Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(6):1770-1778. doi:10.7150/jca.50371 This issue Cite
Research Paper
Preoperative Portal Vein Embolization for Liver Resection: An updated meta-analysis
1. Department of Hepatobiliary and Pancreatic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310009.
2. Key Laboratory of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Tumor of Zhejiang Province, Hangzhou, Zhejiang 310009.
3. Research Center of Diagnosis and Treatment Technology for Hepatocellular Carcinoma of Zhejiang Province, Hangzhou, Zhejiang 310009.
4. Clinical Medicine Innovation Center of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Disease of Zhejiang University, Hangzhou, Zhejiang 310009.
5. Clinical Research Center of Hepatobiliary and Pancreatic Diseases of Zhejiang Province, Hangzhou, Zhejiang 310009.
#These authors contributed equally to this work.
Received 2020-7-6; Accepted 2020-12-26; Published 2021-1-21
Abstract
Background: Portal vein embolization (PVE) is performed before major liver resection to increase liver volume remnant, controversy remains on the adverse effect of PVE on liver tumor patients. The current study highlighted the effect of PVE on the degree of hypertrophy of future liver remnant (FLR) and summarized PVE-related complications, aiming to provide a guideline for surgeons.
Methods: A search of current published studies on PVE was performed. Meta-analysis was conducted to assess the effect of PVE on hypertrophy of FLR and summarized PVE-related complications.
Results: 26 studies including 2335 patients were enrolled in the meta-analysis. All enrolled studies reported data regarding FLR hypertrophy rate, pooled effect size (ES) for FLR hypertrophy rate using a fixed-effect model was 0.105 (95%CI: 0.094-0.117, p=0.000), indicating PVE is favored in inducing FLR hypertrophy. Metatrim method indicated no obvious evidence of publication bias in the present meta-analysis. 247 (10.6%) patients exhibited PVE-related complications, receiving expectant treatment without affecting planned liver resection. Total 1782 patients (76%) underwent a subsequent liver resection after PVE, which is an encouraging result comparing with traditional resection rate in liver tumor patients.
Conclusions: PVE is a safe and effective procedure with a low occurrence of related complications for inducing sufficient hypertrophy of FLR in liver tumor patients, which could elevate the resection rate of liver tumor patients. Careful patient cohort selection is crucial to avoid overuse of PVE in technically resectable patients. Further multiple central clinical trials are conducive to select optimal patient cohorts and provide a guideline for surgeons.
Keywords: portal vein embolization, liver tumor, future liver remnant, liver regeneration, liver resection
Introduction
Liver resection remains the gold standard treatment offering both potential cure and long-term survival to patients with either primary or secondary liver tumors [1,2]. The aim of resection is to offer a curative effect with reservation of a sufficient future liver remnant (FLR) to maintain basic liver function at the same time in patients with liver tumors [3,4]. Unfortunately, at the time of diagnosis, only <25% of patients are suitable for surgical resection [5]. Meanwhile, the resection rate for the liver tumor is just 20%-30% in patients with normal livers even reduced in patients with cirrhotic liver. For up to 45% of liver tumor patients, an extended liver resection is imperative to achieve absolute clear resection margins [6]. One of the reasons for aforementioned unresectability is that the remnant liver is insufficient to support postoperative liver function [7]. Postoperative liver failure is still one of the main causes of death following major liver resection, ranging from 0 to 30%, with insufficient FLR being a limiting factor [4]. In literature, postoperative liver failure is directly associated with the volume of liver remnant [8]. To ensure sufficient liver remnant volume after liver resection, several strategies, including portal vein embolization (PVE), portal vein ligation (PVL), associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure, and selective internal radiation therapy (SIRT), have been recently employed in inducing hypertrophy of FLR [9,10]. Within them, ALPPS procedure has been demonstrated to achieve the greatest increase rate of FLR recently [11]. However, PVE has been sometimes recognized as a more ideal method for inducing a comparable increase rate of FLR with ALPPS procedure as well as its lower morbidity and mortality than ALPPS, which is widely accepted by the majority of centers before major liver resection [12,13].
Portal vein embolization, of which the basic principle involved in occluding a branch of portal venous flow to the liver segments that are planned to resect, subsequently results in ipsilateral hepatic atrophy and compensatory contralateral hypertrophy, was first described by Kinoshita in a hepatocellular carcinoma (HCC) patient in 1986 [12]. Since then, various studies have reported the efficacy of PVE in inducing compensatory hypertrophy of FLR in preparation for liver resection [14-17]. Currently, PVE is usually performed as a routine procedure before any extended liver resection to increase remnant liver volume [18]. Although, many clinical studies have been published on hypertrophy of the FRL in small and large patient cohorts. Controversy remains on the potential adverse effect of PVE on tumor growth. Some studies suggested that PVE also stimulates the growth of liver tumor that is still present within the regenerating liver, regardless of embolized lobe or the non-embolized lobe. Disease progression secondary to PVE may affect surgical strategies and patient outcomes [19,20]. Meanwhile, concerns are also raised as to whether PVE only induces volume change rather than functional gain [21].
Two meta-analyses have been published on the effect of PVE in major liver resection. The first by Abulkhir et al. in 2008 reviewed different techniques (percutaneous transhepatic and transileocolic) of PVE and concluded that PVE is an effective procedure in inducing liver regeneration to prevent postoperative liver failure [21]. Another by Lienden et al. demonstrated that PVE has a high technical and clinical success rate and liver cirrhosis has a negative effect on the hypertrophy induced by PVE [8]. However, there is still no authoritative literature systematically summarized the advantages and adverse effects of PVE. In our present meta-analysis, we mainly highlighted the effect of PVE on the degree of hypertrophy of FLR and summarized PVE-related complications, aiming to provide a guideline for surgeons to make an accurate decision.
Materials and Methods
Search strategy and study selection
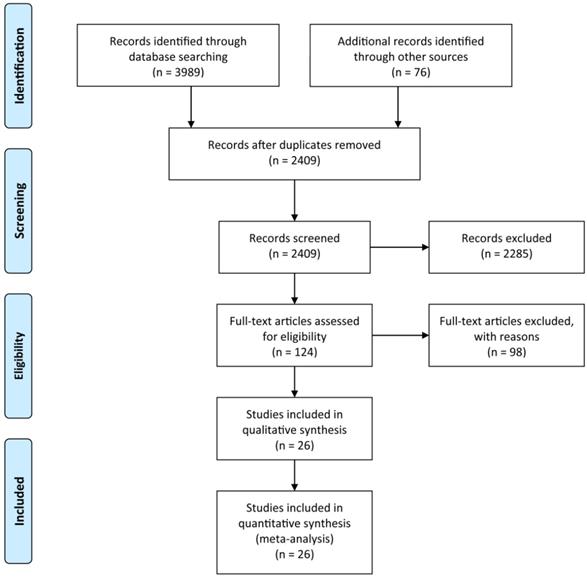
A systematic search of the available published studies on portal vein embolization was conducted in Pubmed, Embase, Medline, PMC, Web of Science, and Cochrane database. Two researchers (Y.H. and W.G.) independently searched publications from 1990 to March 2020 using the following “Mesh Terms”: “portal vein embolization”, “liver resection”, and “liver tumor”. The “related article” function was also used to broaden the search. All abstracts, studies, and citations retrieved were reviewed, including references of these articles. The final selection of the articles was made in consensus by all authors. The detail of literature search strategies is illustrated in Figure 1.
Eligibility criteria
All full text articles were enrolled if they were composed of information on patient characteristics, indications for PVE, techniques, and materials of PVE, the hypertrophy rate of FLR, the successful rate of resection and complications after PVE. Newcastle-Ottawa Quality Assessment Scale (NOS) bias risk tool was used to assess the methodological quality of enrolled studies, and those with a score ≥7 were considered eligible and enrolled in our study. We then extracted the aforementioned clinical parameters from enrolled studies.
Exclusion criteria
We excluded studies if they are reviews, case reports, animal studies, non-English publications, and repetitive publications in different databases. Studies that didn't record patient characteristics, FLR before and after PVE or the hypertrophy rate of FLR, and complications after PVE were also excluded. We also excluded the studies in which appropriate data could not extract from the results.
Statistical analysis
The meta-analysis was performed according to recommendations from the Cochrane Collaboration and the Quality of Reporting of Meta-analyses (QUORUM) guidelines. Single-rate meta-analysis was performed using Stata 12.0 software (Stata Corporation, College Station, TX, USA). The combined effect size (ES) of FLR hypertrophy rate was examined. Combined ES more than 0 favored in the efficacy of PVE and the point estimate of ES was considered to be statistically significant at P < 0.05 level if the 95% confidence interval didn't include the value 0. Heterogeneity among the studies was tested using the p value of Q test and I2 test. When p > 0.1 and I2 ≤50%, a fixed-effect model was used, otherwise a random effect model was selected. A further sensitivity analysis was performed to detect the heterogeneity. Funnel plot, as well as metatrim method, was used to detect the publication bias. P < 0.05 was considered as statistically significant.
Results
Research selection and quality assessment
Based on the aforementioned search strategies, 4065 publications including related articles were searched from the online database. After removing repetitive publications, a total of 2409 records remained. Then, 2285 publications were excluded by screening the titles and abstracts, and 98 of the remaining 124 articles were deleted for various reasons. At last, 26 publications with an NOS score ≥7, including 2335 patients were enrolled in the present meta-analysis (Figure 1). The characteristics of the enrolled studies and clinical parameters of patients in these studies were summarized in Tables 1-3.
FLR hypertrophy rate
All 26 studies reported data regarding FLR hypertrophy rate, pooled ES for FLR hypertrophy rate using a fixed-effect model was 0.105 (95%CI: 0.094-0.117, p=0.000), indicating PVE is favored in inducing FLR hypertrophy. Additionally, the sensitivity analysis demonstrated that there is no study that greatly interfered with the results of the present meta-analysis, suggesting no proof of heterogeneity among the enrolled studies (p value of Q test=0.995, I2=0%) (Figures 2 & 3).
Publication bias
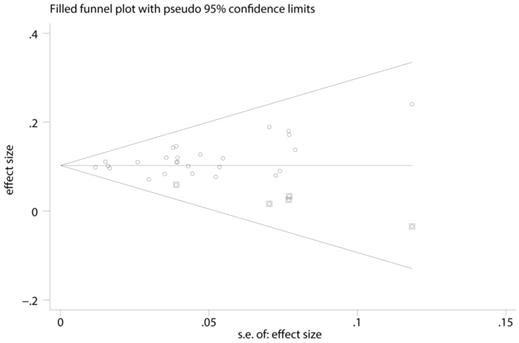
A funnel plot, as well as metatrim method was used to detected publication bias. Before metatrim, pooled ES for FLR hypertrophy rate was 0.105 (95%CI: 0.094-0.117, p=0.000). After metatrim, 5 studies were added into the meta-analysis, and pooled ES for FLR hypertrophy rate was 0.103 (95%CI: 0.092-0.114, p=0.000). Results before and after metatrim are stable and are both statistically significant, which means publication bias is negligible in the present study (Figure 4).
Description of the 26 studies enrolled in the meta-analysis
| Author | Year | Country | Inclusion period | Age | No. of patients | Resection patients | Interval between PVE and surgery | NOS score |
|---|---|---|---|---|---|---|---|---|
| Okabe [46] | 2011 | Japan | 1999-2009 | 58.8 (40-78) | 24 | 19 | 28 (19-63) | 7 |
| Yamashita [2] | 2013 | Japan | 1996-2009 | 61 (35-81) | 64 | 49 | NR | 7 |
| Shindoh [6] | 2013 | America | 1995-2012 | 58 (24-86) | 358 | 282 | 32 (5-385) | 8 |
| Fischman [37] | 2014 | America | 2011-2013 | 59.9 (34-76) | 35 | 27 | 41.6 (26-78) | 9 |
| Luz [41] | 2017 | Brazil | NR | 56.5 (27-86) | 50 | 31 | NR | 7 |
| Alvarez [23] | 2018 | France | 1993-2015 | 60 (24-86) | 431 | 287 | 50 (35-69.5) | 7 |
| Marti [24] | 2017 | America | 2006-2014 | 61 (51.8-68) | 82 | 69 | 37 (20-135) | 8 |
| Tsurusaki [32] | 2018 | Japan | 2010-2016 | 69.5 (45-86) | 19 | 19 | NR | 7 |
| Cotroneo [25] | 2009 | Italy | NR | 66.2 (54-77) | 31 | 24 | NR | 7 |
| Giraudo [33] | 2007 | France | 1997-2006 | 64 (44-88) | 145 | 114 | NR | 7 |
| Ribero [9] | 2007 | America | 1995-2006 | 60 (36-78) | 112 | 78 | NR | 7 |
| Kakizawa [42] | 2006 | Japan | 2001-2005 | 65 (35-81) | 14 | 11 | 22 (14-37) | 8 |
| Beal [48] | 2006 | British | 1999-2002 | 65 (52-74) | 15 | 8 | NR | 7 |
| Elias [16] | 2001 | France | 1987-2000 | NR | 68 | 60 | 30 (24-65) | 7 |
| Madoff [36] | 2003 | America | 1998-2001 | 59 (29-77) | 26 | 16 | NR | 7 |
| Jaberi [44] | 2016 | Canada | 2008-2013 | 61.2 (38-84) | 85 | 60 | NR | 8 |
| Hemming [26] | 2002 | America | 1996-2002 | 61 (31-82) | 39 | 31 | NR | 7 |
| Sofue [43] | 2014 | Japan | 2007-2011 | 68 (45-82) | 83 | 69 | 25 (14-55) | 7 |
| Geisel [38] | 2013 | Germany | 2011-2012 | NR | 75 | 70 | NR | 7 |
| Ratti [27] | 2010 | Italy | 2006-2009 | 63 (37-82) | 62 | 56 | 35 (13-57) | 8 |
| Radeleff [39] | 2008 | Germany | 2001-2006 | 55 (31-68) | 15 | 11 | 49 (34-72) | 9 |
| Cazejust [40] | 2013 | France | 2009-2013 | 63 (38-80) | 63 | 49 | 34 (28-49) | 8 |
| Kuo [17] | 2012 | Australia | 1998-2007 | 60 (46-78) | 25 | 19 | 36 (17-180) | 7 |
| Camelo [45] | 2019 | Portugal | 2013-2017 | 64 (42-84) | 64 | 44 | NR | 7 |
| Loveday [28] | 2018 | America | 2008-2015 | 61.8 (39-80) | 31 | 23 | 8 (4-58) | 9 |
| Yamashita [29] | 2017 | Japan | 1995-2013 | 63 (22-81) | 319 | 256 | NR | 7 |
Abbreviation: NOS: Newcastle-Ottawa Quality Assessment Scale Score; NR: not reported.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for search and selection processes of the meta-analysis.
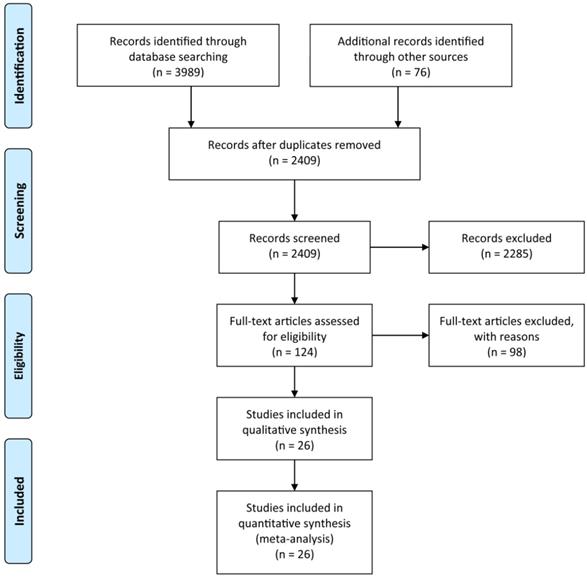
Meta-analysis of effect size (ES) of hypertrophy rate in future liver remnant after PVE.
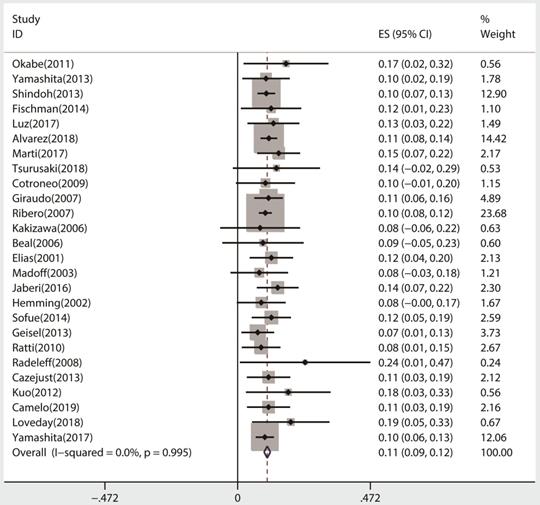
Sensitivity analysis of the meta-analysis.
PVE indications
| Author | PVE indications |
|---|---|
| Okabe [46] | ICG≤10% and FLR<35% or 10%<ICG<20% and FLR<60% |
| Yamashita [2] | FLR≤40% |
| Shindoh [6] | FLR≤20% in patients with normal liver or FLR≤30% in patients with liver fibrosis |
| Fischman [37] | FLR≤20% in patients with normal liver or FLR≤30% in patients with history of chemotherapy or FLR≤40% in patients with liver fibrosis |
| Luz [41] | FLR≤25% in patients with normal liver or FLR≤40% in patients with liver fibrosis |
| Alvarez [23] | FLR≤30% in patients with normal liver or FLR≤40% in patients with liver fibrosis |
| Marti [24] | FLR≤40% |
| Tsurusaki [32] | NR |
| Cotroneo [25] | FLR≤25% |
| Giraudo [33] | FLR≤30% in patients with normal liver or FLR≤40% in patients with liver fibrosis |
| Ribero [9] | FLR≤20% in patients with normal liver or FLR≤30% in patients with history of chemotherapy or FLR≤40% in patients with liver fibrosis |
| Kakizawa [42] | NR |
| Beal [48] | NR |
| Elias [16] | FLR≤30% in patients with normal liver or FLR≤40% in patients with history of chemotherapy |
| Madoff [36] | FLR≤25% |
| Jaberi [44] | FLR<30% in patients with normal liver or FLR<40% in patients with history of chemotherapy |
| Hemming [26] | FLR≤25% in patients with normal liver or FLR≤40% in patients with liver fibrosis |
| Sofue [43] | ICG<15% and FLR<40% |
| Geisel [38] | FLR≤25% in patients with normal liver or FLR≤40% in patients with liver fibrosis |
| Ratti [27] | FLR<30% in patients with normal liver or FLR<40% in patients with history of chemotherapy |
| Radeleff [39] | FLR≤25% in patients with normal liver or FLR≤45% in patients with liver fibrosis |
| Cazejust [40] | FLR≤25% in patients with normal liver or FLR≤30% in patients with history of chemotherapy or FLR≤40% in patients with liver fibrosis |
| Kuo [17] | NR |
| Camelo [45] | NR |
| Loveday [28] | FLR≤40% |
| Yamashita [29] | ICG<10% and FLR≤40% or 10%<ICG≤20% and FLR<50% |
Abbreviation: PVE: portal vein embolization; ICG: indocyanine green; FLR: future liver remnant; NR: not reported.
Baseline characteristic of patients in the enrolled studies
| Details | No. (%) |
|---|---|
| Total no. patients | 2335 |
| Age (year) | 61±14 |
| Pathology | |
| HCC [6,9,17,23,24,25,36,27,28,29,32,33,36,37,38,39,40,43,44,45,46] | 528 (23) |
| CHC [6,9,17,23,25,27,29,32,33,36,37,38,39,40,41,42,43,44] | 558 (24) |
| CLM [2,6,9,16,17,23,25,26,27,29,32,33,36,37,38,39,40,41,42,43,44,45,48] | 1045 (45) |
| Others [6,9,23,25,26,27,32,33,36,38,39,40,41,42,43,44,45] | 204 (8) |
| Embolization materials | |
| Ethanolamine oleate iopamidol [46] | 24 (1) |
| Gelatinpowder+thrombin | 319 (14) |
| +diatrizoate sodium meglumine+gentamicin [2,29] | |
| Microspheres [6] | 358 (15.5) |
| Sodium tetradecyl sulfate foam [24,37] | 75 (3) |
| N-butyl-cyanocrylate | 387 (17) |
| +iodized oil [23,24,41] | |
| Absolute ethanol [23,29,32,43] | 302 (13) |
| Cyanoacrylate glue+iodized oil [25] | 29 (1) |
| PVA+coils [9,25,26,28,36,38,44,45,48] | 334 (14) |
| Isobutyl-2-cyanoacrylate glue+iodized oil [16,33] | 213 (9) |
| Gelatin sponge+iodized oil [42] | 14 (0.6) |
| Enbucrilate tissue adhesive+lipiodol [48] | 12 (0.5) |
| N-butyl-cyanocrylate+amplatzer vascular plug [44] | 45 (1.9) |
| Amplatzer vascular plug+coils [38] | 35 (1.5) |
| Glue+lipiodol+microparticles [27] | 62 (2.7) |
| Ethibloc+lipiodol [39] | 15 (0.6) |
| Trisacryl microspheres+gelform+coils [40] | 63 (2.7) |
| Histoacryl+lipiodol [17,28] | 48 (2) |
| Interval between PVE and surgery (day) | 38.9 |
| Resection post PVE | 1782 (76) |
| No-resection post PVE | 553 (24) |
Abbreviation: HCC: hepatocellular carcinoma; CHC: cholangiocarcinoma; CLM: colorectal liver metastases; PVE: portal vein embolization.
PVE-related complications
Although almost every enrolled study reported the complications, 247 (10.6%) patients exhibited PVE-related complications, of which abdominal pain, fever, and coil displacement are most frequently seen. The overall occurrence rate of complications is infrequent after PVE, and there was no mortality directly associated with PVE. All patients with complications received expectant treatment without affecting subsequent liver resection (Table 4).
Funnel plot, as well as metatrim method, assess the publication bias of the meta-analysis.
Complications related to PVE
| Details | No. |
|---|---|
| Total no. patients | 247 |
| Abdominal pain [16,17,23,25,27] | 69 |
| Fever [16,23,25,27,33,36,45] | 81 |
| Coil displacement [6,9,23,29,33,37,40,41] | 42 |
| Portal vein thrombosis [6,9,23,29,36,41,43,48] | 30 |
| Subcapsular hematoma [6,9,29,32,33,36,39] | 14 |
| Nausea and vomiting [33,44,45] | 12 |
| Hepatic abscess [23,43,44] | 7 |
| Subcapsular biloma [38,41] | 5 |
| Esophageal bleeding [6,9] | 2 |
| Liver failure [23] | 4 |
| Hemoperitoneum [33,45] | 2 |
| Portal hypertension [40] | 4 |
| Systemic sepsis [33] | 1 |
| Bile duct infection [42] | 1 |
| Pseudoaneurysm [43] | 1 |
| Pulmonary embolism [33] | 1 |
| Intrahepatic portovenous shunt [40] | 1 |
| Hepatic artery branch laceration [45] | 1 |
| Bile leak [29] | 2 |
| Bowel obstruction [29] | 1 |
| Hyperbilirubinemia [44] | 1 |
| Idiopathic hepatic venous thrombosis [44] | 1 |
Liver resection after PVE
In the present study, 1782 patients (76%) underwent a subsequent liver resection after PVE, which is an encouraging result comparing with the traditional resection rate in liver tumor patients. The average interval between PVE and surgery was 38.9 days, resembling the results ever reported. 553 patients (24%) failed to undergo operations because of insufficient hypertrophy, local tumor progression, extrahepatic tumor spread and other complications (Table 3).
Discussion
Preoperative PVE has been performed clinically to induce hypertrophy of the contralateral lobe and avoid postoperative liver failure resulted from insufficient remnant liver after resection. The basic principle of PVE is occluding a branch of portal venous flow to the liver segments that are planned to resect, resulting in ipsilateral hepatic atrophy and compensatory contralateral hypertrophy [18]. However, the exact molecular mechanism leading to atrophy of the embolized lobe and hypertrophy of the FLR is still unknown. Recent studies showed that hepatic growth factor (HGF) and transforming growth factor (TGF)-α and -β may play vital roles in contributing to the hypertrophy of the non-embolized lobe [22].
As for the indications, PVE is initially used to increase the resection rate in HCC patients [12]. Over the past two decades, the indications of PVE also include nearly all primary and secondary liver tumors with insufficient FLR before major liver resection [23-29]. Ribero et al. showed a small FLR is strongly associated with postoperative hepatic dysfunction [9]. Hence, the majority of centers use an FLR volume ratio of 25%-30% of the original liver volume as a threshold to select appropriate patients with normal liver function. Nevertheless, most liver tumor patients are usually with the infection of hepatic virus, the history of chemotherapy, liver cirrhosis or fibrosis, and other factors inducing liver dysfunction. A threshold of 35%-45% is preferred by most centers as a minimum FLR volume rate [3,23]. Some Japanese researchers also advocate to select appropriate patients for PVE by the method of indocyanine green (ICG) plasma disappearance or retention rate test at 15 min, which is beneficial to estimate preoperative remnant liver function [30]. Recent researches reported quantitative liver function tests, such as 99Tc-labelled mebrofenin hepatobiliary scintigraphy HBS and 99Tc-galactosyl-human serum albumin (GSA) scintigraphy, are conducive to select appropriate patients for PVE [31].
Several mature techniques for PVE have been introduced, including transileocolic portal vein embolization (TIPE), the percutaneous transhepatic ipsilateral or contralateral PVE technique (PTPE), and intraoperative portal branch embolization [32-34]. It is demonstrated that a greater increase in FRL in PTPE than in surgical TIPE, as well as no difference in the occurrence of major complications [6]. With the advancement of radiological intervention, PTPE becomes the standard technique for PVE with a satisfactory success rate. PTPE can be performed by an ipsilateral or contralateral approach. The ipsilateral approach is preferred by the majority of centers for its advantage of avoiding puncturing the FLR tissue and easier to access to segment Ⅳ, though technically more difficult [35].
Many available commercially embolization materials have been applied for PVE. Polyvinyl alcohol (PVA) particles and N-butyl-cyanoacrylate with coils are mostly used [8,36]. In our meta-analysis, we summarized 26 studies and concluded that apart from both of them, absolute ethanol, microspheres, and gelatin powder are also widely accepted in the majority of centers [37-40]. (Table 3) N-butyl-cyanoacrylate induces severe inflammatory reaction, usually resulting in technical difficulty in surgical resection [14,41]. Gelatin powder is absorbable, producing only transient embolization with the possibility of vascular recanalization [42]. Absolute ethanol has been showed to induce peripheral parenchyma fibrosis and necrosis, and severe abdominal pain sometimes, though producing effective hypertrophy of FLR [43]. PVA particles are easily available and provide persistent occlusion of portal branched with acceptable side effects. Hence, PVA is recommended to apply alone or with other materials in the majority of centers [44,45]. In general, large clinical studies comparing different embolization materials are still necessary to seek the optimal materials.
All patients underwent volumetric assessment by means of CT imaging before PVE and surgery [46]. There is no consensus on the most appropriate waiting time between PVE and surgery. It has been showed that the average interval from PVE to liver resection was 29 days [21]. In our study, the majority of the enrolled studies reported interval between PVE and liver resection, the average interval was 38.9 days (Table 3), which is similar to the results ever reported. To our knowledge, a longer time interval after PVE allows greater growth of FRL. Nevertheless, there is the issue put forward by some surgeons that tumor growth is simultaneously induced by PVE. Accumulating studies demonstrated that tumor progression after PVE is possible in both embolized and non-embolized lobe [19,47]. Additionally, controversy remains as to whether PVE only induces volume change rather than functional gain [21]. In consideration of disease progression after PVE may affect surgical strategies and patient outcomes, more multiple central clinical trials are imperative to come to a consensus on the optimal interval between PVE and liver resection.
Apart from limiting time between PVE and liver resection, post-PVE chemotherapy, or sequential transarterial chemoembolization (TACE) is also recommended to restrict tumor progression by some centers [8]. Beal et al. demonstrated a reduction in tumor size in patients who received chemotherapy after PVE. However, the attendant problem is that less hypertrophy of FRL is observed in patients with a history of chemotherapy [48]. Other studies also showed no significant difference in hypertrophy rate or complications in patients with chemotherapy post PVE [49]. Due to the limited number of current studies and their heterogeneity, more researches are needed to evaluate the effect of chemotherapy on the PVE receptor.
Either the overall technical success (99.3%) or clinical success rate (96.1%) of PVE is extremely high as reported. Patients who experienced failure for the first time also possess the second chance to achieve a successful embolization, which made PVE a safe and effective technique for patients [8]. Although various PVE-related complications have been reported, complications infrequently occurred after PVE and there was no mortality directly associated with PVE. In our present study, 247 (10.6%) patients exhibited PVE-related complications, of which abdominal pain, fever, and coil displacement are most frequently seen (Table 4). All patients with complications received expectant treatment without affecting subsequent liver resection. In our present meta-analysis, 553 patients (24%) failed to undergo a liver resection because of insufficient hypertrophy, local tumor progression, extrahepatic tumor spread, and other PVE-related complications (Table 3). However, comparing with traditional resection rate in liver tumor patients, more patients benefit from PVE and have access to resection with a reduced occurrence of postoperative complications.
Conclusion
Although as one of the emerging methods inducing hypertrophy of FLR, PVE has been expertly used during recent years with an acceptable adverse effect to make more patients able to achieve major liver resection with a high rate of success, which is recommendable for any patients with a small future liver remnant volume when considering liver resection. Our previous teamwork reported that PVE prior to hepatectomy may promote FLR compensatory hypertrophy and an increase in the resectability of primary liver cancer, which could be considered as an independent patient cohort to validate our findings and conclusions in our present meta-analysis [50,51]. The ipsilateral approach is preferred and PVA particles are usually the first choice for PVE. More multiple central clinical trials are needed to determine whether it is necessary to conduct post-PVE chemotherapy and when is the appropriate time to perform the resection. PVE-related complications are infrequently seen and timely expectant treatment is beneficial for patients without affecting subsequent liver resection.
Superiority
To date, this is the first meta-analysis that directly highlighted the degree of hypertrophy of FLR by PVE procedure. Due to the greatest patient cohort and rational analysis method in the present meta-analysis, the statistical power of this meta-analysis and the integrity of the summary were better than any individual research published so far. Additionally, this meta-analysis contributes new convincing information to previous literature, which may provide a promising guideline for other researchers.
Limitations
Meta-analysis has an intrinsic bias introduced by the selection and location of studies. Meanwhile, researchers preferred to report positive findings and studies with significant differences are easy to be published, which may induce publish bias. In our present meta-analysis, most of the enrolled studies are retrospective, of which a long study period may introduce potential confounders. In addition, although a large patient cohort is included, the quality of enrolled studies is uneven, which may result in bias in our result. Hence, more high quality randomized, clinical trials are conducive to select the most appropriate patient cohorts and evaluate the effect of PVE.
Abbreviations
PVE: portal vein embolization; FLR: future liver remnant; PVL: portal vein ligation; SIRT: selective internal radiation therapy; ALPPS: associating liver partition with portal vein ligation for staged hepatectomy; HCC: hepatocellular carcinoma; NOS: Newcastle-Ottawa Quality Assessment Scale; HGF: hepatic growth factor; TGF: transforming growth factor; GSA: 99Tc-galactosyl-human serum albumin; TIPE: transileocolic portal vein embolization; PTPE: percutaneous transhepatic portal vein embolization; PVA: polyvinyl alcohol; TACE: transarterial chemoembolization; ICG: indocyanine green.
Acknowledgements
Author Contributions
Yu Huang and Wenhao Ge proposed the concept and design of this study; Yang Kong and Yuan Ding collected the literature; Yu Huang, Wenhao Ge, Bingqiang Gao and Xiaohui Qian analyzed the data; All authors participated in the manuscript writing and final approval of the manuscript.
Funding
This work is supported by National Natural Science Foundation of China (No.81572307 and 81773096).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Annals of surgery. 2008;247:125-35
2. Yamashita S, Hasegawa K, Takahashi M, Inoue Y, Sakamoto Y, Aoki T. et al. One-stage hepatectomy following portal vein embolization for colorectal liver metastasis. World journal of surgery. 2013;37:622-8
3. Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271-80
4. Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-96
5. Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:8490-9
6. Shindoh J, Tzeng CW, Aloia TA, Curley SA, Huang SY, Mahvash A. et al. Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2014;18:45-51
7. Garcea G, Ong SL, Maddern GJ. Predicting liver failure following major hepatectomy. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2009;41:798-806
8. van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM. et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25-34
9. Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. The British journal of surgery. 2007;94:1386-94
10. Teo JY, Allen JC Jr, Ng DC, Choo SP, Tai DW, Chang JP. et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2016;18:7-12
11. Eshmuminov D, Raptis DA, Linecker M, Wirsching A, Lesurtel M, Clavien PA. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. The British journal of surgery. 2016;103:1768-82
12. Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World journal of surgery. 1986;10:803-8
13. Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P. et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-7
14. de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology (Baltimore, Md). 1996;24:1386-91
15. Kawasaki S, Makuuchi M, Kakazu T, Miyagawa S, Takayama T, Kosuge T. et al. Resection for multiple metastatic liver tumors after portal embolization. Surgery. 1994;115:674-7
16. Elias D, Ouellet JF, De Baère T, Lasser P, Roche A. Preoperative selective portal vein embolization before hepatectomy for liver metastases: long-term results and impact on survival. Surgery. 2002;131:294-9
17. Kuo SC, Azimi-Tabrizi A, Briggs G, Maher R, Harrington T, Samra JS. et al. Portal vein embolization prior to major liver resection. ANZ journal of surgery. 2014;84:341-5
18. Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A. et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Annals of surgery. 2000;231:480-6
19. Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M. et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology (Baltimore, Md). 2001;34:267-72
20. Heinrich S, Jochum W, Graf R, Clavien PA. Portal vein ligation and partial hepatectomy differentially influence growth of intrahepatic metastasis and liver regeneration in mice. Journal of hepatology. 2006;45:35-42
21. Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J. et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Annals of surgery. 2008;247:49-57
22. Hayashi H, Beppu T, Sugita H, Masuda T, Okabe H, Takamori H. et al. Serum HGF and TGF-beta1 levels after right portal vein embolization. Hepatology research: the official journal of the Japan Society of Hepatology. 2010;40:311-7
23. Alvarez FA, Castaing D, Figueroa R, Allard MA, Golse N, Pittau G. et al. Natural history of portal vein embolization before liver resection: a 23-year analysis of intention-to-treat results. Surgery. 2018;163:1257-63
24. Marti J, Giacca M, Alshebeeb K, Bahl S, Hua C, Horn JC. et al. Analysis of Preoperative Portal Vein Embolization Outcomes in Patients with Hepatocellular Carcinoma: A Single-Center Experience. Journal of vascular and interventional radiology: JVIR. 2018;29:920-6
25. Cotroneo AR, Innocenti P, Marano G, Legnini M, Iezzi R. Pre-hepatectomy portal vein embolization: single center experience. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35:71-8
26. Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG. et al. Preoperative portal vein embolization for extended hepatectomy. Annals of surgery. 2003;237:686-91 discussion 91-3
27. Ratti F, Soldati C, Catena M, Paganelli M, Ferla G, Aldrighetti L. Role of portal vein embolization in liver surgery: single centre experience in sixty-two patients. Updates in surgery. 2010;62:153-9
28. Loveday BPT, Jaberi A, Moulton CA, Wei AC, Gallinger S, Beecroft R. et al. Effect of portal vein embolization on treatment plan prior to major hepatectomy for hepatocellular carcinoma. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2019;21:1072-8
29. Yamashita S, Sakamoto Y, Yamamoto S, Takemura N, Omichi K, Shinkawa H. et al. Efficacy of Preoperative Portal Vein Embolization Among Patients with Hepatocellular Carcinoma, Biliary Tract Cancer, and Colorectal Liver Metastases: A Comparative Study Based on Single-Center Experience of 319 Cases. Ann Surg Oncol. 2017;24:1557-68
30. Takasaki T, Kobayashi S, Suzuki S, Muto H, Marada M, Yamana Y. et al. Predetermining postoperative hepatic function for hepatectomies. International surgery. 1980;65:309-13
31. de Graaf W, van Lienden KP, van den Esschert JW, Bennink RJ, van Gulik TM. Increase in future remnant liver function after preoperative portal vein embolization. The British journal of surgery. 2011;98:825-34
32. Tsurusaki M, Oda T, Sofue K, Numoto I, Yagyu Y, Kashiwagi N. et al. The technical aspects of a feasible new technique for ipsilateral percutaneous transhepatic portal vein embolization. The British journal of radiology. 2018;91:20180124
33. Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery. 2008;143:476-82
34. Shimura T, Suehiro T, Suzuki H, Okada K, Araki K, Kuwano H. Trans-ileocecal portal vein embolization as a preoperative treatment for right trisegmentectomy with caudate lobectomy. Journal of surgical oncology. 2007;96:438-41
35. Glantzounis GK, Tokidis E, Basourakos SP, Ntzani EE, Lianos GD, Pentheroudakis G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2017;43:32-41
36. Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness-study in 26 patients. Radiology. 2003;227:251-60
37. Fischman AM, Ward TJ, Horn JC, Kim E, Patel RS, Nowakowski FS. et al. Portal vein embolization before right hepatectomy or extended right hepatectomy using sodium tetradecyl sulfate foam: technique and initial results. Journal of vascular and interventional radiology: JVIR. 2014;25:1045-53
38. Geisel D, Malinowski M, Powerski MJ, Wüstefeld J, Heller V, Denecke T. et al. Improved hypertrophy of future remnant liver after portal vein embolization with plugs, coils and particles. Cardiovasc Intervent Radiol. 2014;37:1251-8
39. Radeleff B, Schawo S, Hoffmann K, Schemmer P, Noeldge G, Kauffmann GW. et al. Efficacy and safety of percutaneous transhepatic portal embolization before right liver resection using an ethibloc/lipiodol mixture: a single-center experience. Digestive surgery. 2008;25:52-9
40. Cazejust J, Bessoud B, Le Bail M, Menu Y. Preoperative portal vein embolization with a combination of trisacryl microspheres, gelfoam and coils. Diagnostic and interventional imaging. 2015;96:57-64
41. Luz JHM, Luz PM, Bilhim T, Martin HS, Gouveia HR, Coimbra É. et al. Portal vein embolization with n-butyl-cyanoacrylate through an ipsilateral approach before major hepatectomy: single center analysis of 50 consecutive patients. Cancer imaging: the official publication of the International Cancer Imaging Society. 2017;17:25
42. Kakizawa H, Toyota N, Arihiro K, Naito A, Fujimura Y, Hieda M. et al. Preoperative portal vein embolization with a mixture of gelatin sponge and iodized oil: efficacy and safety. Acta radiologica (Stockholm, Sweden: 1987). 2006;47:1022-8
43. Sofue K, Arai Y, Shimada K, Takeuchi Y, Kobayashi T, Satake M. et al. Right portal vein embolization with absolute ethanol in major hepatic resection for hepatobiliary malignancy. The British journal of surgery. 2014;101:1122-8
44. Jaberi A, Toor SS, Rajan DK, Mironov O, Kachura JR, Cleary SP. et al. Comparison of Clinical Outcomes following Glue versus Polyvinyl Alcohol Portal Vein Embolization for Hypertrophy of the Future Liver Remnant prior to Right Hepatectomy. Journal of vascular and interventional radiology: JVIR. 2016;27:1897-905.e1
45. Camelo R, Luz JH. Portal Vein Embolization with PVA and Coils before Major Hepatectomy: Single-Center Retrospective Analysis in Sixty-Four Patients. 2019; 2019: 4634309.
46. Okabe H, Beppu T, Ishiko T, Masuda T, Hayashi H, Otao R. et al. Preoperative portal vein embolization (PVE) for patients with hepatocellular carcinoma can improve resectability and may improve disease-free survival. Journal of surgical oncology. 2011;104:641-6
47. Elias D, De Baere T, Roche A. et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. The British journal of surgery. 1999;86:784-8
48. Beal IK, Anthony S, Papadopoulou A, Hutchins R, Fusai G, Begent R. et al. Portal vein embolisation prior to hepatic resection for colorectal liver metastases and the effects of periprocedure chemotherapy. The British journal of radiology. 2006;79:473-8
49. Goéré D, Farges O, Leporrier J, Sauvanet A, Vilgrain V, Belghiti J. Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2006;10:365-70
50. Sun JH, Zhang YL, Nie CH, Li J, Zhou TY, Zhou GH. et al. [Effect of liver cirrhosis on percutaneous selective portal vein embolization for primary liver cancer]. Zhonghua yi xue za zhi. 2013;93:3831-4
51. Sun JH, Zhang YL, Nie CH, Li J, Zhou TY, Zhou GH. et al. Effects of liver cirrhosis on portal vein embolization prior to right hepatectomy in patients with primary liver cancer. Oncology letters. 2018;15:1411-6
Author contact
![]() Corresponding author: Weilin Wang, Department of Hepatobiliary and Pancreatic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, No. 88 Jiefang Road, Hangzhou, Zhejiang, China, 310009; E-mail: wamedu.cn; Tel: +86 0571 87783820; Fax: +86 0571 87068001.
Corresponding author: Weilin Wang, Department of Hepatobiliary and Pancreatic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, No. 88 Jiefang Road, Hangzhou, Zhejiang, China, 310009; E-mail: wamedu.cn; Tel: +86 0571 87783820; Fax: +86 0571 87068001.

 Global reach, higher impact
Global reach, higher impact