemotherapy due to an increased rate of major histological regression of the tumor and improved survival [34,35]. Unfortunately, histological analysis of FLOT4-AIO trial did not include the nodal regression [34]. Therefore, it remains unclear if some of the available preoperative chemotherapy regimens may increase the rate of nodal response.
Various grading systems for different gastrointestinal cancers have the same aim to categorize the number of regressive changes following preoperative cytotoxic treatment and to provide prognostic information [36]. The grading system for advanced gastric cancer proposed by Becker et al. [8] was subsequently confirmed to provide highly valuable prognostic information [9]. Although, this system as all other refers to the regression only in the primary tumor site but not in the LN [36]. This study demonstrated the same system of Becker can be applied to evaluate the nodal regression and it provides even more accurate prognostic information. Therefore, we suggest that Becker system should be adapted to evaluate the histological regression not only in the primary tumor but also in the LN and this information should be implemented to routine pathological reports.
The role of nodal regression following preoperative chemo-/chemoradio- therapy to provide strong prognostic information has been already confirmed in oesophageal adenocarcinoma [11] and rectal cancer [14,37]. However, previous evidence for GC was conflicting [38,39]. A recent study by Zhu et al. concluded that the existence of a residual nodal tumor, rather than nodal regression change is useful to predict the prognosis and suggested unnecessity to routinely investigate nodal regression [38]. Although, the results from this Asian study did not completely refute the prognostic value of nodal regression, but rather showed only complete nodal tumor regression is clinically significant [38]. In contrast, the very recent study by Pereira et al. defined nodal responders as those with less than 43 % of residual tumor and showed improved survival of these patients [39]. Similarly, in our larger-scale study, we defined nodal responders as those with less than 50 % of the residual tumor in at least one of the metastatic LN and showed the improved long-term outcomes for these patients. The reason for such a discrepancy might be different grouping systems used in the different studies. Although our study confirmed, that a widely acknowledged tumor regression grading system by Becker may be adapted to evaluate the nodal response and prognosticate the survival of patients with non-metastatic resectable AGC.
Multivariable Cox regression analysis for overall and disease-free survival.
| HR (95% CI) | p | ||
|---|---|---|---|
| Death | |||
| Lymph node response | lnNR | 1.00 (reference) | |
| lnR | 0.37 (0.14-0.99) | 0.04 | |
| lnNEG | 0.39 (0.14-1.02) | 0.05 | |
| Tumor localization | Upper/middle third | 1.00 (reference) | |
| Lower third | 0.31 (0.10-0.89) | 0.03 | |
| Age (years) | 1.03 (0.98-1.09) | 0.15 | |
| CCI | 1-3 | 1.00 (reference) | |
| ≥4 | 0.74 (0.28-1.95) | 0.55 | |
| Recurrence of disease | |||
| Lymph node response | lnNR | 1.00 (reference) | |
| lnR | 0.57 (0.24-1.34) | 0.20 | |
| lnNEG | 0.132 (0.01-2.47) | 0.17 | |
| ypT | 1-2 | 1.00 (reference) | |
| 3-4 | 3.39 (1.12-10.23) | 0.03 | |
| ypN | N0 | 1.00 (reference) | |
| N+ | 1.79 (0.21-14.97) | 0.59 | |
| Lymphovascular invasion | LV+ | 1.00 (reference) | |
| LV- | 0.93 (0.42-2.01) | 0.85 | |
| Age (years) | 1.05 (0.99-1.11) | 0.07 | |
| CCI | 1-3 | 1.00 (reference) | |
| ≥4 | 0.36 (0.12-1.02) | 0.05 |
lnNR: lymph node non-responders; lnR: lymph node responders; TRG: tumor regression grade; LV: lymphovascular invasion; CCI: Charlson Comorbidity Index.
The main limitations of the present study include the retrospective design, the limited number of patients, and a wide variety of different preoperative chemotherapy regimens used in the study. Despite these drawbacks, we were able to demonstrate, that histologic nodal regression after preoperative chemotherapy should be investigated not only in the primary tumor but also in the lymph nodes. In the future, these regression scores may serve as a surrogate outcome to rapidly evaluate the preoperative treatment efficacy.
Abbreviation
AGC: advanced gastric cancer; TRG: tumor regression grade; LN: lymph node; CT: computed tomography; OS: overall survival; DFS: disease-free survival; HR: hazards ratios; CI: confidence intervals; lnR: nodal responders; lnNR: nodal non-responders; lnNEG: lymph node-negative.
Acknowledgements
This study did not receive any funding.
Data availability statement: Data is available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cunningham D, Allum WH, Stenning SP. et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med. 2006;355:11-20
2. Ychou M, Boige V, Pignon J-P. et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:1715-21
3. Reddavid R, Sofia S, Chiaro P. et al. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J Gastroenterol. 2018;24:274-89
4. Bringeland EA, Wasmuth HH, Grønbech JE. Perioperative chemotherapy for resectable gastric cancer - what is the evidence? Scand J Gastroenterol. 2017;52:647-53
5. Smyth EC, Fassan M, Cunningham D. et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:2721-7
6. Blackham AU, Greenleaf E, Yamamoto M. et al. Tumor regression grade in gastric cancer: Predictors and impact on outcome. J Surg Oncol. 2016;114:434-9
7. Mingol F, Gallego J, Orduña A. et al. Tumor regression and survival after perioperative MAGIC-style chemotherapy in carcinoma of the stomach and gastroesophageal junction. BMC Surg. 2015;15:66
8. Becker K, Mueller JD, Schulmacher C. et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521-30
9. Becker K, Langer R, Reim D. et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253:934-9
10. Ikoma N, Blum M, Chiang Y-J. et al. Race Is a Risk for Lymph Node Metastasis in Patients with Gastric Cancer. Ann Surg Oncol. 2017;24:960-5
11. Davies AR, Myoteri D, Zylstra J. et al. Lymph node regression and survival following neoadjuvant chemotherapy in oesophageal adenocarcinoma. Br J Surg. 2018;105:1639-49
12. Robb WB, Dahan L, Mornex F. et al. Impact of neoadjuvant chemoradiation on lymph node status in esophageal cancer: post hoc analysis of a randomized controlled trial. Ann Surg. 2015;261:902-8
13. Philippron A, Bollschweiler E, Kunikata A. et al. Prognostic Relevance of Lymph Node Regression After Neoadjuvant Chemoradiation for Esophageal Cancer. Semin Thorac Cardiovasc Surg. 2016;28:549-58
14. Choi JP, Kim SJ, Park IJ. et al. Is the pathological regression level of metastatic lymph nodes associated with oncologic outcomes following preoperative chemoradiotherapy in rectal cancer? Oncotarget. 2017;8:10375-84
15. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19
16. Marano L, Polom K, Patriti A. et al. Surgical management of advanced gastric cancer: An evolving issue. Eur J Surg Oncol. 2016;42:18-27
17. Kunz PL, Gubens M, Fisher GA, Ford JM, Lichtensztajn DY, Clarke CA. Long-Term Survivors of Gastric Cancer: A California Population-Based Study. J Clin Oncol. 2012;30:3507-15
18. Yamashita K, Hosoda K, Ema A, Watanabe M. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer. Eur J Surg Oncol. 2016;42:1253-60
19. Akagi T, Shiraishi N, Kitano S. Lymph Node Metastasis of Gastric Cancer. Cancers. 2011;3:2141-59
20. Bausys R, Bausys A, Vysniauskaite I. et al. Risk factors for lymph node metastasis in early gastric cancer patients: Report from Eastern Europe country- Lithuania. BMC Surg. 2017;17:108
21. Martin-Romano P, Sola JJ, Diaz-Gonzalez JA. et al. Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br J Cancer. 2016;115:655-63
22. Schuhmacher C, Gretschel S, Lordick F. et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-8
23. Li W, Qin J, Sun Y-H, Liu T-S. Neoadjuvant chemotherapy for advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2010;16:5621-8
24. Kim D-W, Kwon OK, Yoo M-W. et al. Actual compliance to adjuvant chemotherapy in gastric cancer. Ann Surg Treat Res. 2019;96:185-90
25. Yamashita K, Kurokawa Y, Yamamoto K. et al. Risk Factors for Poor Compliance with Adjuvant S-1 Chemotherapy for Gastric Cancer: A Multicenter Retrospective Study. Ann Surg Oncol. 2017;24:2639-45
26. Xu W, Beeharry MK, Liu W, Yan M, Zhu Z. Preoperative Chemotherapy for Gastric Cancer: Personal Interventions and Precision Medicine. BioMed Res Int. 2016;2016:3923585
27. Napieralski R, Ott K, Kremer M. et al. Combined GADD45A and thymidine phosphorylase expression levels predict response and survival of neoadjuvant-treated gastric cancer patients. Clin Cancer Res. 2005;11:3025-31
28. Bataille F, Rümmele P, Dietmaier W. et al. Alterations in p53 predict response to preoperative high dose chemotherapy in patients with gastric cancer. Mol Pathol MP. 2003;56:286-92
29. Naka A, Takeda R, Shintani M. et al. Organic cation transporter 2 for predicting cisplatin-based neoadjuvant chemotherapy response in gastric cancer. Am J Cancer Res. 2015;5:2285-93
30. Kubo T, Kawano Y, Himuro N. et al. BAK is a predictive and prognostic biomarker for the therapeutic effect of docetaxel treatment in patients with advanced gastric cancer. Gastric Cancer. 2016;19:827-38
31. Blank S, Rachakonda S, Keller G. et al. A retrospective comparative exploratory study on two Methylentetrahydrofolate Reductase (MTHFR) polymorphisms in esophagogastric cancer: the A1298C MTHFR polymorphism is an independent prognostic factor only in neoadjuvantly treated gastric cancer patients. BMC Cancer. 2014;14:58
32. Wu A, Jia Y, Dong B. et al. Apoptosis and KI 67 index correlate with preoperative chemotherapy efficacy and better predict the survival of gastric cancer patients with combined therapy. Cancer Chemother Pharmacol. 2014;73:885-93
33. Jia Y, Ye L, Ji K. et al. Death-associated protein-3, DAP-3, correlates with preoperative chemotherapy effectiveness and prognosis of gastric cancer patients following perioperative chemotherapy and radical gastrectomy. Br J Cancer. 2014;110:421-9
34. Al-Batran S-E, Hofheinz RD, Pauligk C. et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697-708
35. Al-Batran S-E, Homann N, Pauligk C. et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. The Lancet. 2019;393:1948-57
36. Langer R, Becker K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch Int J Pathol. 2018;472:175-86
37. Mirbagheri N, Kumar B, Deb S. et al. Lymph node status as a prognostic indicator after preoperative neoadjuvant chemoradiotherapy of rectal cancer. Colorectal Dis. 2014;16:O339-46
38. Zhu Y-L, Sun Y-K, Xue X-M, Yue J-Y, Yang L, Xue L-Y. Unnecessity of lymph node regression evaluation for predicting gastric adenocarcinoma outcome after neoadjuvant chemotherapy. World J Gastrointest Oncol. 2019;11:48-58
39. Pereira MA, Ramos MFKP, Dias AR. et al. Lymph node regression after neoadjuvant chemotherapy: A predictor of survival in gastric cancer. J Surg Oncol. 2020;121:795-803
Author contact
![]() Corresponding author: MD Augustinas Bausys. Address: Ciurlionio str. 21, Vilnius 03101, Lithuania. Email: abpelikanascom. Telephone: +37062363865.
Corresponding author: MD Augustinas Bausys. Address: Ciurlionio str. 21, Vilnius 03101, Lithuania. Email: abpelikanascom. Telephone: +37062363865.

 Global reach, higher impact
Global reach, higher impact