Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(14):4362-4371. doi:10.7150/jca.57076 This issue Cite
Review
Receptor-interacting protein in malignant digestive neoplasms
Department of General Surgery, Renmin Hospital of Wuhan University, Jiefang Road 238, Wuhan, Hubei 430060, China.
Received 2020-12-12; Accepted 2021-4-22; Published 2021-5-19
Abstract
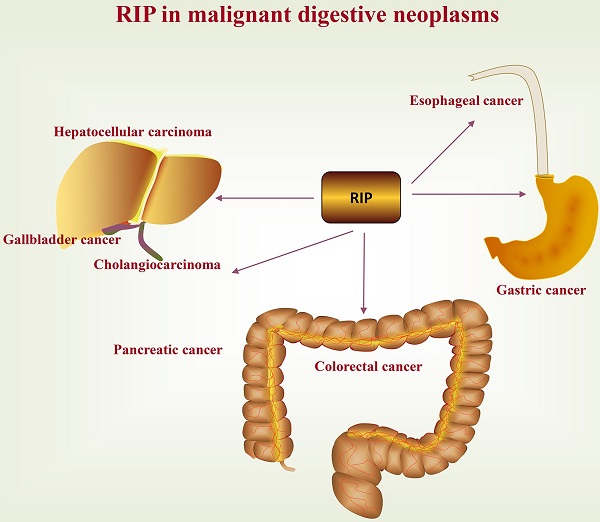
A deep and comprehensive understanding of factors that contribute to cancer initiation, progression, and evolution is of essential importance. Among them, the serine/threonine and tyrosine kinase-like kinases, also known as receptor interacting proteins (RIPs) or receptor interacting protein kinases (RIPKs), is emerging as important tumor-related proteins due to its complex regulation of cell survival, apoptosis, and necrosis. In this review, we mainly review the relevance of RIP to various malignant digestive neoplasms, including esophageal cancer, gastric cancer, colorectal cancer, hepatocellular carcinoma, gallbladder cancer, cholangiocarcinoma, and pancreatic cancer. Consecutive research on RIPs and its relationship with malignant digestive neoplasms is required, as it ultimately conduces to the etiology and treatment of cancer.
Keywords: Receptor Interacting Proteins, malignant digestive neoplasms, Therapeutics, Necroptosis
Introduction
Receptor Interacting Proteins (RIPs), also known as receptor interacting protein kinases (RIPKs), are a seven-member family of serine/threonine and tyrosine kinase-like kinases, including RIP1-7, which acts as a critical sensor of intracellular and extracellular stimuli and plays an important role in cell death, immune response and inflammation [1]. These proteins share a homologous serine-threonine kinase domain (KD), and RIP2 displays an additional tyrosine kinase activity [2]. In addition to the homologous kinase domain, RIP1, the founding member of the family, has a C-terminal death domain (DD) which mediates death-receptor signaling, and a bridging intermediate domain (ID) which includes a RIP homotypic interaction motif (RHIM); RIP2 bears a C-terminal caspase activation and recruitment domain (CARD) and an ID without RHIM; RIP3 only contains a C-terminal RHIM domain alongside the N-terminal KD; RIP4 and RIP5 harbor an ID without RHIM and a C-terminal ankyrin repeat domains (ARD), while RIP6 and RIP7 are structurally less similar to other members. They incorporate many additional domains, such as ARD, lceucine-rich repeats domains (LRRD), Ras (GTPase) of complex proteins (Roc) and C-terminal of Roc (COR) domains, and WD40 repeats domains (WDRD) [2]. For a detailed discussion of the biology of RIPs, we refer readers to a recent review [3]. Here, we mainly review the relevance of RIP to various malignant digestive neoplasms. Consecutive research on RIP and its relationship with malignant digestive neoplasms is needed, as it ultimately conduces to the etiology and treatment of cancer.
Role of RIPs in cell survival, apoptosis, and necrosis
As we all know, apoptosis, a programmed cell death mechanism, acts as a natural barrier to prevent the development of cancer. However, resistance and evasion to apoptosis are also deemed incontrovertible features of cancer [4], and apoptosis resistance usually also contributes to tumorigenesis and drug resistance, leading to chemotherapy failure [5]. Thus, excepting overcoming resistance to apoptosis, the development of methods to induce non-apoptotic forms of programmed cell death is imperative and attractive as an alternative therapy for cancer. Recently, necroptosis, also known as programmed necrosis, was found as a novel and regulable form of cell death that has a morphological similarity to necrosis and a mechanistic similarity to apoptosis [6]. As a combination of apoptosis and necrosis, necroptosis has been shown to have the following dual effects on cancer [7]: on the one hand, the core mediators of this pathway alone or in combination have been thought to contribute to cancer metastasis and progression [8, 9]; On the other hand, it was also reported to be a "fail-safe" mechanism that prevents tumor development when apoptosis is compromised [10, 11].
Currently, RIP1 is one of the most intensively investigated types, which is considered as a crucial molecule in the pathways in regulating cell survival, apoptosis, and necroptosis. RIP1 acts downstream of some receptors, such as tumor necrosis factor receptor (TNFR)1, Toll-like receptor (TLR)3, TLR4, and death receptors (DRs), of which TNFR1 signaling is the most classic pathway[12-15]. Following TNF activation of TNFR1, the latter undergoes trimerization that induces the formation of a receptor proximal membrane-signaling complex (Complex I) via sequential recruitment of RIP1, TNFR1-associated death domain protein (TRADD), TNF receptor-associated factor (TRAF) 2/5, cellular inhibitors of apoptosis (cIAP) 1/2, and Linear ubiquitin chain assembly complex (LUBAC) subunits (E3 ubiquitin ligase complex that includes HOIP, HOIL1, and SHARPIN and adds M1-linked linear polyubiquitin chains on RIP1 and other Complex I components), transforming growth factor (TGF) β-activated kinase 1 (TAK1)/TANK-binding kinase (TAB) 2/3, and nuclear factor kappa B (NF-κB) essential modulator (NEMO)/IκB kinase (IKK) 1/2 complexes [16-19]. Within this complex, RIP1 is a key regulator of cell fate [20]. Subsequently, several ubiquitination and phosphorylation events come up at Complex I that promote cell survival, including the TAK1/MK2(mitogen-activated protein kinase (MAPK)-activated protein kinase 2)-, IKK1/2- and TBK1-dependent phosphorylation of RIP1 (early cell death checkpoint) and the TAK1- and IKK-dependent activation of the NF-kB signaling pathway (late cell death checkpoint) [21-26]. Nuclear translocation of NF-κB transcription factors regulates the expression of proinflammatory and prosurvival genes [27], including pro-survival factor and caspase-8 homolog cellular Fas-associated DD-like interleukin-1β converting enzyme inhibitory protein (c-FLIP) [28], the members of the inhibitor of apoptosis protein (IAP) family [29] and the anti-apoptotic Bcl-2 family [30]. Furthermore, because of the quick internalization of ligand-bound TNFR, those proteins in complex I and their posttranslational modification are consequently changed [31]. For instance, the second mitochondria-derived activator of caspases (Smac) mimetic-induced degradation of cIAPs causes the de-ubiquitination of RIP1 by the de-ubiquitinating enzyme de-ubiquitinating enzyme (CYLD) and/or A20 [32-34]. Consequently, complex I converts into a cytoplasmic death-inducing signaling complex (Complex II), including FADD, TRADD, caspase-8, and RIP1, which is also referred to as “Ripoptosome”, is formed, inducing caspase-8 activation and apoptosis [35, 36]. When the activity of caspase-8 is inhibited by pharmaceutical or genetic intervention, RIP1 associates with RIP3 via their RHIM domains to shape a crucial GLU protein complex (necrosome). This can cause activation and autophosphorylation of RIP3 that in turn recruits the pseudo-kinase mixed lineage kinase like (MLKL). MLKL phosphorylation induces its translocation to the plasma membrane and forms pores that result in necroptotic cell death [15, 37, 38].
RIPs and esophageal cancer
Esophageal cancer (EC) is a serious malignant tumor of the upper gastrointestinal tract with high mortality and poor prognosis [39]. Recently, RIP3 expression level in esophageal squamous cell carcinoma (ESCC) has been described. The cytoplasmic expression of RIP3 mRNA and protein was significantly down-regulated in ESCC tumor tissues. And the expression of RIP3 was more inadequate in the more advanced and invasive tumors [40]. Besides, RIP3 deficiency was associated with poor prognosis and cisplatin resistance in ESCC patients who had received cisplatin chemotherapy, and re-expression of kinase-dead RIP3 can restore cisplatin sensitivity [40]. Further functional analyses found that RIP3 depletion stimulated the DNA repair pathway to potentiate cisplatin chemoresistance. Specifically, the deficient expression of RIP3 upregulated POLD1 and FOS Like Antigen 1 (FOSL1) via activation of the ERK phosphorylation and HSP90/CDC37 complex in ESCC cell lines [40]. POLD1 is the DNA polymerase delta catalytic subunit and involves in nuclear excision repair [41, 42]. Knockdown of POLD1 reduced repair activity and sensitized cells to reagents that cause DNA damage. Also, FOSL1 was upregulated in ESCC tumors, and the down-regulation of FOSL1 expression can inhibit cell proliferation and metastasis [43]. Thus, RIP3 may be a useful marker for predicting chemotherapy sensitivity and prognosis of EC patients. And the RIP3 levels in tumor tissues may guide the chemotherapeutic regimen in the clinic.
RIPs and gastric cancer
Gastric cancer (GC), a highly fatal disease, is a common malignancy type of gastrointestinal tract worldwide, with earlier stages being asymptomatic and difficult to detect, by the time of diagnosis it reaches the advanced stages [44]. A recent study reported [45] that RIP1 was strongly associated with the occurrence and development of GC. The high expression of RIP1 was detected in GC tissues, while low expression of RIP1 was found in the normal gastric tissues [45]. Importantly, immunohistochemistry staining revealed the RIP1 immunoreactivity was positive at the site of invasion with little or no immunoreactivity in the interstitial substance. And the RIP1 expression was significantly positively related to the clinical stage and lymph node metastasis of GC patients, which revealed that RIP1 may promote growth and invasion of GC [45]. Further analysis showed the GC patients with low expression of RIP1 had a better prognosis than the patients with high expression of RIP1. Mechanistic studies found that the RIP1-NF-κB/AP-1-VEGF-C signaling pathways play a vital role in regulating the biological functions of human GC cell lines (HGC and AGS) [45]. Among them, the VEGF-C is an essential factor for lymph node metastasis in the GC [46]. The nude mice model with subcutaneous xenograft tumors further verified the above findings. Thus, RIP1 promotes the growth and invasion of GC in vitro and in vivo, targeting the overexpressed RIP may be used in the treatment of GC patients. Interestingly, the study also found that the expression of RIP1 was significantly higher in GC patients with Helicobacter pylori infection. The reason may be that Helicobacter pylori infection causes inflammation, which increases the RIP1 expression [47].
Also, studies have also found that RIPs may be involved in anti-gastric cancer treatment. Celastrol, isolated from the root of Thunder of God Vine, has anti-inflammatory and anti-cancer activity. Guo et al. confirmed [48] that celastrol can activate the RIP1/RIP3/MLKL pathway by down-regulating biglycan to result in the necroptosis of HGC-27 and AGS cell. Previous research reported that biglycan expression was upregulated in GC tissues to enhance GC invasion [49]. Furthermore, RIP3 knockdown, apoptosis inhibitor (Z-VAD-fmk) or necrostatin-1 (Nec-1), the first well-established necroptosis inhibitor that exclusively suppressed RIP1 activity [50], all can in part rescue celastrol-induced GC cell death. Vetrivel et al. [51] also came up with similar results through in vitro, vivo experiments, and silico molecular docking and simulation studies. They found that prunetin induced necroptosis-mediated human AGS cell death via activating the RIP3, leading to the phosphorylation of MLKL. As the third-generation of platinum anti-cancer drugs, oxaliplatin is widely used in various cancers, which works by binding to DNA to produce oxaliplatin-DNA conjugates. However, the mechanism by which the DNA conjugate kills cells is not fully clear. Recently, Wu et al. [52] demonstrated that oxaliplatin killed human GC cell SGC-7901 primarily by necroptosis relying on RIP1, and Nec-1 significantly inhibited oxaliplatin-induced cell death.
RIPs and colorectal cancer
Colorectal cancer (CRC) is the third most common human cancer and the second leading cause of cancer death worldwide [53]. It is considered a multifactorial disease, whose etiology is relevant to genetic factors, environmental exposure, and inflammatory conditions of the intestinal tract [54]. However, the underlying mechanisms of CRC carcinogenesis remain unclear. Recently, some studies suggested that RIP1 may be involved in CRC colorectal cancer oncogenesis. For instance, Zeng et al. [55] discovered that, compared to normal para carcinoma tissue, the RIP1 protein and mRNA expression levels were upregulated significantly in human CRC tissues, and RIP1 protein levels were positively related to the stage of cancer progression and 3 years mortality rate. Further analysis showed that the mitochondrial Ca2+ uniporter (MCU), an evolutionarily conserved Ca2+ channel, was a crucial interacting directly downstream component of RIP1 in promoting cellular proliferation in the colon cancer cell line of HT29 by increasing energy metabolism and mitochondrial Ca2+ uptake. Besides, the study also found that knockdown of RIP1 or MCU could significantly impede cancer growth in a nude mouse tumor inoculation model. Thus, targeting RIP1 and MCU may be a new strategy for the treatment and prevention of CRC. Moriwaki et al. [56] drew the opposite conclusion. They found that, compared with adjacent normal colon tissues, the expression of RIP1 and RIP3 was remarkably decreased in human colon cancer tissue. As we know, the expression of tumor suppressor genes is often silenced in cancer tissues by epigenetic DNA modifications such as DNA methylation and histone deacetylation [57]. However, Moriwaki et al found that RIP1 and RIP3 expression were suppressed by hypoxia, a hallmark of solid tumor, but not by epigenetic DNA modification. Hypoxia has been implicated to promote cancer progression by activating adaptive transcriptional programs that promote cell survival and angiogenesis [58].
Other studies also found that the expression of RIP3 was significantly decreased in human CRC tissues compared with adjacent normal tissues [11, 40, 59]. The down-regulation expression of RIP3 was associated with some disadvantageous clinicopathologic parameters such as T stage, M stage, and AJCC stage [11], and damaged the colon cancer cells' response to necroptosis triggers [56]. Overexpression of RIP3 can suppress proliferation, migration, and invasion of CRC cell lines in vitro [11]. COX risk model analysis indicates that the expression level of RIP3 was an independent prognostic factor for disease-free survival and overall survival in CRC patients, and RIP3 negative patients had a remarkably lower survival rate than patients with RIP3 positive staining [11]. The downregulation of RIP3 in tumor-infiltrating myeloid-derived suppressor cells (MDSCs) potentiates NF-kB activation and COX-2-derived prostaglandin E2 (PGE2) production. PGE2, in turn, further decreases RIP3 expression level and promotes the immunosuppressive activity of MDSCs and colorectal carcinogenesis [60]. Bozec et al. [59] revealed that mice with RIP3-deficient were highly susceptible to colitis-associated CRC and displayed greater production of tumor-promoting factors and pro-inflammatory mediators. The tumorigenesis of RIP3 deficiency results from uncontrolled activation of AKT, STAT3, NF-κB, and Wnt-β-linked protein signaling pathways that promote the proliferate abnormally of intestinal epithelial cells (IECs) and CRC. Thus, RIP3 may play a key anti-inflammatory and anti-tumoral functions in the intestine. Besides, Conev et al. [61] revealed that metastatic colon cancer patients with a high level of RIP3 expression were related to longer overall survival and progression free survival, and lower risk of disease progression. This study also found that patients with high expression of RIP3 had a significantly higher response rate to 5-fluorouracil based chemotherapy regimens, which indicated RIP3 expression levels might be a potential predictive marker for response rate. However, at the level of RIP3 expression, Liu et al. and He et al. came to the opposite conclusion. They revealed that RIP3 expression levels were upregulated in human and mouse colonic cancers and mouse colitis-associated cancer (CAC) [62, 63], and RIP3 deficiency decreased significantly colitis-associated tumorigenesis. Mechanistic studies revealed that, on the one hand, RIP3 enhanced the proliferation of premalignant intestinal epithelial cells to facilitate the progression of CAC via activating JNK signaling; on the other hand, RIP3 promoted the myeloid cell-induced adaptive immune suppression to contribute to the CAC progression by CXCL1 signaling to increase the number of myeloid-derived suppressor cells and M2c-like macrophage tumor-associated macrophages while decreasing T-cell accumulation, infiltration and activation. Therefore, activation and expansion of T cells by the impediment of RIP3 signaling are a promising way to raise T cell activity and strengthen the effectiveness of cancer immunotherapy [62]. The different expression patterns of RIP1 and RIP3 in colon cancer may be a consequence of its pleiotropic functions in multiple signaling pathways, and the expression of RIP1 and RIP3 in CRC patients deserves further study.
Apart from interfering with the development of CAC, RIP also affects anti-colorectal cancer treatment. Currently, 5-Fluorouracil (5-FU), irinotecan (the active metabolite of which is SN38), and oxaliplatin are the major chemotherapeutic agents in the treatment of CRC. However, CRC patients have a low response rate to these agents, and even with positive treatments, the 5-year survival rate of patients with advanced diseases is < 10%. Thus, increasing these agent sensitivities in resistant tumors remains an urgent challenge in the chemotherapy of CRC. Recently, some scholars have found that RIP1 can affect the sensitivity of anti-colorectal drugs. Metzig et al. [64] revealed that 5-FU and pan-caspase inhibitor Z-VAD-induced necroptosis in CRC cells depends on RIP1 and RIP3, and knockdown of RIP1 and RIP3 significantly rescued cells from 5-FU plus Z-VAD-induced necroptosis. Other scholars have found that the downregulation of RIP1 can increase resistance to topoisomerase inhibitor 7-Ethyl-10-hydroxycamptothecin (SN38) in the colon cancer cell line of HT29 and HCT116 under hypoxic conditions or normoxic, and SN38 alone and in combination with TNF declined the expression RIP1 in the treatment for HT29 cells. RIP1 knockdown HT29-derived tumor xenograft models have demonstrated that RIP1 was required for tumor growth and the tumors that lack RIP1 grow more slowly. Further, the therapeutic benefit of irinotecan was apparent in the control mice, while the tumor mice of RIP1 knockdown had no obvious response to irinotecan [65].
Resibufogenin, derived from toad venom, triggers necroptosis to inhibit growth and metastasis of colorectal cancer via upregulating RIP3 and MLKL. Besides, resibufogenin also activated the expression of glycogen phosphorylase (PYGL), glutamine synthetase (GLUD1), and glutamate dehydrogenase (GLUL) in a RIP3-dependent manner to promote a considerable metabolic burst [66]. This in turn propagates the overgeneration of ROS, thereby favoring mitochondrial dysfunction and necroptosis [67, 68]. Mishra et al. [69] discovered that the overexpression of GLTP, encoding a 24 kD amphitropic lipid transfer protein, can induce cell death by necroptosis in HT-29 colon cancer cells as explained by RIP3-induced the phosphorylation of mixed lineage kinase domain-like protein (pMLKL), elevated intracellular Ca2+ levels and the permeabilization of the plasma membrane by pMLKL oligomerization, but not in HCT-116 or normal colonic cells. To further determine the RIP3 dependence of GLTP-induced HT-29 cell death, the author depleted RIP3 or MLKL, and found the above-mentioned conditions invalidated necroptosis induced by the overexpression of GLTP. Some scholars have also pointed out that 2-methoxy-6-acetyl-7-methyljuglone (MAM) can promote the death of HCT116 and HT29 colon cancer cells, and RIP1/RIP3 complex-induced cytosolic calcium accumulation is a key mediator in MAM-induced necroptosis in human colon cancer cells via mitochondrial ROS production and sustained JNK activation [70].
In addition to necroptosis, RIP3 is also involved in other non-apoptotic programmed cell death pathways that lead to CRC cell death, such as autophagy. Hou et al [71] first discovered that chloroquine (CQ) upregulated the expression of RIP3 in CT26 cancer cells, and RIP3 can participate in CQ-related autophagy. The combination of mRIP3 and CQ had a significant antitumor effect in vitro and in vivo experiments. Furthermore, this combination can enhance lysosomal membrane permeabilization via increasing autophagic flux and inducing RIP3-dependent necroptosis, but apoptosis was not increased.
RIPs and hepatocellular carcinoma
Hepatocellular carcinoma (HCC) accounts for the fourth leading cause of cancer death globally and has highly invasive and aggressive behavior with 5-year survival rates of 3%-11% [72, 73]. Recently, some scholars have found that patients with higher expression of RIP1 in HCC tissues suffered from poor post-surgical survival. The RIP1 expression was significantly increased in HCC tissues than in adjacent normal liver tissues from 81.9% of HCC patients. And RIP1 overexpression was found positively related to advanced TNM staging, intrahepatic metastases, portal vein invasion, and HBV infection [74]. Mechanistic studies revealed that RIP1 exerted its function on HCC progression through the activation of the AKT/Bcl-2/BAX signaling pathway [74]. However, Schneider et al. [75] reached the opposite conclusion. They revealed that low expression of RIP1 and TNF receptor-associated factor 2 (TRAF2) in HCC was related to poor prognosis. Further analysis showed RIP1 deficiency in liver parenchymal cells (LPC) enhanced the degradation of TNF-induced TRAF2, causing liver injury. And the loss of both RIP1 and TRAF2 in LPC promoted the spontaneous development of HCC, which suggests that RIP1 collaborates with TRAF2 to suppresses hepatocarcinogenesis [75]. Besides, Van et al. investigated the role of RIP1 kinase-dependent and -independent functions in hepatic homeostasis, hepatocyte death, liver injury, and hepatocarcinogenesis. This work has demonstrated that kinase-independent RIP1 functions (scaffolding functions) with NF-κB to prevent hepatocyte apoptosis, chronic liver disease, and cancer under steady-state conditions. However, when NEMO is absent, RIP1 kinase activity drives hepatocyte apoptosis, chronic liver disease, and HCC development [76]. Recently, RIP6 has also been found to inhibit the growth of hepatocellular carcinoma cells. The work revealed that, compared to adjacent normal tissues, RIP6 was significantly down-regulated in human HCC tissue. And, the low expression of RIP6 was positively associated with tumor size in HCC patients. Further studies found that RIP6 induced HCC cell apoptosis to suppress cancer progress, and inhibited HCC cell proliferation to further prevent cancer growth [77].
The IKK complex consists of the catalytic subunits IKKα and IKKβ, and the regulatory subunit NEMO. IKKα and IKKβ, known function in NF-κB activation, also directly phosphorylate RIP1 at different regions of the protein to regulate cell viability [78]. Loss of this IKKα/β-dependent RIP1-phosphorylation in LPC impedes compensatory hepatocytes and intrahepatic biliary cell s proliferation, thus inhibiting the development of HCC but inducing biliary cell paucity and lethal cholestasis [79]. Kondylis et al. [80] discovered that mice lacking NEMO in LPC spontaneously develop steatohepatitis and HCC. RIP1 kinase activity-induced hepatocyte apoptosis can drive HCC in NEMOLPC-KO mice. And NEMO prevents hepatocarcinogenesis by inhibiting RIP1 kinase activity-mediated hepatocyte apoptosis via NF-kB-dependent and NF-kB -independent functions [80]. Interestingly, Aigelsreiter et al. [81] reported that loss of NEMO immunoreactivity in a considerable percentage of human HCC tissues, which related to a poor 5-year overall survival. Thus, the inhibition of RIP1 could provide a likely well-tolerated and effective therapy choice for this subset of patients. Besides, Clinical practice has shown that inhibition of RIP1 promotes the anti-tumor effect of pirarubicin by the RIP1-AKT-P21-dependent pathway to overcome chemoresistance in HCC [82].
HepG2/DDP cells, a human liver cancer cell line HepG2 cisplatin-resistant counterpart, are resistant to cisplatin-induced apoptosis due to downregulation of pro-apoptotic genes (Bad and Bax) and upregulation of anti-apoptotic genes (Bcl-XL and Bcl-2) [83]. Zhang et al. [84] discovered that ectopic expression of RIP3 prompted cisplatin-induced HepG2/DDP cell death. And this type of cell death was necroptosis and relied on the RIP1-RIP3-MLKL signaling pathway owing to inhibition of MLKL activity by necrosulfonamide (NSA) or knockdown of RIP1 significantly reduced cisplatin-induced cell death. The author also found that the high level of RIP3 expression was closely related to better response rates to cisplatin clinical treatment in HCC patients. However, the expression of RIP3 was usually inhibited in liver tumor cells due to genomic methylation. Thus, RIP3-dependent activation of necroptosis was also mostly repressed during chemotherapy. A previous study reported that hypomethylating agents could restore RIP3 expression level in some cancer cells, enhancing their sensitivity to chemotherapeutics-induced cell necroptosis [85]. Therefore, the combined use of hypomethylating agents and chemotherapeutics may be a better choice for the treatment of HCC patients. The result of Li et al. [86] and Sun et al. [40] is consistent with the above findings. They also confirmed that the expression of RIP3 is declined in HCC patients, which correlates with myeloid-derived suppressor cell (MDSC) infiltration and poor prognosis. RIP3 deficiency facilitates CXCL1/CXCR2-induced MDSC chemotaxis to promote immune escape and HCC progression. And MDSC is regarded as a major suppressor of T-cell cytotoxicity and a major mechanism adopted by cancers to escape immune surveillance [87, 88]. Other scholars also have reported that RIP3 hinders inflammatory hepatocarcinogenesis but induces cholestasis via controlling caspase-8-and Jun-(N)-terminal kinase (JNK)-dependent compensatory cell proliferation [89].
Liver oval cells (OCs) have highly proliferative activity, which when disrupted may promote HCC [90]. Interestingly, WÓJCIK et al. [91] indicated that, compared with the non-neoplastic OCs, the OCs of neoplastic livers possess high proliferative activity with apoptosis suppression, and their necroptosis potential was preserved, which was reflected by the pronounced RIP3 expression and unchanged RIP1 expression. However, the authors also suggest that although this response strengthens necroptotic cell death, it is not sufficient to disrupt the highly proliferative activity of neoplastic OCs.
RIPs and gallbladder cancer
Gallbladder cancer (CA) is a very rare, highly-lethal, highly invasive and aggressive, and carries a dismal prognosis disease. The 5-year survival rate for all stages of CA patients is about 5% [92, 93]. The high expression of RIP1 was found in the CA tissues, but low expression of RIP1 was detected in the normal gallbladder tissues. The patients with IV~V clinical stage, lymph node metastasis, and gallstones had higher levels of RIP1 expression, which indicated that RIP1 may promote tumor invasion and growth [94]. Notably, gallstones can cause inflammation of the gallbladder, which can increase RIP1 expression [47], while the direct relationship between gallstones and CA has long been established [95]. Intriguingly the subcutaneous xenograft tumors model confirmed that RIP1 promoted tumor growth in nude mice [94]. And silencing of RIP1 in gallbladder cell lines NOZ significantly repressed proliferation and invasiveness by downgrading the RIP1-NF-κB(p65)/C-jun(AP-1)-VEGF-C pathways [94]. Thus, targeting RIP1 for CA may be a promising option.
RIPs and cholangiocarcinoma
Cholangiocarcinoma (CCA) is an aggressive malignancy originating from the epithelium of extrahepatic and intrahepatic bile ducts, leading to high mortality and recurrence/metastasis rates and subsequently poor clinical outcomes, with a relatively low 5-year survival rate (5-10%) [96, 97]. Recently, Sacchi et al. [98] confirmed that RIP played an important role in the growth and treatment of CCA. The RIP3, RIP1, and pMLKL were highly expressed in the intrahepatic CCA patients. Importantly, these necroptosis-associated proteins were found to be negative correlated with the presence of perineural invasion and nodal metastasis. And the patients with higher RIP3 and RIP1 expression have a significantly longer overall survival [98]. Another study analyzing RNA sequencing data from The Cancer Genome Atlas (TCGA) database found that both RIP3 and MLKL mRNA were upregulated in CCA tissues compared to in normal bile ducts, while RIP1 mRNA was similarly expressed between the two groups [99]. However, A clinical study by Xu et al. [100] revealed that, compared to adjacent normal tissues, RIP3 was lower but still moderately expressed in most CCA tissue. Therefore, the relationship between RIP1, RIP3 and the development of CCA needs further study.
Besides, the role of RIPs in the treatment of CCA has also attracted the attention of relevant scholars. They revealed that the Smac mimetic, an inhibitor of apoptosis protein (IAP) antagonist, sensitized CCA cells to low-dose standard chemotherapy, gemcitabine, and induced necroptosis in a RIP1/RIP3/MLKL-dependent manner based on caspase inhibition, but not in non-tumor cholangiocytes [99]. Xu et al. [100] showed that matrine, an alkaloid derived from traditional Chinese medicine Sophora flavescens, can induce necroptosis in CCA cell lines. CCA cells with matrine therapy displayed typical necrosis-like instead of apoptotic morphology changes, and these effects were considerably weakened by Nec-1, but not apoptosis inhibitor z-VAD-fmk. Further analysis showed matrine could enhance RIP3 expression level and the following RIP3/MLKL/ROS signaling pathway, which may contribute to the necroptosis process. And necroptosis was switched to apoptosis after knocking down RIP3 [100]. The findings have pivotal suggestions for designing a new necroptosis-based therapeutic ideal to overcome CCA.
RIPs and pancreatic cancer
Pancreatic cancer (PC) is one of the deadliest solid cancers, with a 5-year survival rate of only about 6%, and patients with PC have the lowest survival rate of all patients with cancer [101, 102]. Recently, Seifert et al. reported that RIP1, RIP3, FADD, caspase 8, and MLKL were highly expressed in pancreatic ductal adenocarcinoma (PDA) [103]. The deletion of RIP3 or inhibition of RIP1 prevented the progression of PDA in mice in vivo and was related to the development of T cell infiltrate and a highly immunogenic myeloid. Mechanistic studies revealed that the immunosuppressive tumor microenvironment related to intact RIP1/RIP3 signal was partially dependent on the expression of necroptosis-induced chemokine attractant CXCL1, and blockade of the CXCL1 can protect against PDA [103]. Besides, cytoplasmic SAP130, a subunit of the histone deacetylase complex, was upregulated in a RIP1/RIP3-dependent manner in PDA. Mincle, SAP130 cognate receptor which can promote sterile inflammation by ligating SAP130 [104, 105], was upregulated in PDA tumor-infiltrating myeloid cells. Mincle deletion can protect against oncogenesis [103]. Thus, the RIP1 and RIP3 promote PC progression through mincle and CXCL1-induced immune suppression. Previous studies have found that PC patients with preoperative serum CEA+/CA125+/CA19-9 ≥ 1000 U/ml usually had poor surgical outcomes and were more likely to develop distant metastases after radical surgery [106]. A recent study [107] reported that RIP4 was one of the most key genes upregulated in this subgroup of patients. Further analysis showed the overexpression of RIP4 significantly stimulated PC cell invasion and migration by the proteasome-mediated phosphatidylethanolamine binding protein 1 (PEBP1) degradation-induced activation of the RAF1/MEK/ERK pathway. Among them, PEBP1, as a physiological endogenous inhibitor of the RAF1/MEK/ERK pathway, can impede the proliferation, migration, and invasion of PC cells [108].
The evasion of apoptosis is a critical mechanism of PC treatment resistance and leads to a poor prognosis [109]. Some studies have found that necroptosis is a novel and alternative treatment strategy to induce programmed cell death in PC cells with apoptosis-resistant. For instance, Hannes et al. [110] revealed that the Smac mimetic BV6 can switch TNF-α into a death signal to induce necroptosis in PC cells when caspase activation was blocked. BV6 promoted the production of TNF-α and the formation of RIP1/RIP3-containing necrosome complex in PC cells. The pharmacological inhibition of RIP1, RIP3, or MLKL remarkably reduced BV6-induced cell death. Also, Doxorubicin sensitized human PC and CRC cells to Smac mimetic through synergistically activating CYLD/RIP1/FADD/caspase-8-dependent apoptosis. And RIP1 is critical for the synergistic induction of apoptosis by Smac mimetic and doxorubicin, while it is irrelevant for cell death caused by doxorubicin alone [111]. Chen et al. [112] found that Shikonin, a naphthoquinone isolated from the Chinese medicinal herb Lithospermum erythrorhizon, can up-regulate RIP1 and RIP3 expression to induce apoptosis and necroptosis in different PC cells, and also strengthen the anti-tumor activity of gemcitabine. Other scholars have proposed that gemcitabine treatment can induce the components of the necrosome by increasing the expression level of RIP1 and RIP3 in PDA in vivo and in vitro [103]. The combinatorial treatment of gemcitabine and CD95 ligand has also been demonstrated to induce apoptotic and RIP1-mediated necroptotic cell deaths in PC cells, and gemcitabine significantly switched CD95L-induced cell death into necroptosis [113].
In addition to chemotherapy and natural compounds that induce necroptosis in PC, metal nanoparticles also can induce necroptotic cell death. Silver nanoparticles, as metal nanoparticles, have received increasing attention and were regarded as a promising anticancer agent due to their unique functions to easily cross biological barriers and penetrate cell membranes to induce apoptosis in cancer cells [114-116]. Based on a study performed by Zielinska et al., in human pancreatic cancer cells (PANC-1 cells), silver nanoparticles can promote both necroptosis and apoptosis and considerably increase the levels of proteins related to necroptosis and autophagy, including RIP1, RIP3, MLKL, and LC3-II. The author also pointed out that silver nanoparticles markedly inhibited the proliferation and reduced the viability of PANC-1 cells. And, PC cells were more remarkably sensitive than nontumor PC to silver nanoparticles-induced cytotoxicity [114]. Thus, silver nanoparticles-induced cell death may provide a new therapeutic strategy to overcome chemoresistance in PC cancer.
Conclusion
Overall, significant progress has been made in the field of RIPs biology, and there is a strong link between RIPs and the development and treatment of gastrointestinal malignant cancers. Continued in-depth research in this area will contribute to enhance our understanding of cancer control. However, as shown in Table 1, the study of RIPs in colorectal cancer, hepatocellular carcinoma, and cholangiocarcinoma remains controversial. In addition, current studies have focused on RIP1 and RIP3 and gastrointestinal malignancies, while no studies have been conducted on RIP2, PIR5, or RIP7. Recent studies have found that RIP2 promotes cell survival by activating NF-ĸB in triple-negative breast cancer cells [117]. Thus, whether RIP2, RIP5, and RIP7 are involved in the development of malignant tumors in the gastrointestinal tract deserves further investigation. Finally, RIPs play an important role in a variety of antitumor therapies (Table 2), either by inducing necroptosis to bypass acquired or intrinsic apoptotic resistance or by increasing sensitivity to antitumor drugs. Thus, RIPs kinases are regarded as potential and promising therapeutic targets for cancer.
Expression of RIPs in malignant digestive neoplasms and its influence on cancer prognosis
| Cancer Type | Expression of RIPs | The influence on prognosis | Reference |
|---|---|---|---|
| Esophageal cancer | Decrease PIR3 expression | Poor prognosis; cisplatin resistance; enhanced tumorigenesis | [40] |
| Gastric cancer | Increase RIP1 expression | Poor prognosis; enhanced tumorigenesis | [45] |
| Colorectal cancer | Increase RIP1 expression | Poor prognosis; enhanced tumorigenesis | [55] |
| Colon cancer | Decrease RIP1/RIP3 expression | Promoted oncogenesis | [56] |
| Colorectal cancer | Decrease RIP3 expression | Poor prognosis; enhanced tumorigenesis | [11, 40, 59] |
| Colorectal cancer | Increase RIP3 expression | Promoted oncogenesis and tumorigenesis | [62, 63] |
| Hepatocellular carcinoma | Increase RIP3 expression | Poor prognosis; enhanced tumorigenesis | [74] |
| Hepatocellular carcinoma | Decrease RIP6 expression | Inhibited tumorigenesis | [77] |
| Hepatocellular carcinoma | Decrease RIP3 expression | Poor prognosis | [40, 86] |
| Gallbladder cancer | Increase RIP1 expression | Enhanced tumorigenesis | [94] |
| Intrahepatic cholangiocarcinoma | increase RIP1/RIP3 expression | Good prognosis; inhibited tumorigenesis | [98] |
| Cholangiocarcinoma | Increase RIP3 expression; while RIP1 remains unchanged | [99] | |
| Cholangiocarcinoma | Decrease RIP3 expression | [100] | |
| Pancreatic cancer | Increase RIP1/RIP3 expression | Promoted oncogenesis | [103] |
| Pancreatic cancer | Increase RIP4 expression | Enhanced oncogenesis and tumorigenesis | [107] |
Compounds that induce cell death in cancer therapy
| Compounds and Agents | Antitumor Mechanism | Cancer Type | Reference |
|---|---|---|---|
| Celastrol | RIP1/RIP3/MLKL pathway dependent | Gastric cancer | [48] |
| Prunetin | RIP3 dependent | Gastric cancer | [51] |
| Oxaliplatin | RIP1 dependent | Gastric cancer | [52] |
| Resibufogenin | Upregulating RIP3 and MLKL | Colorectal cancer | [66] |
| GLTP | Upregulating RIP3 and MLKL | Colorectal cancer | [69] |
| 2-methoxy-6-acetyl-7-methyljuglone | Relying on RIP1/RIP3 complex | Colorectal cancer | [70] |
| Chloroquine | Upregulating RIP3 | Colorectal cancer | [71] |
| Smac mimetic | RIP1/RIP3/MLKL-dependent manner | Cholangiocarcinoma | [99] |
| Matrine | Relying on RIP3/MLKL/ROS signaling pathway | Cholangiocarcinoma | [100] |
| Smac mimetic BV6 | Formatting RIP1/RIP3-containing necrosome complex | Pancreatic cancer | [110] |
| Shikonin | Upregulating RIP1 and RIP3 | Pancreatic cancer | [112] |
| Gemcitabine | Upregulating RIP1 and RIP3 | Pancreatic cancer | [103] |
| Silver nanoparticles | Upregulating RIP1 and RIP3 | Pancreatic cancer | [114] |
Competing Interests
The authors have declared that no competing interest exists.
References
1. Peixoto MS, de Oliveira Galvão MF, Batistuzzo de Medeiros SR. Cell death pathways of particulate matter toxicity. Chemosphere. 2017;188:32-48
2. He S, Wang X. RIP kinases as modulators of inflammation and immunity. Nat Immunol. 2018;19:912-22
3. Cuny GD, Degterev A. RIPK protein kinase family: Atypical lives of typical kinases. Semin Cell Dev Biol. 2021;109:96-105
4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
5. Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153-64
6. Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263-8
7. Seehawer M, Heinzmann F, D'Artista L, Harbig J, Roux PF, Hoenicke L. et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562:69-75
8. Strilic B, Yang L, Albarrán-Juárez J, Wachsmuth L, Han K, Müller UC. et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536:215-8
9. McCormick KD, Ghosh A, Trivedi S, Wang L, Coyne CB, Ferris RL. et al. Innate immune signaling through differential RIPK1 expression promote tumor progression in head and neck squamous cell carcinoma. Carcinogenesis. 2016;37:522-9
10. Höckendorf U, Yabal M, Herold T, Munkhbaatar E, Rott S, Jilg S. et al. RIPK3 Restricts Myeloid Leukemogenesis by Promoting Cell Death and Differentiation of Leukemia Initiating Cells. Cancer cell. 2016;30:75-91
11. Feng X, Song Q, Yu A, Tang H, Peng Z, Wang X. Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma. 2015;62:592-601
12. Annibaldi A, Meier P. Checkpoints in TNF-Induced Cell Death: Implications in Inflammation and Cancer. Trends Mol Med. 2018;24:49-65
13. Ting AT, Bertrand MJM. More to Life than NF-κB in TNFR1 Signaling. Trends Immunol. 2016;37:535-45
14. Peltzer N, Darding M, Walczak H. Holding RIPK1 on the Ubiquitin Leash in TNFR1 Signaling. Trends Cell Biol. 2016;26:445-61
15. Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311-20
16. Ikeda F, Deribe YL, Skånland SS, Stieglitz B, Grabbe C, Franz-Wachtel M. et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature. 2011;471:637-41
17. Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL. et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591-6
18. Kondylis V, Pasparakis M. RIP Kinases in Liver Cell Death, Inflammation and Cancer. Trends Mol Med. 2019;25:47-63
19. Mifflin L, Ofengeim D, Yuan J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat Rev Drug Discov. 2020;19:553-71
20. Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS. et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339-50
21. Jaco I, Annibaldi A, Lalaoui N, Wilson R, Tenev T, Laurien L. et al. MK2 Phosphorylates RIPK1 to Prevent TNF-Induced Cell Death. Molecular cell. 2017;66:698-710.e5
22. Dondelinger Y, Delanghe T, Rojas-Rivera D, Priem D, Delvaeye T, Bruggeman I. et al. MK2 phosphorylation of RIPK1 regulates TNF-mediated cell death. Nat Cell Biol. 2017;19:1237-47
23. Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ. et al. NF-κB-Independent Role of IKKα/IKKβ in Preventing RIPK1 Kinase-Dependent Apoptotic and Necroptotic Cell Death during TNF Signaling. Molecular cell. 2015;60:63-76
24. Xu D, Jin T, Zhu H, Chen H, Ofengeim D, Zou C. et al. TBK1 Suppresses RIPK1-Driven Apoptosis and Inflammation during Development and in Aging. Cell. 2018;174:1477-91.e19
25. Geng J, Ito Y, Shi L, Amin P, Chu J, Ouchida AT. et al. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun. 2017;8:359
26. Menon MB, Gropengießer J, Fischer J, Novikova L, Deuretzbacher A, Lafera J. et al. p38(MAPK)/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection. Nat Cell Biol. 2017;19:1248-59
27. Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203-34
28. Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F. et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517-21
29. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-3
30. Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci U S A. 1999;96:9136-41
31. Fulda S. The mechanism of necroptosis in normal and cancer cells. Cancer Biol Ther. 2013;14:999-1004
32. Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694-9
33. Wright A, Reiley WW, Chang M, Jin W, Lee AJ, Zhang M. et al. Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD. Dev Cell. 2007;13:705-16
34. Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y. et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994-1006
35. Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ. et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311-23
36. Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F. et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Molecular cell. 2011;43:432-48
37. Varfolomeev E, Vucic D. Intracellular regulation of TNF activity in health and disease. Cytokine. 2018;101:26-32
38. Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16:689-97
39. Tan S, Zhang H, Zhang Y, Chen W, D'Souza WD, Lu W. Predicting pathologic tumor response to chemoradiotherapy with histogram distances characterizing longitudinal changes in 18F-FDG uptake patterns. Med Phys. 2013;40:101707
40. Sun Y, Zhai L, Ma S, Zhang C, Zhao L, Li N. et al. Down-regulation of RIP3 potentiates cisplatin chemoresistance by triggering HSP90-ERK pathway mediated DNA repair in esophageal squamous cell carcinoma. Cancer letters. 2018;418:97-108
41. Ogi T, Limsirichaikul S, Overmeer RM, Volker M, Takenaka K, Cloney R. et al. Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Molecular cell. 2010;37:714-27
42. Karkhanis V, Wang L, Tae S, Hu YJ, Imbalzano AN, Sif S. Protein arginine methyltransferase 7 regulates cellular response to DNA damage by methylating promoter histones H2A and H4 of the polymerase delta catalytic subunit gene, POLD1. The Journal of biological chemistry. 2012;287:29801-14
43. Usui A, Hoshino I, Akutsu Y, Sakata H, Nishimori T, Murakami K. et al. The molecular role of Fra-1 and its prognostic significance in human esophageal squamous cell carcinoma. Cancer. 2012;118:3387-96
44. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-48
45. Zhu G, Ye J, Huang Y, Zheng W, Hua J, Yang S. et al. Receptor-interacting protein-1 promotes the growth and invasion in gastric cancer. International journal of oncology. 2016;48:2387-98
46. Choi JH, Oh YH, Park YW, Baik HK, Lee YY, Kim IS. Correlation of vascular endothelial growth factor-D expression and VEGFR-3-positive vessel density with lymph node metastasis in gastric carcinoma. J Korean Med Sci. 2008;23:592-7
47. Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. The Journal of biological chemistry. 2004;279:33185-91
48. Guo D, Zhang W, Yang H, Bi J, Xie Y, Cheng B. et al. Celastrol Induces Necroptosis and Ameliorates Inflammation via Targeting Biglycan in Human Gastric Carcinoma. International journal of molecular sciences. 2019 20
49. Hu L, Duan YT, Li JF, Su LP, Yan M, Zhu ZG. et al. Biglycan enhances gastric cancer invasion by activating FAK signaling pathway. Oncotarget. 2014;5:1885-96
50. Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X. et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313-21
51. Vetrivel P, Kim SM, Ha SE, Kim HH, Bhosale PB, Senthil K. et al. Compound Prunetin Induces Cell Death in Gastric Cancer Cell with Potent Anti-Proliferative Properties: In vitro Assay, Molecular Docking, Dynamics, and ADMET Studies. Biomolecules. 2020 10
52. Wu P, Zhu X, Jin W, Hao S, Liu Q, Zhang L. Oxaliplatin triggers necrosis as well as apoptosis in gastric cancer SGC-7901 cells. Biochemical and biophysical research communications. 2015;460:183-90
53. The global, regional, national burden of colorectal cancer, its attributable risk factors in 195 countries, territories. 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:913-33
54. Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B. et al. Colorectal cancer. Lancet. 2010;375:1030-47
55. Zeng F, Chen X, Cui W, Wen W, Lu F, Sun X. et al. RIPK1 Binds MCU to Mediate Induction of Mitochondrial Ca(2+) Uptake and Promotes Colorectal Oncogenesis. Cancer research. 2018;78:2876-85
56. Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell death & disease. 2015;6:e1636
57. Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726-34
58. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393-410
59. Bozec D, Iuga AC, Roda G, Dahan S, Yeretssian G. Critical function of the necroptosis adaptor RIPK3 in protecting from intestinal tumorigenesis. Oncotarget. 2016;7:46384-400
60. Yan G, Zhao H, Zhang Q, Zhou Y, Wu L, Lei J. et al. A RIPK3-PGE(2) Circuit Mediates Myeloid-Derived Suppressor Cell-Potentiated Colorectal Carcinogenesis. Cancer research. 2018;78:5586-99
61. Conev NV, Dimitrova EG, Bogdanova MK, Kashlov YK, Chaushev BG, Radanova MA. et al. RIPK3 expression as a potential predictive and prognostic marker in metastatic colon cancer. Clinical and investigative medicine Medecine clinique et experimentale. 2019;42:E31-e8
62. Liu ZY, Zheng M, Li YM, Fan XY, Wang JC, Li ZC. et al. RIP3 promotes colitis-associated colorectal cancer by controlling tumor cell proliferation and CXCL1-induced immune suppression. Theranostics. 2019;9:3659-73
63. He GW, Günther C, Thonn V, Yu YQ, Martini E, Buchen B. et al. Regression of apoptosis-resistant colorectal tumors by induction of necroptosis in mice. The Journal of experimental medicine. 2017;214:1655-62
64. Oliver Metzig M, Fuchs D, Tagscherer KE, Gröne HJ, Schirmacher P, Roth W. Inhibition of caspases primes colon cancer cells for 5-fluorouracil-induced TNF-α-dependent necroptosis driven by RIP1 kinase and NF-κB. Oncogene. 2016;35:3399-409
65. Cabal-Hierro L, O'Dwyer PJ. TNF Signaling through RIP1 Kinase Enhances SN38-Induced Death in Colon Adenocarcinoma. Molecular cancer research: MCR. 2017;15:395-404
66. Han Q, Ma Y, Wang H, Dai Y, Chen C, Liu Y. et al. Resibufogenin suppresses colorectal cancer growth and metastasis through RIP3-mediated necroptosis. Journal of translational medicine. 2018;16:201
67. Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332-6
68. Galluzzi L, Kepp O, Kroemer G. RIP kinases initiate programmed necrosis. J Mol Cell Biol. 2009;1:8-10
69. Mishra SK, Stephenson DJ, Chalfant CE, Brown RE. Upregulation of human glycolipid transfer protein (GLTP) induces necroptosis in colon carcinoma cells. Biochimica et biophysica acta Molecular and cell biology of lipids. 2019;1864:158-67
70. Sun W, Wu X, Gao H, Yu J, Zhao W, Lu JJ. et al. Cytosolic calcium mediates RIP1/RIP3 complex-dependent necroptosis through JNK activation and mitochondrial ROS production in human colon cancer cells. Free radical biology & medicine. 2017;108:433-44
71. Hou X, Yang C, Zhang L, Hu T, Sun D, Cao H. et al. Killing colon cancer cells through PCD pathways by a novel hyaluronic acid-modified shell-core nanoparticle loaded with RIP3 in combination with chloroquine. Biomaterials. 2017;124:195-210
72. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424
73. Liang H, Xiong Z, Li R, Hu K, Cao M, Yang J. et al. BDH2 is downregulated in hepatocellular carcinoma and acts as a tumor suppressor regulating cell apoptosis and autophagy. J Cancer. 2019;10:3735-45
74. Wang C, Yao B, Xu M, Zheng X. RIP1 upregulation promoted tumor progression by activating AKT/Bcl-2/BAX signaling and predicted poor postsurgical prognosis in HCC. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:15305-13
75. Schneider AT, Gautheron J, Feoktistova M, Roderburg C, Loosen SH, Roy S. et al. RIPK1 Suppresses a TRAF2-Dependent Pathway to Liver Cancer. Cancer cell. 2017;31:94-109
76. Van TM, Polykratis A, Straub BK, Kondylis V, Papadopoulou N, Pasparakis M. Kinase-independent functions of RIPK1 regulate hepatocyte survival and liver carcinogenesis. The Journal of clinical investigation. 2017;127:2662-77
77. Zhong F, Wu Q, Xia G, Liu L, Yu T. RIP6 Suppresses Tumor Cell Growth in Hepatocellular Carcinoma. Clin Lab. 2020 66
78. Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13
79. Koppe C, Verheugd P, Gautheron J, Reisinger F, Kreggenwinkel K, Roderburg C. et al. IκB kinaseα/β control biliary homeostasis and hepatocarcinogenesis in mice by phosphorylating the cell-death mediator receptor-interacting protein kinase 1. Hepatology (Baltimore, Md). 2016;64:1217-31
80. Kondylis V, Polykratis A, Ehlken H, Ochoa-Callejero L, Straub BK, Krishna-Subramanian S. et al. NEMO Prevents Steatohepatitis and Hepatocellular Carcinoma by Inhibiting RIPK1 Kinase Activity-Mediated Hepatocyte Apoptosis. Cancer cell. 2015;28:582-98
81. Aigelsreiter A, Haybaeck J, Schauer S, Kiesslich T, Bettermann K, Griessbacher A. et al. NEMO expression in human hepatocellular carcinoma and its association with clinical outcome. Hum Pathol. 2012;43:1012-9
82. Huang H, Chen T, Zhou Y, Geng L, Shen T, Zhou L. et al. RIPK1 Inhibition Enhances Pirarubicin Cytotoxic Efficacy through AKT-P21-dependent Pathway in Hepatocellular Carcinoma. International journal of medical sciences. 2018;15:1648-57
83. Liu XY, Liu SP, Jiang J, Zhang X, Zhang T. Inhibition of the JNK signaling pathway increases sensitivity of hepatocellular carcinoma cells to cisplatin by down-regulating expression of P-glycoprotein. Eur Rev Med Pharmacol Sci. 2016;20:1098-108
84. Zhang B, Cao K, Liu Z, Shan W, Wen Q, Wang R. Receptor interacting protein kinase 3 promotes cisplatin-induced necroptosis in apoptosis-resistant HepG2/DDP cells. Neoplasma. 2019. 2019
85. Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, Koo JS. et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015;25:707-25
86. Li YM, Liu ZY, Wang JC, Yu JM, Li ZC, Yang HJ. et al. Receptor-Interacting Protein Kinase 3 Deficiency Recruits Myeloid-Derived Suppressor Cells to Hepatocellular Carcinoma Through the Chemokine (C-X-C Motif) Ligand 1-Chemokine (C-X-C Motif) Receptor 2 Axis. Hepatology (Baltimore, Md). 2019;70:1564-81
87. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253-68
88. Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP. et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234-43
89. Vucur M, Reisinger F, Gautheron J, Janssen J, Roderburg C, Cardenas DV. et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell reports. 2013;4:776-90
90. Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS. et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248-53
91. Wójcik M, Bobowiec R, Lisiecka U, Śmiech A. Expression of receptor interacting protein 1 and receptor interacting protein 3 oval cells in a rat model of hepatocarcinogenesis. Experimental and therapeutic medicine. 2018;15:4448-56
92. Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World journal of gastroenterology. 2017;23:3978-98
93. Baiu I, Visser B. Gallbladder Cancer. Jama. 2018;320:1294
94. Zhu G, Chen X, Wang X, Li X, Du Q, Hong H. et al. Expression of the RIP-1 gene and its role in growth and invasion of human gallbladder carcinoma. Cell Physiol Biochem. 2014;34:1152-65
95. Andia ME, Hsing AW, Andreotti G, Ferreccio C. Geographic variation of gallbladder cancer mortality and risk factors in Chile: a population-based ecologic study. Int J Cancer. 2008;123:1411-6
96. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111
97. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P. et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-80
98. Sacchi D, Sarcognato S, Cillo U, Gringeri E, Fabris L, Di Giunta M. et al. Necroptosis is associated with a better survival in intrahepatic cholangiocarcinoma. Digestive and Liver Disease. 2019;51:e2-e3
99. Akara-Amornthum P, Lomphithak T, Choksi S, Tohtong R, Jitkaew S. Key necroptotic proteins are required for Smac mimetic-mediated sensitization of cholangiocarcinoma cells to TNF-α and chemotherapeutic gemcitabine-induced necroptosis. PloS one. 2020;15:e0227454
100. Xu B, Xu M, Tian Y, Yu Q, Zhao Y, Chen X. et al. Matrine induces RIP3-dependent necroptosis in cholangiocarcinoma cells. Cell Death Discov. 2017;3:16096
101. Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M, Liu L. et al. Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer letters. 2014;346:273-7
102. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30
103. Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D. et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532:245-9
104. Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179-88
105. Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ. et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404-13
106. Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J. et al. A preoperative serum signature of CEA+/CA125+/CA19-9 ≥ 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer. 2015;136:2216-27
107. Qi ZH, Xu HX, Zhang SR, Xu JZ, Li S, Gao HL. et al. RIPK4/PEBP1 axis promotes pancreatic cancer cell migration and invasion by activating RAF1/MEK/ERK signaling. International journal of oncology. 2018;52:1105-16
108. Dai H, Chen H, Liu W, You Y, Tan J, Yang A. et al. Effects of Raf kinase inhibitor protein expression on pancreatic cancer cell growth and motility: an in vivo and in vitro study. J Cancer Res Clin Oncol. 2016;142:2107-17
109. Fulda S. Apoptosis pathways and their therapeutic exploitation in pancreatic cancer. Journal of cellular and molecular medicine. 2009;13:1221-7
110. Hannes S, Abhari BA, Fulda S. Smac mimetic triggers necroptosis in pancreatic carcinoma cells when caspase activation is blocked. Cancer letters. 2016;380:31-8
111. Yang C, Ran Q, Zhou Y, Liu S, Zhao C, Yu X. et al. Doxorubicin sensitizes cancer cells to Smac mimetic via synergistic activation of the CYLD/RIPK1/FADD/caspase-8-dependent apoptosis. Apoptosis: an international journal on programmed cell death. 2020;25:441-55
112. Chen C, Xiao W, Huang L, Yu G, Ni J, Yang L. et al. Shikonin induces apoptosis and necroptosis in pancreatic cancer via regulating the expression of RIP1/RIP3 and synergizes the activity of gemcitabine. American journal of translational research. 2017;9:5507-17
113. Pietkiewicz S, Eils R, Krammer PH, Giese N, Lavrik IN. Combinatorial treatment of CD95L and gemcitabine in pancreatic cancer cells induces apoptotic and RIP1-mediated necroptotic cell death network. Exp Cell Res. 2015;339:1-9
114. Zielinska E, Zauszkiewicz-Pawlak A, Wojcik M, Inkielewicz-Stepniak I. Silver nanoparticles of different sizes induce a mixed type of programmed cell death in human pancreatic ductal adenocarcinoma. Oncotarget. 2018;9:4675-97
115. Sanpui P, Chattopadhyay A, Ghosh SS. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl Mater Interfaces. 2011;3:218-28
116. Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310-6
117. Jaafar R, Mnich K, Dolan S, Hillis J, Almanza A, Logue SE. et al. RIP2 enhances cell survival by activation of NF-ĸB in triple negative breast cancer cells. Biochemical and biophysical research communications. 2018;497:115-21
Author contact
![]() Corresponding authors: Wenhong Deng and Weixing Wang contributed equally to this study. E-mail: wenhongdengedu.cn (DW); sate.llitecom (WW).
Corresponding authors: Wenhong Deng and Weixing Wang contributed equally to this study. E-mail: wenhongdengedu.cn (DW); sate.llitecom (WW).

 Global reach, higher impact
Global reach, higher impact