3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(14):4332-4340. doi:10.7150/jca.55826 This issue Cite
Research Paper
Use of extended HR-HPV Genotyping in improving the Triage Strategy of 2019 ASCCP recommendations in Women with positive HR-HPV diagnosis and Simultaneous LSIL Cytology Results
1. Fujian Provincial Cervical Disease Diagnosis and Treatment Health Center, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou 350001, Fujian, P.R. China.
2. Department of Gynecology, Laboratory of Gynecologic Oncology, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou 350001, Fujian, P.R. China.
3. Department of Pathology, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou 350001, Fujian, P.R. China.
4. Fujian Key Laboratory of Women and Children's Critical Diseases Research, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian 350001, P.R. China.
#These authors contributed equally to this work.
Received 2020-11-12; Accepted 2021-4-21; Published 2021-5-19
Abstract
Objective: According to the 2019 American Society for Colposcopy and Cervical Pathology (ASCCP) recommendations, women with a positive high-risk human papillomavirus (HR-HPV) diagnosis and low-grade cervical intraepithelial lesion (LSIL) cytology result should be referred for further colposcopy examination. However, this strategy results in over-treatment in several cases. In this study, we assessed the performance of extended HR-HPV genotyping in women with a simultaneous positive HR-HPV and LSIL diagnosis with the aim of improving the current triage strategy.
Methods: This study was an observational analysis of women from the Fujian Province Cervical Lesion Screening Cohorts (FCLSCs). Women who were HR-HPV-positive and had a cytological examination of LSIL, which were followed up with colposcopy and biopsy, from 2015 to 2018 were included. The study endpoint was defined as the detection of histological cervical intraepithelial neoplasia grade 2 or worse (CIN2+). We combined HR-HPV genotypes according to the prevalence rate in histological CIN2+ and ranked them from high to low to establish HR-HPV genotyping models. Outcomes were assessed with respect to sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and colposcopy referral rate.
Results: Overall, 56,788 women undergoing preliminary screening for HR-HPV genotyping were included in this study. Among them, 10,499 women positive for HR-HPV underwent a cytology examination, and 902 women with LSIL cytology diagnosed and subsequent biopsy results were included in the final evaluation. Among these patients, 25.1% (226/902) were found to have CIN2+ in histology. HPV-16, -58, -52, -18, -33, and -31 infections were the most common genotypes, and HPV-16, -18, -58, -33, and -31 (odds ratio [OR] = 5.41, 2.98, 1.38, 1.24, and 1.21, respectively) were associated with the potential for histological CIN2+, from the highest to lowest. In the detection of CIN2+ lesions in HR-HPV-positive LSIL women of different HR-HPV genotyping models, the extended HPV 16/18/31/33/52/58 genotyping model was found to have better efficacy with higher sensitivity (92.9%) and NPV (93.0%), but a significantly lower colposcopy referral rate (74.7%) than the ASCCP-recommended HR-HPV non-genotyping model.
Conclusion: For HR-HPV-positive women with LSIL, the HPV 16/18/31/33/52/58 genotyping model can serve as an alternative approach to the ASCCP recommendations, potentially reducing the unnecessary colposcopy referral burden in China.
Keywords: human papillomavirus, genotyping, low-grade cervical intraepithelial lesion, cervical intraepithelial neoplasia
Introduction
Cervical cancer is the fourth most frequent cause of death in women [1], with the majority of cases occurring in low- or middle-income countries [2]. Fortunately, early detection and preventative measures have been shown to be successful in reducing the progression of premalignant cervical lesions [3]. Among all cancer types, cervical lesions are among the most efficiently controlled through appropriate screening measures [4, 5]. Currently, effective screening methods include human papillomavirus (HPV) testing, cytology, and colposcopy [6].
Since the publication of the 2015 American Society for Colposcopy and Cervical Pathology (ASCCP) interim guidance, HPV testing has been used as the primary screening strategy for cervical cancer [7]. Nevertheless, in most cases, the infections are benign and can be resolved within 2 years without the need for invasive treatment [8, 9]. Secondary triage of HPV-positive women is recommended during clinical management, including cytology, which allows deferral of colposcopy for low-risk cases, reducing the long follow-up interval [10].
Low-grade squamous intraepithelial lesion (LSIL), as defined by the Bethesda system, is the second most common cervical abnormality in cytological results [11, 12]. According to the most recent 2019 ASCCP risk-based management consensus guidelines for the management of cervical cancer screening abnormalities, HPV-positive women with LSIL have an immediate cervical intraepithelial neoplasia (CIN) 3+ risk of over 4.0%, which necessitates a colposcopy referral [13]. However, most LSIL cases do not develop into a clinically significant disease. A previous study demonstrated that the prevalence of high-risk (HR)-HPV could reach 76% in women with LSIL [14], indicating high sensitivity but poor specificity. A large number of negative colposcopy/biopsy results were reported in women with positive HPV and LSIL, which poses a challenge for the limited capacity of colposcopy in most hospital systems and subsequent adverse effects from overtreatment [15, 16]. Hence, risk stratification strategies that can better identify underlying or incipient CIN2, CIN3, adenocarcinoma in situ (AIS), or cancer (CIN2+) with less resource waste are needed in HPV-positive patients with LSIL. However, most of the related articles published to date have mainly investigated HPV-positive patients with LSIL using the cytology as a primary screening method but not the HPV testing [17-21]. Moreover, no study has yet addressed application of the 2019 ASCCP guidelines in such patients.
Extensive evidence indicates that the persistent infection of HPV promotes the progression of cervical cancer [22, 23]. However, individual HPV types differ enormously in their relative carcinogenic potential. Thirteen HPV genotypes, including HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66, are categorized as causative agents of cancer; HPV 68 is considered to be probably carcinogenic [23]. According to a previously published study, the baseline infection rate of HR-HPV genotypes among women with baseline normal cytology but who subsequently developed CIN3+ during a follow-up period of 11.5 years was as follows: 19.4% HPV-16, 11.7% HPV-18, 13.3% HPV-31, 13.3% HPV-33, 10.7% HPV-52, 9.9% HPV-39, 8.6% HPV-35, 7.9% HPV-58, 7.9% HPV-45, 7.9% HPV-59, 7.7% HPV-51, 7.2% HPV-68, and 6.2% HPV-56 [24]. This information provides a basis for the selection of HPV genotypes for the appropriate triaging of women with HR-HPV and LSIL cytology.
The purpose of this retrospective study was to assess the potential of extended HR-HPV genotyping in identifying underlying histological CIN2+ in women with a positive HR-HPV and LSIL finding. Furthermore, we aimed to assess the efficacy of different HR-HPV genotyping models to optimize the 2019 ASCCP risk-based management consensus guidelines for these patients to achieve a reduction in the unnecessary use of colposcopy resources.
Methods
Patients
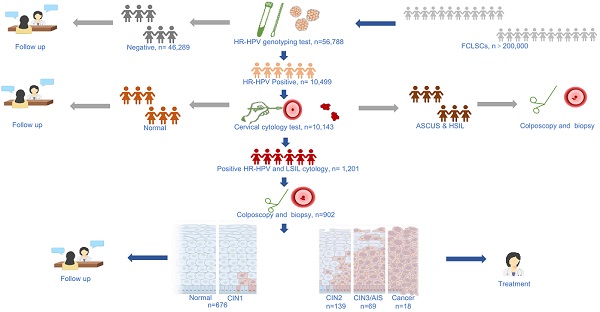
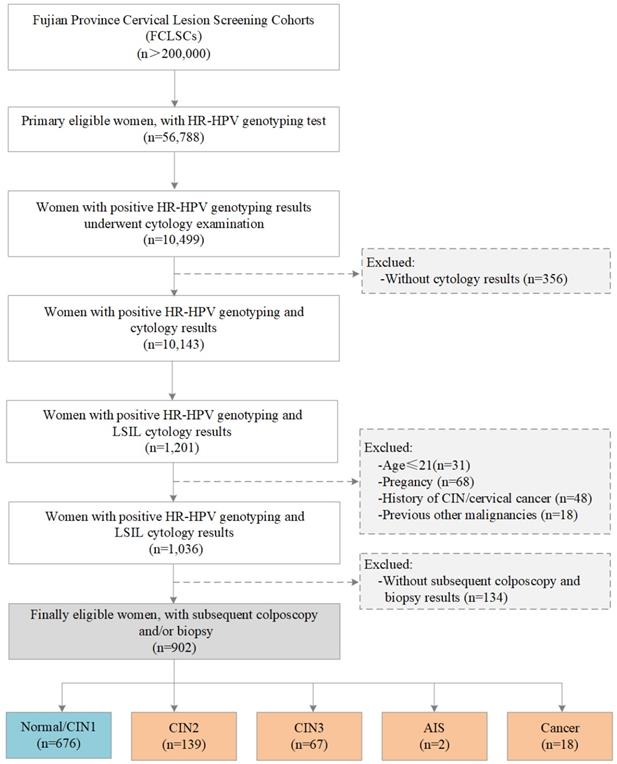
The study population was selected from the Fujian Province Cervical Lesion Screening Cohorts (FCLSCs) with more than 200,000 cases, including a provincial-level hospital, nine municipal-level hospitals, and more than 500 community health service centers [25, 26]. All participants were required to satisfy the following characteristics: (1) history of sexual activity, (2) willingness to undergo cervical cancer screening with cervical cytology and an HR-HPV genotyping test, (3) no history of severe immunodeficiency disease, and (4) provision of written informed consent. From January 2015 to December 2018, women from the FCLSCs who underwent HR-HPV genotyping were initially included. Women with positive HR-HPV genotyping results subsequently underwent a cytology examination. Women without cytology results were excluded. Subsequent participants were also excluded according to the following criteria: (1) age less than 21 years, (2) pregnancy, (3) history of CIN/cervical cancer, (4) other previous malignancies, and (5) no subsequent colposcopy and biopsy results. The final eligibility criteria included women with an HR-HPV-positive diagnosis with HR-HPV genotyping, LSIL cytology, and subsequent colposcopy and/or biopsy results (Figure 1). The Ethics Committees of the Fujian Maternity and Child Health Hospital approved this study (2014-045).
Flow chart for the selection of patients in this study. Abbreviations: LSIL, low-grade squamous intraepithelial lesion; HR-HPV, high-risk human papillomavirus; CIN, cervical intraepithelial neoplasia; AIS, adenocarcinoma in situ.
Collection of basic information and cervical specimens
Prior to registration, each woman provided informed consent. An experienced doctor conducted a confidential interview with a questionnaire to collect basic information, including the patient's history of medication, cervix-related diseases and treatments, other malignancies, education background, smoking and drinking habits, and reproduction history. All eligible individuals underwent gynecological examinations. Exfoliated cervical cells from the ecto- or endo-cervical canals were obtained with a cytobrush. For HPV genotyping, specimens were stored using a preservation solution in 2 ml vials at -20 °C. For cytology, samples were stored using ThinPrep® PreservCyt® (Hologic, Waltham, MA, USA) in 20 ml vials at 4 °C. Samples were subsequently transferred to the laboratory and cytological site where HR-HPV genotyping and cytology were conducted.
HR-HPV genotyping test
Polymerase chain reaction-reverse dot blot (PCR-RDB) was used for the analysis of HR-HPV, including 14 HR-HPV genotypes (HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -66, and -68), in cervical exfoliated cells (Yaneng® Biosciences, ShenZhen, China). All detection procedures were conducted according to the manufacturer's instructions [27].
Cytology
An auto-image system (Hologic, Inc., San Diego, CA, USA) was used for cervical cytological examinations. All slides prepared for the cytological examinations were analyzed independently by two experienced cytopathologists and were diagnosed according to the Bethesda system [28]. If there was divergence in the diagnosis, the cervical samples were re-evaluated to reach a consensus.
Histology
HR-HPV-infected participants who were also diagnosed with LSIL were subsequently referred for colposcopy and/or biopsy within 10 weeks. Colposcopy results that were deemed normal had no requirement for a biopsy. In contrast, participants with abnormal colposcopy results received an immediate biopsy of visible lesions. The results were interpreted in accordance with the CIN system [29], including normal, CIN1, CIN2, CIN3, AIS, and cancer. If a sample was diagnosed with a primary histology result of CIN2+, the sample was reviewed by another independent histopathologist. Any discrepancy was discussed and resolved by a second histological examination until consensus was reached.
Statistical analysis
The referral rate was determined by dividing the number of HR-HPV-infected LSIL patients by the overall number of LSIL patients. The mean and standard deviation (SD) of the classified variables were assessed. The values and percentages were calculated. The study endpoint was defined as the detection of histological CIN2+ [10]. We combined HR-HPV genotypes according to the prevalence rate in LSIL patients whose biopsy showed CIN2+, ranked from highest to lowest, to establish the HR-HPV genotyping model. According to the guidelines [15], HPV-16- and/or 18-positive patients should be further examined via colposcopy; thus, HPV 16/18 was divided into a separate, priority HR-HPV genotyping model. We assessed the potential of different HR-HPV genotyping models in detecting underlying CIN2+ with respect to their sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), which were then compared to the gold-standard histological diagnosis. The odds ratio (OR) for the incidence of CIN2+ in women with LSIL, accounting for age and HR-HPV positivity, was also calculated. p values < 0.05 (two-sided) were regarded as statistically significant. Statistical analysis was performed using Stata 14 software (Stata Corp., College Station, TX, USA).
Results
Baseline characteristics of women with HR-HPV infection and LSIL
A total of 56,788 women with HR-HPV genotyping results who also underwent cervical cytology were recruited for this study. Among these, 10,499 (18.5%) women with positive HR-HPV genotyping results underwent cytology examination, and 356 (3.4%) HR-HPV-positive women without cytology results were excluded. Among the remaining 10,143 (17.9%) HR-HPV-positive women with cytology results, 1,201 (2.1%) women were HR-HPV-positive with both HR-HPV genotyping and LSIL cytology results. After exclusions, 1,036 (1.8%) women were eligible for the study. 134 (12.9%) of whom without colposcopy and/or biopsy results were lost to follow-up and excluded, and the remaining 902 women (87.1%) with colposcopy biopsies were included for final evaluation. The baseline features of the participants are described in Table 1. The average age of the enrolled participants was 38.27 ± 9.86 years (range, 21-74 years). Of the 902 women, 63.2% had received higher education, 97.3% denied a history of smoking, 83.5% denied a history of drinking, and 33.0% had more than two pregnancies.
Positivity rate of HR-HPV genotypes among HR-HPV-positive participants with LSIL
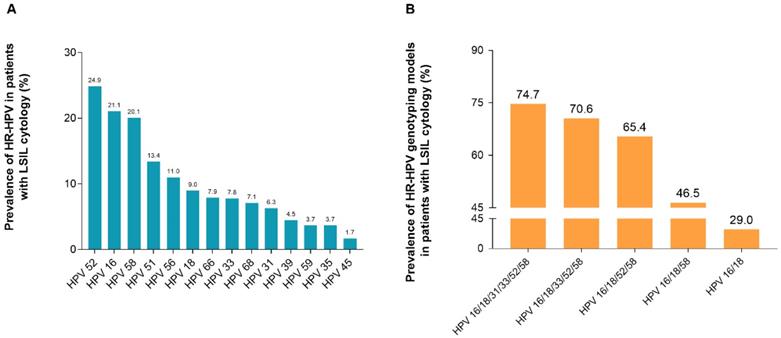
Among all HR-HPV-positive participants with LSIL, the most prevalent HR-HPV genotype was HPV-52, accounting for 24.9% (225/902), followed by HPV-16 (190/902, 21.1%), HPV-58 (181/902, 20.1%), HPV-51 (121/902, 13.4%), and HPV-56 (99/902, 11.0%) (Figure 2, Supplement Table S1).
Positivity rate of HR-HPV genotypes among participants with LSIL cytology. (A) Prevalence rate of HR-HPV genotypes in 902 women with LSIL. (B) Prevalence rate of HR-HPV genotyping models in 902 women with LSIL. HPV 16/18: women with HPV 16 and/or HPV 18 infection; HPV 16/18/58: women with any infection of HPV-16, -18, -58; HPV 16/18/52/58: women with any infection of HPV-16, -18, -52, -58; HPV 16/18/33/52/58: women with any infection of HPV-16, -18, -33, -52, -58; HPV 16/18/31/33/52/58: women with any infection of HPV-16, -18, -31, -33, -52, -58. Abbreviations: HR-HPV, high-risk human papillomavirus; LSIL, low-grade squamous intraepithelial lesion.
Baseline characteristics of 902 women with positive HR-HPV and LSIL
| Variates | n (n=902) | Mean (x±s) or prevalence (%) |
|---|---|---|
| Age (years) | ||
| 21-74 | 902 | 38.27±9.86 |
| 21-30 | 216 | 26.59±2.68 |
| 31-40 | 337 | 35.26±2.87 |
| 41-50 | 248 | 44.89±2.67 |
| 51-65 | 92 | 55.83±3.71 |
| >65 | 9 | 69.33±2.96 |
| Education degree | ||
| Lower education | 332 | 36.8 |
| Higher education | 570 | 63.2 |
| Drinking | ||
| Yes | 149 | 16.5 |
| No | 753 | 83.5 |
| Smoking | ||
| Yes | 24 | 2.7 |
| No | 878 | 97.3 |
| Pregnancy | ||
| ≤2 | 604 | 67.0 |
| >2 | 298 | 33.0 |
Abbreviations: LSIL, low-grade squamous intraepithelial lesion; HR-HPV, high-risk human papillomavirus.
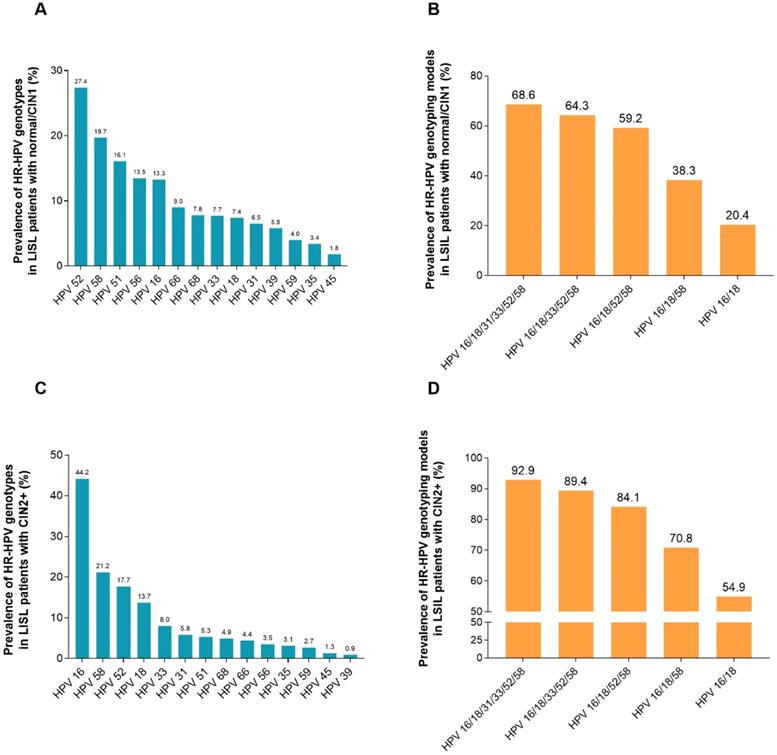
Figure 3 (Supplement Table S2) listed the HR-HPV infection rates in HR-HPV-infected women with LSIL and a CIN2+ biopsy. Overall, 25.1% (226/902) women with HR-HPV infection and simultaneous cytology results of LSIL were found to have CIN2+ in histology. The HR-HPV positivity rate in participants with LSIL and a biopsy confirming CIN2+ status was the highest in HPV-16 (44.2%), followed by HPV-58 (21.2%), HPV-52 (17.7%), HPV-18 (13.7%), HPV-33 (8.0%), HPV-31 (5.8%), HPV-51 (5.3%), HPV-68 (4.9%), HPV-66 (4.4%), HPV-56 (3.5%), HPV-59 (3.1%), HPV-35 (3.1%), HPV-45 (1.3%), and HPV-39 (0.9%). The infection rate of HR-HPV increased with the increase in the HR-HPV genotyping models combining more HR-HPV genotypes. Of the participants with LSIL, 92.9% (p < 0.001) can be confirmed as having histological CIN2+ under the HPV genotyping model HPV 16/18/31/33/52/58. However, the rate was only 54.9% (p < 0.001) for the HPV 16/18 genotyping model.
Odds ratio for histological CIN2+ with different HR-HPV genotypes in participants with LSIL
Table 2 presents the predictive factors for CIN2+ in women with LSIL. After adjusting for age, education level, smoking, drinking, and pregnancies, the infection of HPV-16 was the greatest risk factor for the occurrence of CIN2+ (OR, 5.41; 95% CI, 3.58-8.18; p < 0.001) in women with LSIL. Other HR-HPV genotypes that were correlated with the occurrence of CIN2+ were as follows: HPV-18 (OR, 2.98; 95% CI, 1.69-5.25; p < 0.001), HPV-58 (OR, 1.38; 95% CI, 0.87-2.20; p = 0.168), HPV-33 (OR, 1.24; 95% CI, 0.66-2.31; p = 0.503), and HPV-31 (OR, 1.21; 95% CI, 0.58-2.51; p = 0.611). The remaining HR-HPV genotypes (HPV-35, -39, -45, -51, -52, -56, -59, -66, -68) were reported to have no significant correlation with the occurrence of CIN2+. Moreover, we evaluated the risk of CIN2+ in women with LSIL, according to the HR-HPV genotyping models. The risk assessment of HR-HPV genotyping models was as follows: HPV 16/18 genotyping model (OR, 5.38, 95% CI, 3.83-7.55; p < 0.001), HPV 16/18/58 genotyping model (OR, 4.13, 95% CI, 2.95-5.77; p < 0.001), HPV 16/18/52/58 genotyping model (OR, 3.96, 95% CI, 2.66-5.90; p < 0.001), HPV 16/18/33/52/58 genotyping model (OR, 4.93, 95% CI, 3.11-7.80; p < 0.001), and HPV 16/18/31/33/52/58 genotyping model (OR, 6.19, 95% CI, 3.61-10.63; p < 0.001).
The accuracy of HR-HPV genotyping models in triaging HR-HPV-positive women with simultaneous LSIL
The sensitivities and NPVs were higher in HR-HPV genotyping models that combined more HR-HPV genotypes, with a decrease in the specificity and PPV. The sensitivity and NPV rates for the extended HPV 16/18/31/33/52/58 genotyping model were 92.9% and 93.0%, respectively, and represented the highest values observed for the detection of underlying CIN2+ pathology, followed by the extended HPV 16/18/33/52/58, HPV 16/18/52/58, HPV 16/18/58, and HPV 16/18 genotyping models. In addition, the colposcopy referral rate of women with the extended HPV 16/18/31/33/52/58 genotyping model was only 74.7% (Table 3).
Discussion
According to the most current ASCCP risk-based management consensus guidelines, HPV-positive women whose cytology testing reveals LSIL are recommended for further colposcopy because of the greater than 4.0% risk for an immediate CIN3+ [15]. However, it should be emphasized that different types of HR-HPV have varying potential with respect to the progression of CIN [23]. Triaging all HPV-positive LSIL women to conduct colposcopies will lead to a significant cost burden and overtreatment of patients. Here, we evaluated the correlation between different HR-HPV genotypes and underlying CIN2+ in HPV-positive patients who also had LSIL based on the newly revised 2019 ASCCP risk-based management consensus guidelines, in order to reduce unnecessary colposcopy referrals. Previous reports had focused mainly on HR-HPV or HPV 16/18 [30-32], or investigated a population with cytology as a primary screening method. No study has assessed application of the 2019 ASCCP guidelines in women who are HR-HPV-positive with a LSIL cytology until now. Thus, to the best of our knowledge, this is the first investigation on this population with respect to the 2019 ASCCP guidelines. The present results revealed that the extended HPV 16/18/31/33/52/58 genotyping model had better sensitivity and NPV than other HR-HPV genotyping models with respect to CIN2+ status; moreover, the rate of colposcopies was lower than that of transferring all positive HR-HPV for colposcopies.
Odds ratio of histological CIN2+ according to different HR-HPV genotypes among 902 women with LSIL cytology
| Variates | OR | ORadjust (95% CI)a | p-value |
|---|---|---|---|
| Age | |||
| 21-30 | 1 (R) | 1 (R) | |
| 31-40 | 0.77 (0.15-3.86) | 0.47 (0.08-2.69) | 0.393 |
| 41-50 | 0.89 (0.18-4.36) | 0.64 (0.11-3.61) | 0.612 |
| 51-65 | 1.93 (0.39-9.47) | 1.35 (0.24-7.62) | 0.737 |
| >65 | 1.61 (0.32-8.24) | 1.43 (0.24-8.44) | 0.697 |
| HPV16 | |||
| Negative | 1(R) | 1(R) | |
| Positive | 5.17(3.66-7.29) | 5.41(3.58-8.18) | <0.001 |
| HPV18 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 1.99 (1.24-3.20) | 2.98 (1.69-5.25) | <0.001 |
| HPV31 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.88 (0.46-1.66) | 1.21 (0.58-2.51) | 0.611 |
| HPV33 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 1.04 (0.759-1.82) | 1.24 (0.66-2.31) | 0.503 |
| HPV35 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.91 (0.38-2.14) | 1.02 (0.40-2.62) | 0.961 |
| HPV39 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.18 (0.04-0.73) | 0.13 (0.03-0.61) | 0.010 |
| HPV45 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.91 (0.26-3.26) | 0.80 (0.20-3.19) | 0.752 |
| HPV51 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.37 (0.20-0.69) | 0.42 (0.22-0.83) | 0.012 |
| HPV52 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.57 (0.39-0.84) | 0.75 (0.48-1.16) | 0.194 |
| HPV56 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.57 (0.29-1.13) | 0.26 (0.12-0.59) | 0.001 |
| HPV58 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 1.10 (0.76-1.60) | 1.38 (0.87-2.20) | 0.168 |
| HPV59 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.66 (0.27-1.61) | 0.64 (0.24-1.23) | 0.383 |
| HPV66 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.47 (0.24-0.93) | 0.58 (0.28-1.23) | 0.157 |
| HPV68 | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 0.60 (0.31-1.17) | 0.81 (0.39-1.69) | 0.579 |
| HPV16/18b | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 4.74 (3.44-6.54) | 5.38 (3.83-7.55) | <0.001 |
| HPV16/18/58c | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 3.90 (2.82-5.41) | 4.13 (2.95-5.77) | <0.001 |
| HPV16/18/52/58d | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 3.64 (2.47-5.37) | 3.96 (2.66-5.90) | <0.001 |
| HPV16/18/33/52/58e | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 4.66 (2.97-7.32) | 4.93 (3.11-7.80) | <0.001 |
| HPV16/18/31/33/52/58f | |||
| Negative | 1 (R) | 1 (R) | |
| Positive | 6.00 (3.52-10.52) | 6.19 (3.61-10.63) | <0.001 |
Note: a: OR values were adjusted for age, education level, smoking, drinking, and number of pregnancy; b: Women with any infection of HPV-16, -18; c: Women with any infection of HPV-16, -18, -58; d: Women with any infection of HPV-16, -18, -52, -58; e: Women with any infection of HPV-16, -18, -33, -52, -58; f: Women with any infection of HPV-16, -18, -31, -33, -52, -58.
Abbreviations: OR, odds ratio; CI, confidence interval; R; reference; LSIL, low-grade squamous intraepithelial lesion; HR-HPV, high-risk human papillomavirus.
Prevalence of HR-HPV genotypes based on the histological diagnosis of 902 women with LSIL cytology. (A) Prevalence of HR-HPV genotypes in LSIL patients with biopsy showing normal/CIN1. (B) Prevalence of HR-HPV genotyping models in LSIL patients with biopsy showing normal/CIN1. HPV 16/18: women with HPV 16 and/or HPV 18 infection; HPV 16/18/58: women with any infection of HPV-16, -18, -58; HPV 16/18/52/58: women with any infection of HPV-16, -18, -52, -58; HPV 16/18/33/52/58: women with any infection of HPV-16, -18, -33, -52, -58; HPV 16/18/31/33/52/58: women with any infection of HPV-16, -18, -31, -33, -52, -58. (C) Prevalence of HR-HPV genotypes in LSIL patients with biopsy showing CIN2+. (D) Prevalence of HR-HPV genotyping models in LSIL patients with biopsy showing CIN2+. Abbreviations: HR-HPV, high-risk human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; CIN, cervical intraepithelial neoplasia; CIN2+, cervical intraepithelial neoplasia grade 2 or worse.
The accuracy of different HR-HPV genotyping models to triage women with HR-HPV positive and simultaneous LSIL to detect underlying CIN2+
| HR-HPV genotyping models | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Referral ratea (%) |
|---|---|---|---|---|---|
| HPV16/18b | 54.9 (48.1-61.4) | 79.6 (76.3-82.5) | 47.3 (41.2-53.6) | 84.1 (80.9-86.8) | 29.0 (262/902) |
| HPV16/18/58c | 70.8 (64.3-76.5) | 61.7 (57.9-65.3) | 38.2 (33.5-43.0) | 86.3 (82.9-89.2) | 46.5 (419/902) |
| HPV16/18/52/58d | 84.0 (78.5-88.5) | 40.8 (37.1-44.6) | 32.2 (28.5-36.2) | 88.5 (84.2-91.7) | 65.4 (590/902) |
| HPV16/18/33/52/58e | 89.4 (84.4-92.9) | 35.7 (32.1-39.4) | 31.7 (28.1-35.5) | 90.9 (88.7-94.0) | 70.6 (637/902) |
| HPV16/18/31/33/52/58f | 92.9 (88.5-95.8) | 31.4 (27.9-35.0) | 31.2 (27.7-34.8) | 93.0 (88.6-95.8) | 74.7 (674/902) |
Note: a: The rate of referred to colposcopy in LSIL women; b: Women with any infection of HPV-16, -18; c: Women with any infection of HPV-16, -18, -58; d: Women with any infection of HPV-16, -18, -52, -58; e: Women with any infection of HPV-16, -18, -33, -52, -58; f: Women with any infection of HPV-16, -18, -31, -33, -52, -58.
Abbreviations: HR-HPV, high-risk human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; CIN2+, cervical intraepithelial neoplasia grade 2 or worse; PPV, positive predictive value; NPV, negative predictive value.
In our previous study, the most prevalent HR-HPV genotypes identified in the Chinese population were HPV-16, -52, -58, -18, -53, and -33 [27]. However, we found that HPV-16, -18, -58, -52, -31, and -33, ranked from the highest to lowest, were the most prevalent HR-HPV genotypes in women with LSIL whose biopsy revealed CIN2+. In these patients, the detection rates of HPV-16, -18, -58, -52, -33, and -31 were 44.2%, 13.7%, 21.2%, 17.7%, 8.0%, and 5.8%, respectively. Recent data suggest that the most pathogenic HPV genotypes can be used to identify women who are at high risk of CIN3 [33, 34]. These findings indicated a higher risk of underlying CIN2+ in patients with simultaneous HPV-16, -18, -31, -33, -52, or -58 infection, which emphasizes the importance of detecting specific HR-HPV genotypes.
We also evaluated the risk of CIN2+ associated with different HR-HPV genotypes. HPV-16 infection was found to have the highest correlation with the detection of CIN2+ in histology. HPV-18, HPV-58, HPV-33, and HPV-31 were also found to be associated with the incidence of histological CIN2+. Combining these HR-HPV genotypes in HR-HPV genotyping models increased the risk of having potential histological CIN2+. According to our results, the HPV 16/18/31/33/52/58 model showed an estimated OR of 6.19 (95% CI, 3.61-10.63) for histological CIN2+.
Women with positive HPV results and LSIL face a significant risk of developing cervical precancerous lesions [35]. The sensitivity of HR-HPV testing can reach 100.0%, but at the expense of significant losses in specificity (20.3%) [36]. This is important because a test with poor specificity can overestimate the risk of cervical cancer, which can induce anxiety and overtreatment, among other adverse effects [37, 38]. Therefore, recommendation of colposcopy and/or biopsy for patients with LSIL who are positive for an HPV infection that falls into the HPV 16/18/31/33/52/58 genotyping model may provide optional management of patient care and hospital resources. All HR-HPV genotyping models were evaluated for the effectiveness of detecting underlying CIN2+ in LSIL patients with respect to sensitivity, specificity, PPV, and NPV. In agreement with previous results, triaging women with LSIL using the extended HPV 16/18/31/33/52/58 genotyping model showed higher sensitivity (92.9% vs. 54.9%) and lower specificity (31.4% vs. 79.6%) than obtained when using the HPV 16/18 genotyping model, leading to reduced risk of misdiagnosis [32]. In addition, the extended HPV 16/18/31/33/52/58 genotyping model was also more sensitive to the detection of CIN2+ than any other genotyping model because of the high sensitivity (92.9%). In addition, according to the 2019 ASCCP risk-based management consensus guidelines [13], all HR-HPV-infected women with LSIL had a risk slightly above 4.0% for CIN3+, and were therefore recommended to undergo immediate colposcopy. However, the extended HPV 16/18/31/33/52/58 model indicated a significantly lower referral rate (74.7%), but high sensitivity (92.9%) and NPV (93.0%), which could significantly reduce the burden for health care systems with limited colposcopy capacity but reduced misdiagnosis. Hence, colposcopy referral according to the extended HPV 16/18/31/33/52/58 genotyping model is believed to be a beneficial alternative triaging management approach for Chinese patients.
A potential limitation of this study included the small number of participants with a CIN3+ biopsy. Using histological CIN3+ as the main study outcome can result in an unstable and imprecise sensitivity result. Therefore, our analysis was restricted to CIN2+ as the study endpoint. A second limitation is the enrollment of participants from a single region. In addition, the study lacked large-scale follow-up, which will be conducted in future work.
In conclusion, with a high sensitivity and NPV, but lower referral rates, the extended HPV 16/18/31/33/52/58 genotyping model can provide an alternative to current triaging management of HR-HPV-positive women with LSIL, based on ASCCP guidelines, resulting in a significant reduction in unnecessary referrals for subsequent testing. Future work should focus on the evaluation and validation of this model in large-scale follow-up and different populations.
Abbreviations
HR-HPV: high-risk human papillomavirus; LSIL: low-grade squamous intraepithelial lesion; ASCCP: American Society for Colposcopy and Cervical Pathology; AIS: adenocarcinoma in situ; CIN: cervical intraepithelial neoplasia; CIN2+: cervical intraepithelial neoplasia grade 2 or worse; PCR-RDB: Polymerase chain reaction reverse dot blot; SD: standard deviation; PPV: positive predictive value; NPV: negative predictive value; OR: odds ratio; CI: confidence interval.
Supplementary Material
Supplementary tables.
Acknowledgements
The authors would like to thank the FCLSCs investigators for their contributions to this trial. Above all, we are grateful to all patients who participated in this study. This work was supported by grants from the Fujian Provincial Natural Science Foundation of China (grant no. 2017J01232), Fujian Provincial Maternity and Children's Hospital Natural Science Foundation (grant no. YCXM18-18), Science and Technology Innovation Startup Fund of Fujian Provincial Maternity and Children's Hospital (grant no. YCXM20-19), Fujian Provincial Health and Education Joint Project (grant no. 2019-WJ-05), Fujian Provincial Health Commission Innovation Project (grant no. 2019-CX-7) and Fujian Provincial Maternity and Child Health Hospital Fund (grant no. 13-27).
Author Contributions
Huifeng Xue and Hangjing Gao: Data collection from references, writing and review. Jinwen Zheng, Yaojia Chen, Jiancui Chen and Diling Pan: writing, revised article for important intellectual content. Binhua Dong and Pengming Sun: Designing of article, review and final approval.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424
2. Mishra GA, Pimple SA, Shastri SS. An overview of prevention and early detection of cervical cancers. Indian J Med Paediatr Oncol. 2011;32:125-32
3. Nobbenhuis MA, Walboomers JM, Helmerhorst TJ. et al. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20-5
4. Quinn M, Babb P, Jones J, Allen E. Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics. Bmj. 1999;318:904-8
5. Arbyn M, Roelens J, Simoens C. et al. Human papillomavirus testing versus repeat cytology for triage of minor cytological cervical lesions. Cochrane Database Syst Rev. 2013;2013:Cd008054
6. Boardman LA, Goldman DL, Cooper AS, Heber WW, Weitzen S. CIN in pregnancy: antepartum and postpartum cytology and histology. J Reprod Med. 2005;50:13-8
7. Huh WK, Ault KA, Chelmow D. et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-82
8. Schiffman M, Wentzensen N. A Suggested Approach to Simplify and Improve Cervical Screening in the United States. J Low Genit Tract Dis. 2016;20:1-7
9. Bosch FX, Broker TR, Forman D. et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl 6):G1-31
10. Perkins RB, Guido RS, Castle PE. et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24:102-31
11. Garrett LA, McCann CK. Abnormal cytology in 2012: management of atypical squamous cells, low-grade intraepithelial neoplasia, and high-grade intraepithelial neoplasia. Clin Obstet Gynecol. 2013;56:25-34
12. Sundström K, Lu D, Elfström KM. et al. Follow-up of women with cervical cytological abnormalities showing atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion: a nationwide cohort study. Am J Obstet Gynecol. 2017;216:48.e1-e15
13. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors: Erratum. J Low Genit Tract Dis. 2020
14. Arbyn M, Martin-Hirsch P, Buntinx F, Van Ranst M, Paraskevaidis E, Dillner J. Triage of women with equivocal or low-grade cervical cytology results: a meta-analysis of the HPV test positivity rate. J Cell Mol Med. 2009;13:648-59
15. Berkowitz RP. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;122:393
16. Velentzis LS, Caruana M, Simms KT. et al. How will transitioning from cytology to HPV testing change the balance between the benefits and harms of cervical cancer screening? Estimates of the impact on cervical cancer, treatment rates and adverse obstetric outcomes in Australia, a high vaccination coverage country. Int J Cancer. 2017;141:2410-22
17. Pity IS, Abdo HM, Goreal AA. Human Papillomavirus Genotyping among Different Cervical Smears in Duhok/Iraq. Asian Pac J Cancer Prev. 2019;20:2059-64
18. Luo HX, Du H, Liu ZH, Zhang LJ, Wang C, Wu RF. Evaluation of CIN2+/CIN3+ risk of different HPV subtypes infection combined with abnormal cytology status. Chin J Cancer. 2018;40:232-8
19. Kussaibi H, Al Dossary R, Ahmed A, Muammar A, Aljohani R. Correlation of High-Risk HPV Genotypes with Pap Test Findings: A Retrospective Study in Eastern Province, Saudi Arabia. Acta Cytol. 2020 p:1-8
20. Kjær SK, Munk C, Junge J, Iftner T. Carcinogenic HPV prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ASCUS/LSIL, HSIL, or cervical cancer: what is the potential for prevention? Cancer Causes Control. 2014;25:179-89
21. Ciszek B, Heimrath J, Ciszek M. The application of human papilloma virus genotyping for the identification of neoplasm lesions in the cervix of women with abnormal cytology smears. Adv Clin Exp Med. 2012;21:759-66
22. Muñoz N, Bosch FX, de Sanjosé S. et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-27
23. Bouvard V, Baan R, Straif K. et al. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 2009;10:321-2
24. Thomsen LT, Frederiksen K, Munk C, Junge J, Iftner T, Kjaer SK. Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. Int J Cancer. 2015;137:193-203
25. Kang Y, Sun P, Mao X, Dong B, Ruan G, Chen L. PCR-reverse dot blot human papillomavirus genotyping as a primary screening test for cervical cancer in a hospital-based cohort. J Gynecol Oncol. 2019;30:e29
26. Dong B, Sun P, Ruan G. et al. Type-specific high-risk human papillomavirus viral load as a viable triage indicator for high-grade squamous intraepithelial lesion: a nested case- control study. Cancer Manag Res. 2018;10:4839-51
27. Sun P, Song Y, Ruan G. et al. Clinical validation of the PCR-reverse dot blot human papillomavirus genotyping test in cervical lesions from Chinese women in the Fujian province: a hospital-based population study. J Gynecol Oncol. 2017;28:e50
28. Bergeron C. The 2001 Bethesda system. Salud Publica Mex. 2003;45(Suppl 3):S340-4
29. Waxman AG, Chelmow D, Darragh TM, Lawson H, Moscicki AB. Revised terminology for cervical histopathology and its implications for management of high-grade squamous intraepithelial lesions of the cervix. Obstet Gynecol. 2012;120:1465-71
30. Xu L, Benoy I, Cuschieri K, Poljak M, Bonde J, Arbyn M. Accuracy of genotyping for HPV16 and 18 to triage women with low-grade squamous intraepithelial lesions: a pooled analysis of VALGENT studies. Expert Rev Mol Diagn. 2019;19:543-51
31. Arbyn M, Xu L, Verdoodt F. et al. Genotyping for Human Papillomavirus Types 16 and 18 in Women With Minor Cervical Lesions: A Systematic Review and Meta-analysis. Ann Intern Med. 2017;166:118-27
32. Kulasingam SL, Hughes JP, Kiviat NB. et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. Jama. 2002;288:1749-57
33. Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1157-64
34. Schiffman M, Khan MJ, Solomon D. et al. A study of the impact of adding HPV types to cervical cancer screening and triage tests. J Natl Cancer Inst. 2005;97:147-50
35. Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. J Low Genit Tract Dis. 2008;12:1-7
36. Gold MA, Thomas MA, Huh WK, Sarto GE, Day SP. High-risk human papillomavirus detection in women with low-grade squamous intraepithelial lesions or higher-grade cytology using the Cervista HPV HR test. J Low Genit Tract Dis. 2013;17:51-7
37. Arbyn M, Ronco G, Anttila A. et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88-99
38. Kyrgiou M, Athanasiou A, Kalliala IEJ. et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst Rev. 2017;11:Cd012847
Author contact
![]() Corresponding authors: Pengming Sun, M.D., Ph.D., Professor, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, 18 Daoshan Road, Fuzhou 350001, Fujian, P.R. China (Phone: +86-591-87558732; Fax: +86-591-87551247; E-mail: fmsun1975edu.cn); Binhua Dong, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, 18 Daoshan Road, Fuzhou 350001, Fujian, P.R. China (Phone: +86-591-87558732; Fax: +86-591-87551247; E-mail: dbh18-jycom; pandaedu.cn).
Corresponding authors: Pengming Sun, M.D., Ph.D., Professor, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, 18 Daoshan Road, Fuzhou 350001, Fujian, P.R. China (Phone: +86-591-87558732; Fax: +86-591-87551247; E-mail: fmsun1975edu.cn); Binhua Dong, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, 18 Daoshan Road, Fuzhou 350001, Fujian, P.R. China (Phone: +86-591-87558732; Fax: +86-591-87551247; E-mail: dbh18-jycom; pandaedu.cn).

 Global reach, higher impact
Global reach, higher impact