3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(10):2903-2911. doi:10.7150/jca.51027 This issue Cite
Research Paper
Clinical characteristics and outcomes in HIV-associated diffuse large B-cell lymphoma in China: A retrospective single-center study
1. Department of Hematology, the First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, Nanjing, 210029, China.
2. Key Laboratory of Hematology of Nanjing Medical University, Nanjing, 210029, China.
3. Department of Infectious Diseases, Yunnan Provincial Infectious Diseases Hospital/Yunnan AIDS Care Center, Kunming, 650000, China.
*These authors contributed equally to this work.
Received 2020-7-23; Accepted 2021-3-3; Published 2021-3-15
Abstract
Human immunodeficiency virus (HIV) infection is associated with an increased risk of aggressive lymphoma, especially diffuse large B cell lymphoma (DLBCL). There are few data regarding HIV-associated DLBCL in China. Therefore, we analyzed the characteristics and outcomes of patients with HIV-associated DLBCL from our center. We retrospectively studied HIV-infected patients with DLBCL from 2011 to 2019. Data on HIV infection and lymphoma characteristics, treatments and outcomes were retrieved and analyzed. In 78 patients with HIV-associated DLBCL, most had poor performance status (PS) (74%), elevated lactate dehydrogenase (LDH) levels (95%), B symptoms (74%), advanced Ann Arbor stages (81%), bulky diseases (64%) and extranodal involvement (70%) at diagnosis. The median CD4+ T cell count was 162/µl, and 26 patients were already on combination antiretroviral therapy (cART) treatment at diagnosis of DLBCL. Elevated whole blood EBV DNA copy number was detected in 38 patients (66%, 38/58). Of the 45 patients evaluated at the end of treatment, 26 (58%) achieved CR, 6 (13%) achieved PR and 6 (13%) experienced progressive disease. The 2-year progression-free survival (PFS) and overall survival (OS) rates were 56.4% and 62.7%, respectively. Factors associated with decreased PFS and OS in univariate analysis were unfavorable PS and high international prognostic index. Elevated EBV DNA copy number was inclined to be associated with worse outcome. We did not observe a significant difference in survival between R-EPOCH and R-CHOP regimens. In our population, patients with HIV-associated DLBCL presented with aggressive characteristics and exhibited poor survival outcomes, even in the modern cART era.
Keywords: diffuse large B-cell lymphoma, HIV, outcomes
Introduction
Human immunodeficiency virus (HIV) infection is associated with an increased incidence of malignancy. HIV-associated diffuse large B cell lymphoma (DLBCL) and Burkitt's lymphoma (BL) are among the most common cancers in HIV-positive patients [1-3]. Other non-Hodgkin's lymphomas (NHLs), including primary effusion lymphoma (PEL), plasmablastic lymphoma, KSHV-associated multicentric Castleman's disease, primary central nervous system (CNS) lymphoma and classic Hodgkin's lymphoma (cHL), tend to be diagnosed in HIV patients as well [1]. HIV strongly contributes to NHL mortality, particularly in acquired immunodeficiency syndrome (AIDS)-defining subtypes [4]. With the introduction of combination antiretroviral therapy (cART), the incidence of HIV-associated lymphoma has decreased, and survival outcome have improved [4, 5].
DLBCL is the most common subtype of lymphoid malignancy in adults. It represents approximately 30% of NHL cases without HIV infection and 45% of cases with HIV-related lymphoma [6-8]. In a Spanish study, compared to HIV-uninfected DLBCL patients, HIV-infected patients presented more aggressive features with a poorer performance status, more frequent B symptoms and more advanced Ann Arbor stages [5]. In the above study, when treated with the standard-of-care regimen rituximab plus cyclophosphamide, hydroxydaunorubicin, vincristine and prednisolone (R-CHOP), HIV-infected patients exhibited similar disease-free survival but significantly worse overall survival (OS) compared to those without HIV infection [5]. In contrast, a French study revealed that HIV-infected patients with DLBCL showed survival outcomes similar to those of HIV-negative DLBCL patients [9].
Infections with Epstein-Barr virus (EBV), hepatitis B virus (HBV), and/or hepatitis C virus (HCV) have been previously reported in DLBCL, and the former two viruses independently predict poor prognosis [10-13]. In HIV-infected patients with DLBCL, it has been shown that elevated EBV load predicts inferior survival outcome; however, few data on HIV and HBV/HCV coinfection in patients with DLBCL have been published [13].
The HIV epidemic appears to have an obvious regional distribution in China, occurring frequently in Sichuan Province and Yunnan Province [14, 15]. To date, only a few studies have described the clinical features of Chinese AIDS patients with DLBCL [16, 17]. Herein, we retrospectively analyzed the characteristics and outcomes of Chinese HIV-infected DLBCL patients in the Yunnan Provincial Infectious Diseases Hospital/Yunnan AIDS Care Center.
Methods
Patients
A total of 104 cases of HIV-infected individuals with DLBCL were newly diagnosed between 2011 and 2019 at the Yunnan Provincial Infectious Diseases Hospital/Yunnan AIDS Care Center, which is the largest HIV/AIDS referral hospital in Southwest China. This study was approved by the institutional review board of Yunnan Provincial Infectious Diseases Hospital and was conducted according to the Declaration of Helsinki. DLBCL diagnosis was established in accordance with the 2008 WHO classification and was classified using the Hans classification algorithm. Clinical data were collected from the medical records. HIV-infected patients diagnosed with DLBCL who did not receive chemotherapy were excluded (n=25). After one patient was excluded due to treatment with GemOx (gemcitabine and oxaliplatin) regimen, 78 patients treated with CHOP like regimen with or without rituximab (CHOP ± R) or EPOCH (etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin) regimen with or without rituximab (EPOCH ± R) were finally included in the analysis. Data on patient demographic characteristics (gender and age), HIV-related characteristics (HIV transmission route, years of HIV infection at DLBCL diagnosis, CD4+ cell count at DLBCL diagnosis and date of cART initiation), lymphoma-related characteristics (cell-of-origin subtype, Eastern Cooperative Oncology Group (ECOG) performance status score, serum lactate dehydrogenase (LDH), B symptoms, extranodal sites, Ann Arbor stage, international prognostic index (IPI) score, bulky tumor, Ki 67, BCL-2 expression by immunohistochemistry, bone marrow involvement and CNS-IPI score) and other related characteristics (EBV load, HBV load, HCV load, comorbidities, time from first complain to diagnosis) were available.
In this study, the cART regimen included two nucleoside reverse transcriptase inhibitors and one nonnucleoside reverse transcriptase inhibitor. After the diagnosis of DLBCL was initiated, patients were switched to cART treatment with tenofovir, lamivudine, and efavirenz or raltegravir potassium. Patients with a CD4+ cell count below 200/μl received trimethoprim/sulphamethoxazole as a prophylactic agent against Pneumocystis jirovecii. HIV-1 RNA load was monitored during chemotherapy. For patients with positive hepatitis B surface Ag (HBsAg), prophylaxis was not used because both tenofovir and lamivudine have antiviral activity against HBV. HBV DNA (cutoff ≥ 5×102 copies/ml) and HCV RNA loads (cutoff ≥ 5×102 IU/ml) were assessed during chemotherapy as well. Laboratory monitoring of plasma EBV DNA (cutoff ≥ 5×103 copies/ml) load was performed in some patients.
Response assessment
Interim evaluation was performed after completion of three or four cycles of chemotherapy. One month after completion of all treatments, the efficacy was evaluated. Computed tomography (CT) or 18F-fluorodexyglucose positron emission tomography (PET) was performed for radiological evaluation. Brain magnetic resonance imaging (MRI) was used to assess CNS involvement. The 2007 revised Cheson criteria were performed to define complete response (CR), partial response (PR), progressive disease (PD) and relapse.
Statistical analysis
All statistical data were analyzed using SPSS software, version 21 or GraphPad Prism 8. Continuous variables are presented as the median with the first and third quartiles, and categorical variables are presented as numbers and percentages. Progression-free survival (PFS) was defined as the time from DLBCL diagnosis to progression, relapse or death from any cause. Overall survival (OS) was defined as the time from DLBCL diagnosis to last follow-up or death from any cause. Survival curves were plotted using the Kaplan-Meier method, and the log-rank test was used for comparison. Cox proportional regression models were performed for univariate and multivariate analyses of outcomes. A P-value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 78 patients were included in this analysis. Baseline clinical features of HIV-infected patients are summarized in Table 1. Of these patients, the median age was 43, and 67 (86%) were male. The main HIV transmission group was heterosexuals. Among 78 patients, 29 had a known HIV infection history of more than 1 year at the time of DLBCL diagnosis, and in 37 patients, DLBCL and HIV infection were diagnosed concomitantly (difference less than three months). The median CD4+ T cell count at DLBCL diagnosis was 162/μl (range 6-559) in these 78 patients, of whom 10 had a CD4+ T cell count less than 50/μl. At DLBCL diagnosis, 26 patients were already on cART treatment. The median time from patients' first complaint to diagnosis was 2 months. Most patients (74%) had a poor performance status (PS) (ECOG PS 2-4), and elevated LDH at diagnosis was present in 95% of patients. Seventy-four percent of patients had B symptoms, 81% had an advanced Ann Arbor stage (III-IV), and 64% had bulky tumors at diagnosis. Fifty-five patients (70%) exhibited extranodal involvement, of whom twenty-six possessed more than 2 extranodal sites. The most frequent extranodal sites were the gut (n=30), stomach (n=12), liver (n=10), bone (n=7), pancreas (n=7) and/or kidney (n=6). One-third of patients exhibited a higher central nervous system IPI (CNS-IPI) at diagnosis. A Ki-67 proliferation index higher than 90% was observed in 22 cases. Overall, 30 samples (39%) showed positive BCL2 expression in tumor cells (>50%), 29 (37%) were negative and 19 (24%) were unknown. Among 58 cases with information on EBV status, EBV load was elevated (5×103 copies/ml) in 38 (66%). Of all patients, eight (10%) presented with positive HBsAg and eight (10%) with positive anti-HCV antibody.
Clinical characteristics of HIV-infected patients with DLBCL
| N=78 (%) | Median (1st -3rd quartile) | |
|---|---|---|
| Demographics | ||
| Gender | ||
| Male | 67 (86%) | |
| Female | 11 (14%) | |
| Age (years) | 43 (38.0-52.0) | |
| HIV-related characteristics | ||
| HIV transmission route | ||
| Intravenous drug use | 6 (8%) | |
| Heterosexual | 64 (82%) | |
| Homosexual | 7 (9%) | |
| Mother to child | 1 (1%) | |
| Years of HIV infection at DLBCL diagnosis | ||
| <1 year | 49(63%) | 0.08 (0.03-0.24) |
| ≥1 year | 29(37%) | 5.23 (2.40-9.05) |
| CD4 cell count at DLBCL diagnosis (/μl) | ||
| <50 | 10 (13%) | 25.0 (16.5-42.8) |
| 50~199 | 37 (47%) | 122.0 (92.0-157.0) |
| 200~499 | 27 (35%) | 278.0 (236.0-327.0) |
| ≥500 | 4 (5%) | 541.5 (524.0-553.0) |
| cART initiation | ||
| cART prior to chemotherapy | 26 (35%) | 1.5 (0.4-4.4) |
| cART with chemotherapy (years) | 52 (65%) | |
| Lymphoma-related characteristics | ||
| Cell-of-origin subtype | ||
| GC | 49 (63%) | |
| Non-GC | 29 (37%) | |
| ECOG performance status score | ||
| 0-1 | 20 (26%) | |
| 2-4 | 58 (74%) | |
| LDH above normal | 74 (95%) | 360.5 (267.3-731.5) |
| B symptoms | 58 (74%) | |
| Extra-nodal sites | ||
| 0 | 23 (30%) | |
| 1 | 29 (37%) | |
| ≥2 | 26 (33%) | |
| Ann Arbor stage | ||
| I-II | 15 (19%) | |
| III-IV | 63 (81%) | |
| aaIPI score | ||
| 0-1 | 11 (14%) | |
| 2-3 | 59 (76%) | |
| IPI score | ||
| 0-1 | 0 | |
| 2 | 1 (1%) | |
| 3-5 | 7 (9%) | |
| Bulky tumor (≥7.5 cm) | 50 (64%) | |
| Ki 67 >90% | 22 (28%) | |
| BCL-2 expression positive by IHCa | 30 (51%) | |
| Bone marrow involvement | 15 (19%) | |
| CNS-IPI score | ||
| 0-3 | 51 (65%) | |
| 4-6 | 27 (35%) | |
| Other related characteristics | ||
| EBV (whole blood) b | ||
| <5×103 copies/ml | 20 (34%) | |
| ≥5×103 copies/ml | 38 (66%) | 4.04×104 (1.06×104-8.96×104) |
| HBV | ||
| HBsAg positive | 8 (10%) | |
| HBV-DNA load (copies/ml) | ||
| <5×102 | 6 (75%) | |
| ≥5×102 | 2 (25%) | |
| HCV | ||
| Anti-HCV IgG positive | 8 (10%) | |
| HCV-RNA load (IU/ml) | ||
| <5×102 | 2 (25%) | |
| ≥5×102 | 6 (75%) | |
HIV: human immunodeficiency virus; DLBCL: diffuse large B-cell lymphoma; cART: combination antiretroviral therapy; GC: germinal center; ECOG: Eastern Cooperative Oncology Group; LDH: lactate dehydrogenase; B symptoms: fever, night sweats, weight loss, fatigue, and swelling in lymph nodes; IHC: immunohistochemistry; CNS: central nervous system; a: numbers of missing values, a: 19. b: numbers of missing values, b: 20.
DLBCL treatment
Fifty-three (53/78) patients received the CHOP±R regimen, among whom twenty-five received six to eight cycles. Twenty-four of these 53 patients received R-CHOP, twenty-one received the CHOP regimen, and five received CHOP followed by R-CHOP or vice versa, depending on their financial situation at that time. Two patients received first-line R-CHOP, progressed at interval evaluation; one was changed to the GemOx regimen, and the other was switched to the R-GDP (gemcitabine, dexamethasone and carboplatin) regimen. One patient who received 4 cycles of R-CMOP (cyclophosphamide, mitoxantrone, vincristine and prednisone) was switched to R-GOD (gemcitabine, oxaliplatin and dexamethasone) plus oral lenalidomide due to PD. Twenty-five patients (age ≤60) with high risk (aaIPI scores 2-3) received regimens, including the DA-EPOCH±R (etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin) regimen. Among these, 9 patients received the R-DA-EPOCH regimen, and 13 patients received one or two cycles of R-CHOP followed by R-DA-EPOCH. Fifty-six of 78 patients received intrathecal methotrexate and cytarabine for central nervous system prophylaxis. Radiotherapy with 40-50 Gy was given in eleven patients with bulky tumors, among whom 4 patients received radiotherapy as consolidation after chemotherapy with CR, and 7 were treated after four cycles of chemoradiotherapy with additional cycles afterwards. Five patients were given autologous hematopoietic stem cell transplant (autoSCT) following chemotherapy.
Treatment response and outcomes
Among sixty-seven HIV-infected DLBCL patients who were evaluable in the interim analysis, the overall response rate (ORR) was 88%, including 14 (21%) CR and 45 (67%) PR. Three patients experienced PD, and five patients died before the interval evaluation. One died from hemorrhage, two from serious infection and two from rapid disease progression. Of the 45 patients evaluated at the end of treatment, 26 (58%) achieved CR, 6 (13%) achieved PR and 6 (13%) experienced progressive disease (Table 2). The other 7 patients died during chemotherapy before response could be evaluated from sepsis (n=4), rapid disease progression (n=2) or heart failure (n=1). No one included in our cohort presented with baseline CNS involvement. With over 70% of patients receiving CNS prophylaxis, only one patient experienced CNS relapse 4 months after reaching CR and died soon thereafter.
Evaluation following chemotherapy in HIV-infected patients with DLBCL
| Interval evaluationa (N=78) | At the end of treatment (N=62) | |
|---|---|---|
| Able to evaluate | N=67 | N=45 |
| Complete response | 14 (21%) | 26 (58%) |
| Partial response | 45 (67%) | 6 (13%) |
| Stable disease | 0 | 0 |
| Progressive disease | 3 (4%) | 6 (13%) |
| Deathb | 5 (8%) | 7 (16%) |
| Unable to evaluate | N= 11 | N=17 |
| Ongoing treatment | 0 | 6 |
| Having stopped treatmentc | 11 | 11 |
HIV: human immunodeficiency virus; DLBCL: diffuse large B-cell lymphoma;
a: Response evaluation after three or four chemotherapy cycles; b: Death during the treatment; c: Stop treatment before be able to evaluate response to chemotherapy could be attributed to personal willingness. Four cases end up in losing to follow-up and one patient committed suicide before or at the time of interval evaluation. Eight cases end up in losing to follow-up at the end of treatment.
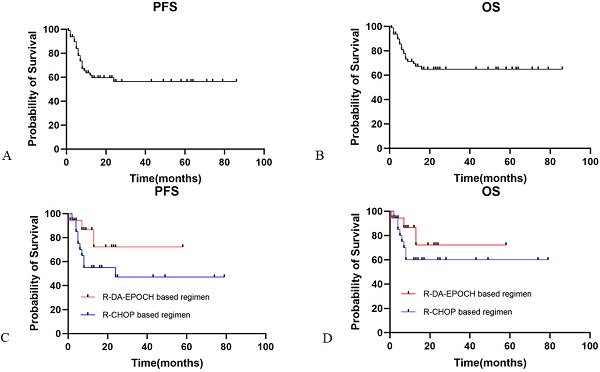
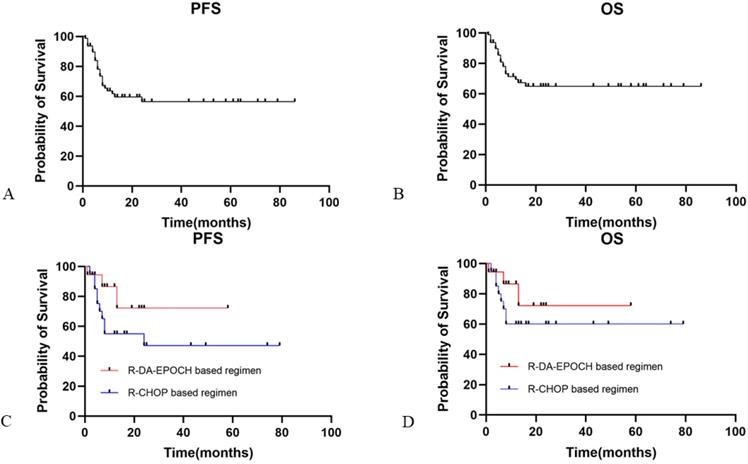
The median PFS and OS for HIV-infected DLBCL patients in our study was not reached. The overall 2-year PFS and OS rates were 56.4% and 62.7%, respectively (Fig. 1A and 1B). ECOG performance status score ≥2 (HR: 6.01, 95% CI [2.71-13.33]) and IPI ≥3 (HR: 3.43, 95% CI [1.59-7.41]) were predictive of worse PFS, while ECOG performance status score ≥2 (HR: 5.5, 95% CI [0.60-50.19]) and IPI ≥3 (HR: 2.72, 95% CI [0.55-13.46]) were associated with worse OS. In contrast, in a multivariate Cox regression model, no HIV-related characteristics were associated with PFS or OS. Neither HBV nor HCV coinfection was associated with prognosis. Elevated plasma EBV DNA copy number was inclined to be associated with worse outcome (PFS and OS: p=0.08 and p=0.06, respectively).
When we evaluated the relationship stratified by aaIPI between treatment and clinical outcomes, for high aaIPI risk patients, the R-EPOCH-based regimen (n=18) was not significantly favored over the R-CHOP-based regimen (n=20) for either PFS (Fig. 1C; p=0.15) or OS (Fig. 1D; p=0.28).
Discussion
Yunnan is located in Southwest China, bordering Myanmar, Laos and Vietnam, and has been regarded as the major site of the HIV infection epidemic for a long time [14, 15]. Over the decades, people living with HIV/AIDS have been increasing in Yunnan, and it remains one of the provinces with the highest number of HIV-infected patients in China [18].
DLBCL is the most common pathological subtype of NHL in both the general population and in people living with HIV/AIDS [1-3, 19]. A large database study shows that HIV infection continues to be an independent risk factor for death among patients with lymphoma [20]. We analyzed the clinical features and survival outcomes of HIV-infected DLBCL in the setting of cART in our series. Consistent with other recent studies, HIV-infected patients with DLBCL presented with poor performance status, a high frequency of B symptoms, elevated LDH, advanced Ann Arbor stages, and high aaIPI scores [5, 6, 9, 17, 20]. Moreover, bulky disease was frequent. EBV infection is found in approximately 10% of HIV-negative DLBCL [21]; however, in our study, 66% of patients with HIV-positive DLBCL exhibited elevated plasma EBV DNA load, suggesting a higher rate of EBV coinfection. In contrast, the frequency of HBV infection in HIV-infected patients with DLBCL was similar to that of their HIV-uninfected counterparts reported in the Chinese population [11]. Likewise, the frequency of HCV infection was close to that of HIV-negative DLBCL in the Caucasian population but lower than that in the Asian population [22, 23]. Unlike a previous study in which HIV had been diagnosed a median of 15 years previously and in which nearly 80% of patients had received cART at DLBCL diagnosis [9], patients in our study experienced late HIV detection and late cART exposure. This may partially be attributed to decreased awareness of HIV testing and reduced initiative for medical care assistance in high-risk HIV populations.
Progression-free and overall survival in HIV-infected patients with DLBCL. Panel A showed the PFS for HIV-infected DLBCL patients. Panel B showed the OS for HIV-infected DLBCL patients. Panel C showed PFS for HIV-infected DLBCL patients with R-DA-EPOCH based regimen and R-CHOP based regimen. Panel D showed OS for HIV-infected DLBCL patients with R-DA-EPOCH based regimen and R-CHOP based regimen. PFS: progression free survival; OS: overall survival; DLBCL: diffuse large B cell lymphoma.
Cox univariable and multivariable analyses of PFS and OS in HIV-infected patients with DLBCL
| N | PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | P | Multivariate analysis | P | Univariate analysis | P | Multivariate analysis | P | ||
| HR, 95%CI | HR, 95%CI | HR, 95%CI | HR, 95%CI | ||||||
| Gender | |||||||||
| Male | 67 | 0.75, [0.26-2.19] | 0.56 | 0.61, [0.19-1.94] | 0.31 | ||||
| Female | 11 | ||||||||
| Age (years) | |||||||||
| ≤60 | 70 | 1.12, [0.36-3.52] | 0.85 | 0.87, [0.24-3.12] | 0.82 | ||||
| >60 | 8 | ||||||||
| Years of HIV infection at DLBCL diagnosis | |||||||||
| <1 year | 49 | 1.06, [0.50-2.25] | 0.89 | 1.06, [0.46-2.43] | 0.90 | ||||
| ≥1 year | 29 | ||||||||
| CD4 cell count at DLBCL diagnosis | |||||||||
| <200 (/μl) | 47 | 1.55, [0.74-3.26] | 0.25 | 1.60, [0.70-3.62] | 0.27 | ||||
| ≥200 (/μl) | 31 | ||||||||
| cART initiation | |||||||||
| cART prior to chemo | 26 | 0.59, [027-1.27] | 0.21 | 0.50, [0.21-1.18] | 0.16 | ||||
| cART with chemo | 52 | ||||||||
| Cell-of-origin subtype | |||||||||
| GC | 49 | ||||||||
| Non-GC | 29 | 1.71, [0.78-3.75] | 0.14 | 1.78, [0.75-4.20] | 0.16 | ||||
| ECOG performance status score | |||||||||
| 0-1 | 20 | ||||||||
| 2-4 | 58 | 6.01, [2.71-13.33] | 0.004 | 4.47, [0.85-23.59] | 0.08 | 10.54, [4.45-24.96] | 0.003 | 5.50, [0.60-50.19] | 0.13 |
| LDH >UNL | 74 | 1.16, [0.18-7.48] | 0.88 | 0.28 | |||||
| B symptoms | 58 | 1.22 [0.54-2.76] | 0.64 | 2.10, [0.87-5.05] | 0.16 | ||||
| Extra-nodal sites | |||||||||
| 0-1 | 52 | ||||||||
| ≥2 | 26 | 0.88, [0.40-1.95] | 0.75 | 0.90 [0.38-2.14] | 0.82 | ||||
| Ann Arbor stage | |||||||||
| I-II | 15 | ||||||||
| III-IV | 63 | 2.50, [1.04-6.02] | 0.11 | 3.15, [1.20-8.23] | 0.10 | ||||
| IPI score (all patients) | |||||||||
| 0-2 | 25 | ||||||||
| 3-5 | 53 | 3.43, [1.59-7.41] | 0.010 | 1.53, [0.45-5.21] | 0.50 | 6.30, [2.74-14.47] | 0.004 | 2.72, [0.55-13.46] | 0.22 |
| Bulky tumor (≥7.5 cm) | 50 | 1.17, [0.53-2.57] | 0.69 | 1.51, [0.64-3.56] | 0.31 | ||||
| Bone marrow involvement | 15 | 1.47, [0.56-3.80] | 0.37 | 1.08, [0.39-2.96] | 0.87 | ||||
| Ki 67 >90% | 22 | 0.60, [0.27-1.33] | 0.25 | 0.60, [0.25-1.42] | 0.29 | ||||
| BCL-2 expression by IHC | 30 | ||||||||
| positive | 30 | 1.59, [0.66-3.82] | 0.31 | 1.23, [0.49-3.27] | 0.63 | ||||
| negative | 29 | ||||||||
| CNS-IPI score | |||||||||
| 0-3 | 51 | 0.88, [0.40-1.93] | 0.74 | 0.79, [0.34-1.87] | 0.58 | ||||
| 4-6 | 27 | ||||||||
| EBV (whole blood)a | |||||||||
| <5×103 copies/ml | 20 | ||||||||
| ≥5×103 copies/ml | 38 | 2.09, [0.84-5.18] | 0.08 | 2.75, [0.98-7.71] | 0.06 | ||||
| HBV | |||||||||
| HBsAg positive | 8 | 1.06, [0.31-3.59] | 0.93 | 1.20, [0.33-4.41] | 0.76 | ||||
| HBsAg negative | 70 | ||||||||
| HCV | |||||||||
| Anti-HCV positive | 8 | 0.80, [0.22-2.96] | 0.75 | 0.44, [0.11-1.79] | 0.40 | ||||
| Anti-HCV negative | 70 | ||||||||
HIV: human immunodeficiency virus; DLBCL: diffuse large B-cell lymphoma; PFS: progression free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; cART: combination antiretroviral therapy; GC: germinal center; ECOG: Eastern Cooperative Oncology Group; LDH: lactate dehydrogenase; UNL: upper normal limit; B symptoms: fever, night sweats, weight loss, fatigue, and swelling in lymph nodes; CNS: central nervous system; a: numbers of missing values, a: 20.
Before the introduction of the anti-CD20 antibody rituximab, HIV-infected patients with DLBCL treated with CHOP therapy showed a similar response rate and survival compared to their HIV-uninfected counterparts [24, 25]. Several retrospective studies of DLBCL patients with HIV infection treated with R-CHOP have been reported. In one study from Spain, HIV-infected DLBCL patients had a CR rate of 69% and a 5-year OS rate of 56%, similar to those of 81% and 74%, respectively, in HIV-uninfected patients [5]. Similarly, in a report from France, survival outcomes of HIV-infected DLBCL patients (2-year PFS & OS: 81% and 81%) did not differ from those of HIV-uninfected patients (2-year PFS & OS: 71% and 83%) [9]. Moreover, Sparana et al concluded that R-EPOCH was an effective regimen in HIV-associated NHL, achieving a CR rate of 73% [26]. In our study, a total of 78 HIV-infected DLBCL patients treated between 2011 and 2019 were analyzed. Most patients received the CHOP±R regimen, and several patients were given EPOCH±R based on their disease status and financial situation. Additionally, a cooperative group prospective trial reported the safety and efficacy of autoSCT in HIV-positive lymphoma, including DLBCL [27]. Here, five patients who underwent autoSCT after CR were lymphoma free. At the end of treatment, 58% of evaluable patients had achieved CR. The 2-year PFS was 56.4%, and the OS rate was 62.7%. The response rate and survival outcome appear to be lower than those in some studies. One of the reasons might be the decreased use of rituximab. Medical insurance has not covered the cost of rituximab until recent years. In addition, Yunnan is a remote province and relatively economically backward; thus, some patients could likely not afford their treatment, while a few exhibited low compliances and did not adhere to the recommended course of treatment. As a result, the high rate of loss to follow-up (20%) decreased the reliability of our results.
R-EPOCH is the preferred regimen for treating HIV-DLBCL, HIV-HHV8-positive DLBCL and HIV-primary effusion lymphoma (PEL) under current National Comprehensive Cancer Network guidelines based on multiple clinical trials and retrospective studies. In a pooled analysis of clinical trials for HIV-associated aggressive B-cell NHL comparing R-EPOCH (AMC034) to R-CHOP (AMC010), R-EPOCH resulted in superior outcomes compared to R-CHOP [28]. In another study, treatment with R-EPOCH seemingly compared favorably to treatment with R-CHOP in HIV-associated DLBCL (p=0.05) [29]. Recently, the histone deacetylase inhibitor vorinostat (VOR), combined with R-EPOCH, was reported to be tolerable and seemingly efficacious in patients with aggressive HIV-NHL [30]. In our small sample size comparison, we did not observe a significant difference in survival between the R-EPOCH and R-CHOP regimens. However, all of these studies were conducted in a post hoc manner.
CNS involvement has been recognized to be more common in AIDS-related lymphomas [31]. A retrospective review of databases from clinical trials showed that CNS involvement at baseline was not associated with shortened overall survival, but CNS relapse was associated with a reduced median OS of 1.6 months [31]. Similarly, one patient in our study experienced CNS relapse and died soon thereafter. Although introduction of a prognostic model that is used for assessing the risk of CNS disease in DLBCL has guided CNS prophylaxis in the general DLBCL population, the value of this prognostic model in HIV-infected patients with DLBCL remains unclear. A survival difference was not observed with this prognostic model in our data, as most patients received intrathecal prophylaxis, suggesting the role of routine CNS prophylaxis in overcoming the unfavorable prognosis of high CNS-IPI.
It has been shown that HIV-related factors, such as low CD4+ cell count and prior history of AIDS, are no longer predictive of worse survival outcome in the cART era [32, 33], consistent with our results. We were not able to analyze the HIV viral load at diagnosis due to the lack of baseline level testing. It is one of the limitations of our study. On the other hand, levels of HIV-1 RNA load were negative in all subjects during chemotherapy. We found that unfavorable performance status and high IPI score were associated with poor outcomes. Interestingly, elevated plasma EBV DNA load exhibited borderline significance associated with poor outcomes. It has been widely accepted that EBV plays an important role in the pathogenesis of lymphomas, such as BL, cHL, DLBCL and natural killer (NK)/T-cell lymphoma [34]. In the setting of HIV infection, EBV infection is more frequent and positive in 30-60% of cases compared to approximately 10% in general DLBCL patients [6, 13, 35, 36]. We found that 66% of our DLBCL cases presented with elevated EBV DNA, consistent with the previously reported prevalence of EBV load in HIV-associated DLBCL [13]. Its high frequency makes EBV a possible factor contributing to the development of HIV-associated lymphomas and is responsible for driving more aggressive behavior [35]. In contrast to the fact that blood EBV DNA is not predictive of outcomes in HIV-associated HL [37], previous studies have demonstrated that tumor EBV infection status is an independent adverse predictive factor of survival among patients with HIV-infected DLBCL [38, 39]. Muncunill et al. observed that plasma EBV load exerted a negative prognostic impact and could be used as an early predictor of HIV-related lymphoma [13]. In this study, we showed that elevated whole blood EBV load was inclined to be an independent negative predictor for both PFS and OS, suggesting measurement of plasma EBV DNA for risk stratification. In HIV-negative DLBCL, HBsAg-positive patients exhibited worse clinical features and poor outcomes, and the presence of HCV conveyed inferior OS when accompanied by impaired liver function [11, 40], whereas our results indicated that HIV and HBV/HCV coinfection in DLBCL did not predict outcomes. Further studies with larger sample sizes are needed to more thoroughly investigate these findings.
A limitation of this study is the small sample size, although this is the largest series reported in China thus far. Moreover, missing data might have led to imprecise estimates. The chemotherapeutic regimens combined in some cases may also have led to difficulties in interpreting the results.
This study provides important real-world data on the clinical characteristics and outcomes of HIV-infected DLBCL patients in China. We conclude that survival outcomes remain poor, and additional therapeutic approaches are warranted in this population.
Acknowledgements
We thank all the faculty members in our departments and all the patients, and their families involved in the current study.
Author Contributions
JZW analyzed the data and drafted the manuscript. YM contributed to the analysis and interpretation of data and revised the manuscript. JZW, YM and HYM contributed to the conception of the study. JHL revised the manuscript. XL, JCL, CQ, PFT, XCW and XQD participated in clinical data collection. WX, JYL and HYM reviewed the manuscript and provided suggestions. All authors read and approved the final manuscript.
Compliance with ethical standards
This study was approved by Yunnan Provincial Infectious Diseases Hospital/Yunnan AIDS Care Center institutional review board and conducted according to the declaration of Helsinki. Informed consent was obtained from all participants.
Funding
This study was supported by National Natural Science Foundation of China (81370657, 81470328, 81600130, 81770166, 81720108002), Project of National Key Clinical Specialty, National Science & Technology Pillar Program (2014BAI09B12), Jiangsu Provincial Special Program of Medical Science (BL2014086 and BE2017751), National Science and Technology Major Project (2018ZX09734007), Science Foundation for Youths of Jiangsu Province (BK20171079), and National Science & Technology Key Program (2017ZX10202101-001-009).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yarchoan R, Uldrick TS. HIV-Associated Cancers and Related Diseases. N Engl J Med. 2018;378:2145
2. Meister A, Hentrich M, Wyen C, Hubel K. Malignant lymphoma in the HIV-positive patient. Eur J Haematol. 2018;101:119-26
3. Gopal S, Patel MR, Yanik EL, Cole SR, Achenbach CJ, Napravnik S. et al. Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst. 2013;105:1221-9
4. Howlader N, Shiels MS, Mariotto AB, Engels EA. Contributions of HIV to Non-Hodgkin Lymphoma Mortality Trends in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1289-96
5. Baptista MJ, Garcia O, Morgades M, Gonzalez-Barca E, Miralles P, Lopez-Guillermo A. et al. HIV-infection impact on clinical-biological features and outcome of diffuse large B-cell lymphoma treated with R-CHOP in the combination antiretroviral therapy era. AIDS. 2015;29:811-8
6. Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50:74-87
7. Schommers P, Hentrich M, Hoffmann C, Gillor D, Zoufaly A, Jensen B. et al. Survival of AIDS-related diffuse large B-cell lymphoma, Burkitt lymphoma, and plasmablastic lymphoma in the German HIV Lymphoma Cohort. Br J Haematol. 2015;168:806-10
8. Miao Y, Medeiros LJ, Xu-Monette ZY, Li J, Young KH. Dysregulation of Cell Survival in Diffuse Large B Cell Lymphoma: Mechanisms and Therapeutic Targets. Frontiers in Oncology. 2019;9:107
9. Besson C, Lancar R, Prevot S, Algarte-Genin M, Delobel P, Bonnet F. et al. Outcomes for HIV-associated diffuse large B-cell lymphoma in the modern combined antiretroviral therapy era. AIDS. 2017;31:2493-501
10. Wu L, Ehlin-Henriksson B, Zhou X, Zhu H, Ernberg I, Kis LL. et al. Epstein-Barr virus (EBV) provides survival factors to EBV(+) diffuse large B-cell lymphoma (DLBCL) lines and modulates cytokine induced specific chemotaxis in EBV(+) DLBCL. Immunology. 2017;152:562-73
11. Deng L, Song Y, Young KH, Hu S, Ding N, Song W. et al. Hepatitis B virus-associated diffuse large B-cell lymphoma: unique clinical features, poor outcome, and hepatitis B surface antigen-driven origin. Oncotarget. 2015;6:25061-73
12. Tsutsumi Y, Nakayama C, Kamada K, Kikuchi R, Kudo D, Ito S. et al. Efficacy and prognosis of antiviral therapy on hepatitis C following treatment of lymphoma in HCV-positive diffuse large-cell lymphoma. Ann Hematol. 2017;96:2057-61
13. Muncunill J, Baptista MJ, Hernandez-Rodriguez A, Dalmau J, Garcia O, Tapia G. et al. Plasma EBV-load as an early biomarker and prognostic factor of HIV-related lymphomas. Clin Infect Dis. 2019;68:834-43
14. Lu L, Jia M, Ma Y, Yang L, Chen Z, Ho DD. et al. The changing face of HIV in China. Nature. 2008;455:609-11
15. Jia M, Luo H, Ma Y, Wang N, Smith K, Mei J. et al. The HIV epidemic in Yunnan Province, China, 1989-2007. J Acquir Immune Defic Syndr. 2010;53(Suppl 1):S34-40
16. Xiao J, Du S, Dai G, Gao G, Yang D, Zhao H. Efficacy and tolerability of chemotherapy in Chinese patients with AIDS-related Burkitt lymphoma and diffuse large B-cell lymphoma: An observational study. Sci Rep. 2017;7:1905
17. Shen Y, Zhang R, Liu L, Shen Y, Song W, Qi T. et al. Clinical and prognostic analysis of 78 patients with human immuno-deficiency virus associated non-Hodgkin's lymphoma in Chinese population. Infect Agent Cancer. 2017;12:7
18. Chen M, Jia MH, Ma YL, Luo HB, Chen HC, Yang CJ. et al. The changing HIV-1 genetic characteristics and transmitted drug resistance among recently infected population in Yunnan, China. Epidemiol Infect. 2018;146:775-81
19. Ji Y, Lu H. Malignancies in HIV-Infected and AIDS Patients. Adv Exp Med Biol. 2017;1018:167-79
20. Han X, Jemal A, Hulland E, Simard EP, Nastoupil L, Ward E. et al. HIV Infection and Survival of Lymphoma Patients in the Era of Highly Active Antiretroviral Therapy. Cancer Epidemiol Biomarkers Prev. 2017;26:303-11
21. Healy JA, Dave SS. The Role of EBV in the Pathogenesis of Diffuse Large B Cell Lymphoma. Curr Top Microbiol Immunol. 2015;390:315-37
22. de Sanjose S, Benavente Y, Vajdic CM, Engels EA, Morton LM, Bracci PM. et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008;6:451-8
23. Chen YY, Huang CE, Liang FW, Lu CH, Chen PT, Lee KD. et al. Prognostic impact of hepatitis C virus infection in patients with diffuse large B-cell lymphoma treated with immunochemotherapy in the context of a novel prognostic index. Cancer Epidemiol. 2015;39:382-7
24. Navarro JT, Lloveras N, Ribera JM, Oriol A, Mate JL, Feliu E. The prognosis of HIV-infected patients with diffuse large B-cell lymphoma treated with chemotherapy and highly active antiretroviral therapy is similar to that of HIV-negative patients receiving chemotherapy. Haematologica. 2005;90:704-6
25. Weiss R, Mitrou P, Arasteh K, Schuermann D, Hentrich M, Duehrsen U. et al. Acquired immunodeficiency syndrome-related lymphoma: simultaneous treatment with combined cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy and highly active antiretroviral therapy is safe and improves survival-results of the German Multicenter Trial. Cancer. 2006;106:1560-8
26. Sparano JA, Lee JY, Kaplan LD, Levine AM, Ramos JC, Ambinder RF. et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115:3008-16
27. Alvarnas JC, Le Rademacher J, Wang Y, Little RF, Akpek G, Ayala E. et al. Autologous hematopoietic cell transplantation for HIV-related lymphoma: results of the BMT CTN 0803/AMC 071 trial. Blood. 2016;128:1050-8
28. Barta SK, Lee JY, Kaplan LD, Noy A, Sparano JA. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. Cancer. 2012;118:3977-83
29. Barta SK, Xue X, Wang D, Tamari R, Lee JY, Mounier N. et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood. 2013;122:3251-62
30. Ramos JC, Sparano JA, Rudek MA, Moore PC, Cesarman E, Reid EG. et al. Safety and Preliminary Efficacy of Vorinostat With R-EPOCH in High-risk HIV-associated Non-Hodgkin's Lymphoma (AMC-075). Clin Lymphoma Myeloma Leuk. 2018;18:180-90 e2
31. Barta SK, Joshi J, Mounier N, Xue X, Wang D, Ribera JM. et al. Central nervous system involvement in AIDS-related lymphomas. Br J Haematol. 2016;173:857-66
32. Miralles P, Berenguer J, Ribera JM, Rubio R, Mahillo B, Tellez MJ. et al. Prognosis of AIDS-related systemic non-Hodgkin lymphoma treated with chemotherapy and highly active antiretroviral therapy depends exclusively on tumor-related factors. J Acquir Immune Defic Syndr. 2007;44:167-73
33. Barta SK, Samuel MS, Xue X, Wang D, Lee JY, Mounier N. et al. Changes in the influence of lymphoma- and HIV-specific factors on outcomes in AIDS-related non-Hodgkin lymphoma. Ann Oncol. 2015;26:958-66
34. Vockerodt M, Yap LF, Shannon-Lowe C, Curley H, Wei W, Vrzalikova K. et al. The Epstein-Barr virus and the pathogenesis of lymphoma. J Pathol. 2015;235:312-22
35. Linke-Serinsoz E, Fend F, Quintanilla-Martinez L. Human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV) related lymphomas, pathology view point. Semin Diagn Pathol. 2017;34:352-63
36. Morton LM, Kim CJ, Weiss LM, Bhatia K, Cockburn M, Hawes D. et al. Molecular characteristics of diffuse large B-cell lymphoma in human immunodeficiency virus-infected and -uninfected patients in the pre-highly active antiretroviral therapy and pre-rituximab era. Leuk Lymphoma. 2014;55:551-7
37. Ul-Haq I, Dalla Pria A, Suardi E, Pinato DJ, Froeling F, Forni J. et al. Blood Epstein-Barr virus DNA does not predict outcome in advanced HIV-associated Hodgkin lymphoma. Med Oncol. 2018;35:53
38. Chao C, Silverberg MJ, Martinez-Maza O, Chi M, Abrams DI, Haque R. et al. Epstein-Barr virus infection and expression of B-cell oncogenic markers in HIV-related diffuse large B-cell Lymphoma. Clin Cancer Res. 2012;18:4702-12
39. Chao C, Silverberg MJ, Chen LH, Xu L, Martinez-Maza O, Abrams DI. et al. Novel tumor markers provide improved prediction of survival after diagnosis of human immunodeficiency virus (HIV)-related diffuse large B-cell lymphoma. Leuk Lymphoma. 2018;59:321-9
40. Dlouhy I, Torrente MA, Lens S, Rovira J, Magnano L, Gine E. et al. Clinico-biological characteristics and outcome of hepatitis C virus-positive patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Ann Hematol. 2017;96:405-10
Author contact
![]() Corresponding author: Dr Haiyan Min, Department of Infectious Diseases, Yunnan Provincial Infectious Diseases Hospital/Yunnan AIDS Care Center, Anning highway 28km, Kunming, 650000, China. E-mail: 454755295com.
Corresponding author: Dr Haiyan Min, Department of Infectious Diseases, Yunnan Provincial Infectious Diseases Hospital/Yunnan AIDS Care Center, Anning highway 28km, Kunming, 650000, China. E-mail: 454755295com.

 Global reach, higher impact
Global reach, higher impact