Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(10):2807-2814. doi:10.7150/jca.51445 This issue Cite
Research Paper
The relationship between the severity of pulmonary fibrosis and the lung cancer stage
1. Division of Pulmonology, Department of Internal Medicine, Institute of Chest Diseases, Severance Hospital, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea.
2. Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, 82 Gumi-ro, 173 Beon-gil, Bundang-gu, Seongnam-si, Gyeonggi-do, 463-707, Republic of Korea.
Received 2020-8-3; Accepted 2021-2-25; Published 2021-3-14
Abstract
Background: The incidence of idiopathic pulmonary fibrosis (IPF) and mortality related to the disease have steadily increased in recent years. The risk of cancer is approximately eight times higher in IPF patients than in the general population. The purpose of this study is to determine whether the severity of IPF is related to the time interval between IPF diagnosis and lung cancer diagnosis and to the stage of lung cancer at diagnosis.
Methods: In this retrospective cohort study, we reviewed the medical records of patients with lung cancer after IPF diagnosis from two tertiary hospitals in South Korea between 2003 and 2018. We identified 61 patients diagnosed with lung cancer at least 3 months after being diagnosed with IPF.
Results: The included patients had a mean age of 71.0 years, and all but one were men (98.4%). The interval between IPF diagnosis and lung cancer diagnosis was not related to the gender-age-physiology (GAP) stage (p=0.662). However, in cox proportional hazard models, a higher GAP stage was significantly correlated with an advanced lung cancer stage (odds ratio 11.1, p=0.003).
Conclusions: The lung cancer stage at diagnosis was higher in patients with a higher GAP stage than in those with a lower GAP stage. Physicians should consider implementing more frequent surveillance with computed tomography scans for patients with advanced IPF.
Keywords: idiopathic pulmonary fibrosis, lung cancer, GAP stage
Introduction
The incidence of idiopathic pulmonary fibrosis (IPF), a debilitating fibrotic lung disease of unknown origin, is steadily increasing, as is the mortality associated with the condition [1]. The prognosis of patients with IPF is poor, and there is no curative treatment. The median survival time following the diagnosis is approximately 3 years [2]. Although the causes of IPF remain unknown, some risk factors have been identified, including advanced age; smoking; and inhalation of stone, metal, wood, and organic dust [3]. IPF and lung cancer (LC) share risk factors (e.g., smoking and advanced age), and pulmonary fibrosis itself is a risk factor for LC [4-7]. The relative risk of IPF patients developing LC is approximately eight times higher than that of the general population [8], and the reported prevalence of LC in patients with IPF ranges from 2.7% to 31.3% [6, 9]. The incidence of LC also increases with advancement in the clinical course of IPF; the cumulative incidence at 10 years of follow-up exceeds 50% [9-11].
Pulmonary fibrosis and LC also share multiple genetic, molecular, and cellular processes that predispose patients to the diseases [4, 12]. Although the precise properties of fibrosis that lead to the development of carcinoma are unknown, one possibility is that progressive scarring causes lymphatic obstruction, which results in a local increase of potentially carcinogenic material [7]. Takahashi et al. [13] reported high concentrations of carcinoembryonic antigen (CEA) in the bronchoalveolar lavage fluid of patients with fibrosing alveolitis, particularly in patients with associated LC. These high CEA levels may be a marker of premalignant metaplasia and hyperplasia and may predict a greater risk of pulmonary carcinoma during the clinical course of pulmonary fibrosis [7]. This is consistent with the observed distribution of the severity of fibrotic lesions in patients with IPF, implicating that the inflammatory procedure and bronchiolar squamous metaplasia may contribute to the pathogenesis of cancer [7]. Considering their high incidence, mortality rates, and shared pathogenesis and risk factors, clarification of the relationship between IPF and LC and their clinical features is essential for establishing diagnostic and therapeutic strategies.
Previous studies have highlighted the clinical risk factors associated with LC development in IPF patients and examined the clinical characteristics and survival of patients with both conditions (IPF-LC). There is abundant research on the epidemiological and mechanistic links between IPF and LC [14], and many researchers have focused on the incidence, location, or histologic type of the cancer [9, 15, 16]. However, the above-mentioned relationships make it likely that the more severe the IPF, the earlier the cancer will develop or progress to an advanced stage. IPF-LC patients are difficult to treat and have poorer clinical outcomes than patients with IPF or LC alone. Therefore, it is important to identify IPF-LC patients in early stages of the process and provide prompt management. However, little is known about the diagnostic and therapeutic management of these patients. Even the most recent American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Society (ATS/ERS/JRS/ALAT) guidelines, updated in 2018, do not address this crucial issue [17]. In this study, we investigated whether the severity of IPF is related to the time interval between IPF diagnosis and LC diagnosis and to the stage of the cancer.
Materials and Methods
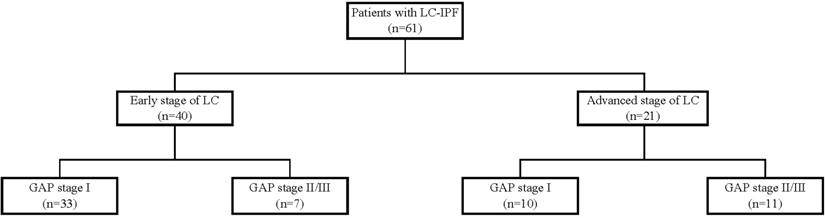
In this study, we reviewed medical records from two tertiary hospitals in South Korea. Eligible patients had been diagnosed with IPF between 2003 and 2018 and diagnosed with LC at least 3 months later. Figure 1 shows the data collection process. A total of 61 eligible IPF patients were identified. Of these patients, 40 had early stage LC (stages I to IIIA, limited stage of small cell LC) and 21 had advanced stage LC (stage IIIB and higher, extensive stage of small cell cancer). Patients' gender-age-physiology (GAP) index scores were classified into stages I-III, with higher scores indicating greater severity [18]. Based on the gender, age, predicted forced vital capacity (FVC), and diffusing capacity of the lung for carbon monoxide (DLco), the GAP index assesses mortality risk levels. Due to the small number of patients in the GAP stage III group (n=5), this group was combined with the GAP stage II group (n=13).
Clinical and laboratory data were collected from patients' medical records. Data on age, sex, body mass index (BMI), smoking history, pulmonary function test results, underlying diseases, Eastern Cooperative Oncology Group (ECOG) performance status, histological type of cancer, date and cause of death, and the clinical and/or pathologic staging of LC were collected for all patients. Smoking history was categorized into three groups (never, former [a person who had smoked at least 100 cigarettes or cigars during their lifetime and who had quit smoking at the time of the interview], and current-smoker); cumulative smoking amount was calculated in pack-years. Overall survival time was calculated from the date of LC diagnosis to the date of death or last follow-up. Pulmonary function tests were performed at the time of IPF diagnosis.
Patient recruitment flow chart.
IPF was diagnosed using the criteria for the usual interstitial pneumonia (UIP) pattern as described by the ATS/ERS/JRS/ALAT [17]: sub-pleural, basal, predominantly reticular abnormality or honeycombing, with or without traction bronchiectasis, and the presence of a consistent UIP pattern. Detailed histories were obtained regarding patients' IPF and serologic tests performed to exclude connective tissue disease. A chest computed tomography (CT) scan showing a definite UIP pattern was considered confirmatory of IPF. When the CT scans were not definitive for the presence or absence of IPF, these diagnoses were confirmed at each hospital by a team of specialists in pulmonary medicine, radiology, and pathology.
Categorical variables were compared using Pearson's chi-square test and continuous variables using independent sample t-test to compare baseline characteristics of GAP I and GAP II/III patients. Results for continuous variables are reported as mean with standard deviation, while categorical variables are reported as numbers and percentages. We further investigated the relationships between clinical parameters and mortality using Cox proportional hazard models with stepwise selection of variables found to be significant in the univariate regression analysis. As age, gender, FVC, and DLco are included in the GAP index, they were excluded from the proportional regression and hazard models. A p-value less than 0.05 was used to indicate statistical significance. All statistical analyses were performed using IBM SPSS Statistics (version 25.0).
This research protocol was approved by the Institutional Review Board of Severance Hospital, South Korea (IRB No. 4-2018-0770) and the Institutional Review Board and Ethics Committee of Seoul National University Bundang Hospital (IRB No. B-1707/411-402). The study design was approved by the appropriate ethics review boards, and the requirement to obtain informed patient consent was waived.
Results
Baseline characteristics
Baseline characteristics of the study subjects are shown in Table 1. Patients had a median age of 71.0±7.7 years, all but one were men (98.4%), and 54 (88.5%) were either past or current smokers. The cumulative smoking amount (pack-years) was higher among patients with an advanced GAP stage. Hypertension and diabetes mellitus were the most common comorbidities in the early GAP stage group, but tuberculosis and chronic obstructive lung disease (COPD) were diagnosed more frequently in the advanced group.
Patient baseline characteristics and comorbidities according to GAP stage
| Variable | GAP I | GAP II/III | Total | p-value |
|---|---|---|---|---|
| Total patients, n (%) | 43 (70.5) | 18 (29.5) | 61 (100.0) | |
| Age (years) | 70.6 ± 8.5 | 72.0 ± 5.6 | 71.0 ± 7.7 | 0.524 |
| Sex, male (%) | 42 (97.7) | 18 (100.0) | 60 (98.4) | 0.527 |
| BMI, kg/m2 | 23.9 ± 2.4 | 22.6 ± 4.0 | 23.5 ± 3.0 | 0.139 |
| GAP score | 2.7 ± 0.6 | 5.1 ± 1.3 | 3.4 ± 1.4 | <0.001 |
| Smoking exposure, No. (%) | 0.467 | |||
| Never | 5 (11.6) | 2 (11.1) | 7 (11.5) | |
| Former | 31 (72.1) | 15 (83.3) | 46 (75.4) | |
| Current | 7 (16.3) | 1 (5.6) | 8 (13.1) | |
| Smoking amount, (Pack-years) | 35.6 ± 23.8 | 47.8 ± 39.8 | 39.4 ± 29.9 | 0.152 |
| FVC, % predicted | 94.8 ± 15.1 | 75.0 ± 24.0 | 86.8 ± 21.3 | 0.004 |
| DLco, % predicted | 74.3 ± 16.7 | 67.0 ± 20.9 | 71.0 ± 18.7 | 0.308 |
| ECOG | 0.001 | |||
| 0/1/2/3 | 10/22/6/2 | 4/6/4/1 | 14/28/10/3 | |
| Medical history, n (%) | ||||
| Hypertension | 23 (53.5) | 7 (38.9) | 30 (49.2) | 0.298 |
| Diabetes mellitus | 14 (32.6) | 2 (11.1) | 16 (26.2) | 0.085 |
| Cardiovascular disease | 2 (4.7) | 2 (11.1) | 4 (6.6) | 0.442 |
| Rheumatic disease | 2 (4.7) | 1 (5.6) | 3 (4.9) | 0.883 |
| Prior tuberculosis | 3 (7.0) | 6 (33.3) | 9 (14.8) | 0.009 |
| COPD | 6 (14.0) | 5 (27.8) | 11 (18.0) | 0.204 |
| Other malignancy | 9 (21.0) | 4 (22.2) | 13 (21.3) | 0.748 |
| Overall mortality, n (%) | 25 (58.1) | 15 (83.3) | 40 (65.6) | 0.140 |
| Median OS, months | 20.8 ± 18.3 | 11.1 ± 12.6 | 18.2 ± 17.4 | 0.065 |
Abbreviations: GAP staging system, gender (G), age (A), forced vital capacity (FVC), and diffusing capacity of carbon monoxide (DLco); BMI, body mass index; COPD, chronic obstructive lung disease.Data are presented as median, interquartile range, or frequency (%).
Table 2 shows the patients' specific characteristics of LC and IPF. There was no significant difference between the GAP groups regarding the diagnostic interval between IPF and LC diagnoses (1445.4 days vs. 1281.1 days). Adenocarcinoma was the most common histology in GAP stage I patients; however, squamous cell carcinoma was more common in stage II/III patients. The most frequent histologic types in early-stage LC patients were squamous cell carcinoma (39.1%) and adenocarcinoma (35.4%); however, adenocarcinoma (32.1%), was more common than squamous cell carcinoma (25.0%) in advanced-stage LC patients.
Relationship between lung cancer stage and GAP stage
Table 3 shows the univariate regression results for associations between advanced LC stage and clinical factors. A higher LC stage had a significant negative association with BMI (odds ratio [OR]=0.793, 95% confidence interval [CI]=0.649-0.971, p=0.025), but a significant positive association with higher GAP stage (OR =5.186, 95% CI=1.589-16.920, p=0.006). Age, smoking status, predicted FVC, predicted DLco, and IPF treatment did not significantly affect LC stage.
Characteristics of LC and IPF according to GAP stage
| Variable | GAP I | GAP II/III | Total | p-value |
|---|---|---|---|---|
| Time from IPF to LC* (day) | 1445.4 ±1420.1 | 1281.1±983.4 | 1396.9±1300.5 | 0.662 |
| The number of CT scans§ | 2.63 (2.0, 19.0) | 2.41 (2.0,7.0) | 2.56 (2.0,19.0) | 0.714 |
| Period from IPF to LC/CT count (day) | 626.1± 599.5 | 581.2±484.4 | 612.9±564.4 | 0.780 |
| Cancer treatment, n (%) | ||||
| Operation | 23 (54.5) | 3 (16.7) | 26 (42.6) | |
| Cytotoxic chemotherapy | 22 (51.2) | 3 (16.7) | 25 (41.0) | |
| Targeted/Immunotherapy | 6 (14.0) | 1 (5.6) | 7 (11.5) | |
| CCRT | 11 (25.6) | 0 (0.0) | 11 (18.0) | |
| Radiotherapy alone | 5 (11.6) | 3 (16.7) | 8 (13.1) | |
| Conservative care | 2 (4.7) | 5 (27.8) | 7 (11.5) | |
| Clinical lung cancer stage, n (%) | 0.005 | |||
| I to IIIA (early) | 33 (82.5) | 7 (17.5) | 40 (65.6) | |
| IIIB to IV (advanced) | 10 (47.6) | 11 (52.4) | 21 (34.4) | |
| IPF treatment, n (%) | 8 (18.6) | 7 (38.9) | 15 (24.6) | 0.162 |
| Histologic type, n (%) | 0.063 | |||
| Small cell carcinoma | 10 (23.3) | 4 (22.2) | 14 (23.0) | |
| Adenocarcinoma | 18 (41.9) | 4 (22.2) | 22 (36.1) | |
| Squamous cell carcinoma | 14 (32.6) | 9 (50.0) | 23 (37.7) | |
| Other | 1 (2.3) | 1 (5.6) | 2 (3.3) | |
Abbreviations: GAP staging system, gender (G), age (A), forced vital capacity (FVC), and diffusing capacity of carbon monoxide (DLco); CT, computed tomography; CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; RT, radiotherapy; CTx, chemotherapy; OS, overall survival.Data are presented as median, interquartile range, or frequency (%). *Time from IPF to LC, time gap between diagnosis of lung cancer and diagnosis of IPF (days). §The number of CT scan was described with mean (minimum, maximum value).
Results from the univariate logistic regression analysis of clinical factors associated with advanced lung cancer stage
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Age (years) | 1.037 | 0.965-1.115 | 0.317 |
| BMI, kg/m2 | 0.793 | 0.649-0.971 | 0.025 |
| Ever smoker | 0.814 | 0.125-5.306 | 0.830 |
| FVC % predicted | 0.984 | 0.961-1.009 | 0.211 |
| DLco % predicted | 0.999 | 0.977-1.021 | 0.929 |
| GAP stage (Stage I vs. II/III) | 5.186 | 1.589-16.920 | 0.006 |
| IPF treatment | 0.889 | 0.223-3.542 | 0.867 |
| Time Gap between diagnosis of lung cancer and diagnosis of IPF, days | 1.000 | 1.000-1.001 | 0.514 |
Abbreviations: GAP stage system, gender (G), age (A), forced vital capacity (FVC), and diffusing capacity of carbon monoxide (DLco); BMI, body mass index; OR, odds ratio; CI, confidence interval.
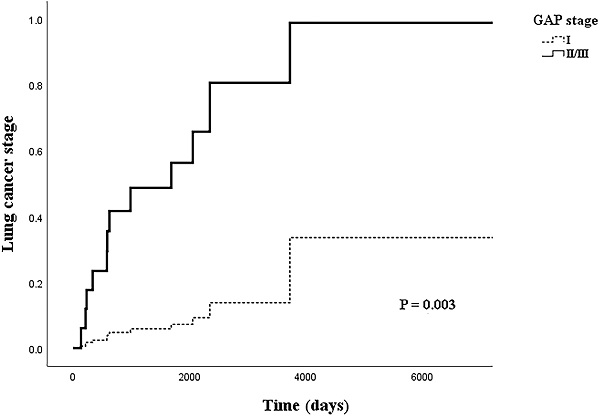
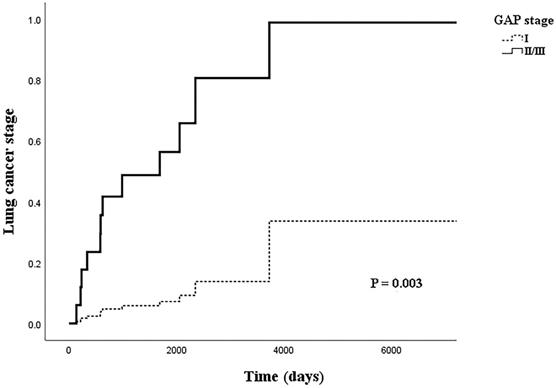
Table 4 shows the relationship between LC stage and clinical factors in the multivariate logistic regression model. In the logistic regression model, the adjusted OR (aOR) for BMI with LC stage had weakened slightly (aOR=0.710, 95% CI: 0.508-0.992, p=0.045). However, the OR for the higher GAP stage group more than doubled from that of the univariate model (aOR=12.158, 95% CI: 1.868-79.138, p=0.009). Figure 2 shows the cox regression analysis of LC stage by GAP stage; a similar result was achieved from the proportional hazard model when adjusted for cancer histology and IPF treatment (aOR=11.121, 95% CI: 2.311-53.532, p=0.003).
Survival outcomes
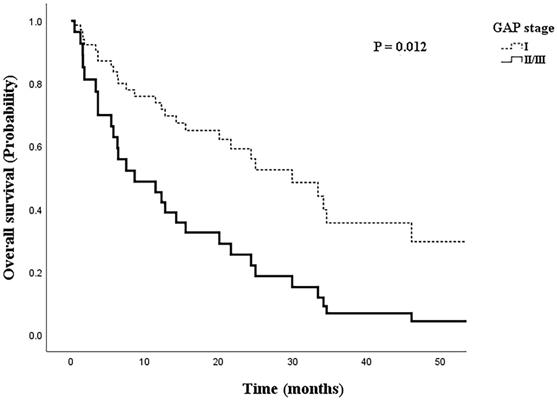
Overall mortality was 58.1% in the early GAP stage group and 83.3% in the advanced GAP stage group, and the mean overall survival time was 20.8±18.3 months in the early GAP stage group and 11.1±12.6 months in the advanced GAP stage group. Figure 3 shows the Cox regression analysis of survival probability by GAP stage, with adjustments for IPF treatment, smoking status, BMI, and histology. There was a significantly lower survival rate in the advanced GAP stage group (aOR=2.860, 95% CI: 1.257-6.508, p=0.012).
Among patients with early stage LC, overall survival was higher for those in the GAP stage I group after adjustment for cancer histology, BMI, smoking status, and IPF treatment, although the difference was not significant (Figure 4A, aOR=1.613, 95% CI: 0.502-5.184, p=0.422). However, the survival of patients with advanced stage LC was significantly higher for those in GAP stage I than in the advanced GAP stages (Figure 4B, aOR=10.412, 95% CI: 1.421-76.285, p=0.021).
Table 5 shows the causes of death in the GAP groups. Cancer progression was the main cause of death in the advanced GAP group (38.9%); however, pneumonia (16.3%) and AE-IPF (16.3%) were also dominant in the early GAP stage group.
Results from the multivariate logistic regression analysis of clinical factors associated with early vs. advanced lung cancer stage
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| BMI, kg/m2 | 0.710 | 0.508-0.992 | 0.045 |
| Ever smoker | 0.787 | 0.091-6.799 | 0.828 |
| GAP stage (Stage I vs. II/III) | 12.158 | 1.868-79.138 | 0.009 |
| IPF treatment | 0.558 | 0.078-4.010 | 0.562 |
Abbreviations: GAP stage system, gender (G), age (A), forced vital capacity (FVC), and diffusing capacity of carbon monoxide (DLco); BMI, body mass index; OR, odds ratio; CI, confidence interval.
Comparison of the causes of death according to GAP stage
| Cause of death, n (%) | GAP I (n=43) | GAP II/III (n=18) | Total (n=61) |
|---|---|---|---|
| Pneumonia | 7 (16.3) | 3 (16.7) | 10 (16.4) |
| RT/CTx pneumonitis | 2 (4.7) | 1 (5.6) | 3 (4.9) |
| Cancer progression | 7 (16.3) | 7 (38.9) | 14 (23.0) |
| AE-IPF | 7 (16.3) | 3 (16.7) | 10 (16.4) |
| *Other causes | 2 (4.7) | 1 (5.6) | 3 (4.9) |
| Total | 25 (58.1) | 15 (83.3) | 40 (65.6) |
*Other causes: Subdural hemorrhage and chronic renal failure in GAP I group; systemic viral infection in GAP II/III group.
Abbreviations: RT, radiotherapy; CTx, chemotherapy; AE-IPF, acute exacerbation of idiopathic pulmonary fibrosis.
Discussion
IPF and LC have common risk factors, and patients with both conditions are known to have a worse prognosis than patients with either condition. This study involved patients with LC that developed after the diagnosis of IPF. Our results showed that IPF severity is associated with the LC stage, but not the interval between IPF and LC diagnoses.
Cox regression model of lung cancer stage by GAP stage, adjusted for idiopathic pulmonary fibrosis treatment and histology.
Cox regression model of survival probability by GAP stage, adjusted for IPF treatment, smoking status, body mass index, and histology.
The demographic and clinical characteristics of the patients in this study are similar to those reported in previous studies. Most patients were older men with tobacco exposure [11, 19]. Although Ozawa et al. [11] did not find differences in survival rates between IPF patients with and without LC, recent studies have reported worse survival outcomes among IPF-LC patients [9, 19, 20]. Tomassetti et al. [9] reported that the development of LC in IPF patients significantly decreased their median overall survival (IPF with LC: 38.7 months, IPF without LC: 63.9 months; hazard ratio = 5.0; 95% CI: 2.91-8.57; p < 0.001). Tzouvelekis et al. [21] reported the median survival of IPF-LC patients to be 27.4 and 14.3 months from the time of IPF and LC diagnoses, respectively.
In this report, we have described the differences in clinical features and stages of LC between patients with early and advanced GAP stages. A significant result of our study was the ability to establish an association between IPF severity and LC stage, showing that GAP stage had an independent, positive association with LC stage. This suggests that patients with an advanced GAP stage require more frequent CT scans than the general population in addition to close observation at their routine follow-ups. Even if the LC stage differed according to the GAP stage, there was no significant difference in the time interval between IPF and LC diagnoses (904 days in GAP stage I vs. 959 days in GAP stage II/III).
These two diseases share biological signaling pathways and microenvironments that have been shown to disrupt tissue architecture and lead to dysfunction, contributing to carcinogenesis and fibrosis (e.g., transforming growth factor beta, platelet-derived growth factor, vascular endothelial growth factor, and fibroblast growth factor) [22]. Considering the theory that LC developing next to or within fibrosis can cause cellular metaplasia and the longstanding hypothesis that the pathogenesis of tissue damage and abnormal repair are common processes in both IPF and LC, it is reasonable that patients with IPF are vulnerable to the development of cancer [19, 23]. Generally, the fibrotic area is broader in patients with an advanced GAP stage than in those with an early GAP stage; therefore, the malignancy would be aroused concomitantly in the widespread areas and result in higher stages of LC.
Cox regression model of survival probability adjusted for IPF treatment, smoking status, body mass index, and histology. A. Patients with early stage lung cancer by gender-age-physiology (GAP) stage. B. Patients with advanced stage lung cancer by GAP stage.
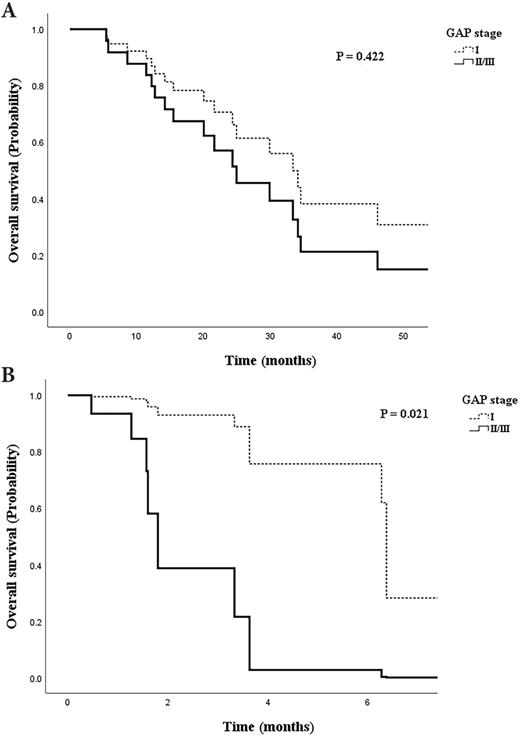
In patients with early stage LC, the overall survival was higher (though not significantly) in patients with GAP stage I than in those with GAP stage II/III (Figure 4A). However, survival in patients with advanced LC stage differed significantly according to the GAP stage (Figure 4B). This suggests that when LC is at an advanced stage, the disease course could be affected by the GAP stage, which itself is determined by the severity of fibrosis, residual pulmonary functions, and age. A previous study of retrospective data suggested a beneficial effect of preoperative pirfenidone on the incidence of postoperative acute exacerbations in patients with adenocarcinoma and IPF [24]. A recent study also suggested that antifibrotic agents should not be discontinued during the diagnostic or therapeutic work-up of LC patients, as benefits seem to outweigh the risk of unfavorable outcomes [1]. Our data also suggest that IPF severity affects survival and that clinicians should consider maintaining IPF and anti-cancer treatments, even when the cancer is at an advanced stage. A consensus statement regarding the treatment of these patients needs to be developed.
Conventional chemotherapy and radiotherapy can have deleterious effects on patients with IPF [6, 25, 26], and optimal treatment regimens have not been established. Considering the high incidence and difficulties encountered in treating patients with IPF-LC, those with more severe pulmonary fibrosis are more difficult to treat because of the higher risk of acute exacerbation. The high incidence of LC and its impact on patient survival underscores the importance of clinicians recognizing the predictive factors of LC development and the need for thorough surveillance protocols.
Although our results showed that fibrosis severity may affect LC stage, there was no clinical effect of IPF treatment for delaying LC development (OR 0.889, p=0.867, 95% CI=0.223-3.542). This may have been a reason behind the small number of patients undergoing IPF treatment (n= 8 vs. 7 in both groups, respectively). A cohort study with a larger sample size is needed.
The main strength of our study is that we highlighted the relationship between IPF severity and LC severity, which has rarely been studied.
We recognize that this study did have several limitations: in particular, the small sample size and use of retrospective data. However, we collected the data from two tertiary care hospitals to improve their generalizability. Second, due to the study's retrospective nature, we could not provide a guide for the optimal CT scan interval for LC surveillance in the different GAP groups.
Conclusion
To our knowledge, this study is the first to show a clear relationship between IPF severity and LC severity. In particular, we found that LC stage is affected by the progression of fibrosis, with higher GAP stages resulting in more advanced cancer stages. These results can provide guidance for treating IPF patients; more frequent checkups and CT scans are warranted to enable early detection of cancer development. Considering the high incidence of LC and its impact on the survival of IPF patients, frequent surveillance and close observation are needed from the time of initial IPF diagnosis. Large and prospective multicenter studies will be key to the development of protocols for the optimal management of patients with IPF-LC.
Abbreviations
FVC: forced vital capacity; GAP: gender-age-physiology; DLco: diffusing capacity of the lung for carbon monoxide; BMI: body mass index; ECOG: Eastern Cooperative Oncology Group; COPD: chronic obstructive lung disease; OS: overall survival; IPF: idiopathic pulmonary fibrosis; LC: lung cancer; CT: computed tomography; CEA: carcinoembryonic antigen; IQR: interquartile range; CI: confidence interval.
Acknowledgements
We are grateful to Dr. M.J. Song (Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul, Korea) and Dr. S.W. Moon (Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea) for their assessment of the patients' clinical data.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1C1B5043991). The funding grant was used as a partial income of a researcher who only participated in collecting retrospective data but was not included in the authors list.
Author contributions
Administrative support: S.H.L and H.I.Y; Provision of study materials or patients: M.S.P. and J.H.L; Collection and assembly of data: H.J.J, Y.S.K., J.C., J.H.L. and C.T.L; Data analysis and interpretation: H.J.J, S.H.L and H.I.Y; Manuscript writing: All authors; Final approval of manuscript: All authors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Tzouvelekis A, Spagnolo P, Bonella F, Vancheri C, Tzilas V, Crestani B. et al. Patients with IPF and lung cancer: diagnosis and management. The Lancet Respiratory Medicine. 2018;6:86-8
2. Vancheri C, du Bois RM. A progression-free end-point for idiopathic pulmonary fibrosis trials: lessons from cancer. European Respiratory Journal. 2013;41:262-9
3. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine. 2011;183:788-824
4. Ballester B, Milara J, Cortijo J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int J Mol Sci. 2019;20:593
5. Artinian V, Kvale PA. Cancer and interstitial lung disease. Current Opinion in Pulmonary Medicine. 2004 10
6. Karampitsakos T, Tzilas V, Tringidou R, Steiropoulos P, Aidinis V, Papiris SA. et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulmonary Pharmacology & Therapeutics. 2017;45:1-10
7. Bouros D, Hatzakis K, Labrakis H, Zeibecoglou K. Association of malignancy with diseases causing interstitial pulmonary changes. Chest. 2002;121:1278-89
8. Hubbard R, Venn A, Lewis S, Britton J. Lung Cancer and Cryptogenic Fibrosing Alveolitis. American Journal of Respiratory and Critical Care Medicine. 2000;161:5-8
9. Tomassetti S, Gurioli C, Ryu JH, Decker PA, Ravaglia C, Tantalocco P. et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015;147:157-64
10. Park J, Kim DS, Shim TS, Lim C-M, Koh Y, Lee SD. et al. Lung cancer in patients with idiopathic pulmonary fibrosis. European Respiratory Journal. 2001;17:1216-9
11. Ozawa Y, Suda T, Naito T, Enomoto N, Hashimoto D, Fujisawa T. et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology. 2009;14:723-8
12. Meyer EC, Liebow AA. RELATIONSHIP OF INTERSTITIAL PNEUMONIA HONEYCOMBING AND ATYPICAL EPITHELIAL PROLIFERATION TO CANCER OF THE LUNG. Cancer. 1965;18:322-51
13. Takahashi H, Nukiwa T, Matsuoka R, Danbara T, Natori H, Arai T. et al. Carcinoembryonic antigen in bronchoalveolar lavage fluid in patients with idiopathic pulmonary fibrosis. Jpn J Med. 1985;24:236-43
14. Guyard A, Danel C, Théou-Anton N, Debray M-P, Gibault L, Mordant P. et al. Morphologic and molecular study of lung cancers associated with idiopathic pulmonary fibrosis and other pulmonary fibroses. Respiratory research. 2017;18:120 -
15. Antoniou KM, Tomassetti S, Tsitoura E, Vancheri C. Idiopathic pulmonary fibrosis and lung cancer: a clinical and pathogenesis update. Current opinion in pulmonary medicine. 2015;21:626-33
16. Lee T, Park JY, Lee HY, Cho Y-J, Yoon HI, Lee JH. et al. Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respiratory medicine. 2014;108:1549-55
17. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ. et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. American journal of respiratory and critical care medicine. 2018;198:e44-e68
18. Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS. et al. A Multidimensional Index and Staging System for Idiopathic Pulmonary Fibrosis. Annals of Internal Medicine. 2012;156:684-91
19. Yoo H, Jeong B-H, Chung MJ, Lee KS, Kwon OJ, Chung MP. Risk factors and clinical characteristics of lung cancer in idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med. 2019;19:149 -
20. Yoon JH, Nouraie M, Chen X, Zou RH, Sellares J, Veraldi KL. et al. Characteristics of lung cancer among patients with idiopathic pulmonary fibrosis and interstitial lung disease - analysis of institutional and population data. Respir Res. 2018;19:195
21. Tzouvelekis A, Karampitsakos T, Gomatou G, Bouros E, Tzilas V, Manali E. et al. Lung cancer in patients with Idiopathic Pulmonary Fibrosis. A retrospective multicenter study in Greece. Pulmonary Pharmacology & Therapeutics. 2020;60:101880
22. Kinoshita T, Goto T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int J Mol Sci. 2019;20:1461
23. Vancheri C. Common pathways in idiopathic pulmonary fibrosis and cancer. European Respiratory Review. 2013;22:265-72
24. Iwata T, Yoshida S, Fujiwara T, Wada H, Nakajima T, Suzuki H. et al. Effect of Perioperative Pirfenidone Treatment in Lung Cancer Patients With Idiopathic Pulmonary Fibrosis. The Annals of Thoracic Surgery. 2016;102:1905-10
25. Kreuter M, Ehlers-Tenenbaum S, Schaaf M, Oltmanns U, Palmowski K, Hoffmann H. et al. Treatment and outcome of lung cancer in idiopathic interstitial pneumonias. Sarcoidosis, vasculitis, and diffuse lung diseases: official journal of WASOG/World Association of Sarcoidosis and Other Granulomatous Disorders. 2015;31:266-74
26. Isobe K, Hata Y, Sakamoto S, Takai Y, Shibuya K, Homma S. Clinical characteristics of acute respiratory deterioration in pulmonary fibrosis associated with lung cancer following anti-cancer therapy. Respirology. 2010;15:88-92
Author contact
![]() Corresponding authors: Sang Hoon Lee, MD, PhD, Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea. Tel: +82.2-2228-1955, E-mail: cloud9ac; Ho Il Yoon, MD, PhD, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, 82 Gumi-ro, 173 Beon-gil, Bundang-gu, Seongnam-si, Gyeonggi-do, 463-707, Republic of Korea. Tel: +82.31-31-787-7036, Fax: +82.31-787-4052, E-mail: dextro70com.
Corresponding authors: Sang Hoon Lee, MD, PhD, Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea. Tel: +82.2-2228-1955, E-mail: cloud9ac; Ho Il Yoon, MD, PhD, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, 82 Gumi-ro, 173 Beon-gil, Bundang-gu, Seongnam-si, Gyeonggi-do, 463-707, Republic of Korea. Tel: +82.31-31-787-7036, Fax: +82.31-787-4052, E-mail: dextro70com.

 Global reach, higher impact
Global reach, higher impact