Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(15):4710-4721. doi:10.7150/jca.58873 This issue Cite
Research Paper
MicroRNA-455-5p Contributes to Cholangiocarcinoma Growth and Mediates Galangin's Anti-Tumor Effects
1. Department of Cardiology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China.
2. Department of Pharmacy, The Affiliated Changsha Hospital of Hunan Normal University, Changsha, Hunan, China.
3. Department of Cardiology, Xiangya Hospital, Central South University, Changsha, Hunan, China.
Received 2021-1-31; Accepted 2021-5-22; Published 2021-6-4
Abstract
Fully understanding the mechanism of how Cholangiocarcinoma (CCA) development and discovering promising therapeutic drugs are important to improve patients' survival time. This study identifies that microRNA-455-5p (miR-455-5p) targets protein phosphatase 1 regulatory subunit 12A (PPP1R12A), an effect that represses mitogen-activated protein kinase (MAPK) and PI3K/AKT pathway activation, thereby controlling CCA cells survival and metastasis. Moreover, miR-455-5p expression is reduced in CCA tissues and negative correlation with PPP1R12A and PPP1R12A knockdown phenotypic mimics miR-455-5p' effects on CCA cells. Furthermore, we demonstrate that galangin inhibits CCA growth both in vitro and in vivo, which is associated with increased miR-455-5p and repressed PPP1R12A expression. In support, overexpression of miR-455-5p abrogates those galangin-mediated anti-CCA effects. These findings establish an essential role of miR-455-5p in CCA development and galangin may provide a potential therapeutic adjuvant agent for anti-CCA treatment.
Keywords: cholangiocarcinoma, microRNA, galangin, survival, metastasis
Introduction
Cholangiocarcinoma (CCA) is a high malignant bile duct system tumor originating from the bile duct epithelial cells and accounts for about 3% of digestive system tumors. Evidence from clinic and experimental studies demonstrated that CCA is a very poor prognostic malignancy with a 5-year survive rate less than 5% due to its very early peripheral infiltration and distant metastasis [1-6]. Traditional chemotherapy drugs, such as gemcitabine and platinum, have been showed to increase the median survival around 8-12 months [7-9]. Yet, this still far from patient's anticipation and those chemotherapy drugs can also cause multiple serious side effects [10]. Moreover, more than 70% of CCA patients are diagnosed in an advanced stage due to extraordinary invasiveness and not suit for surgical resection or liver transplantation [11-16]. Therefore, an urgent needed in clinic to fully discover the mechanism behind CCA development and progression and promising targets for CCA treatment.
Accumulating evidence suggest that natural products derived from traditional Chinese herbal medicines are emerging as promising adjuvant drug to improve chemotherapy efficiency [17]. For example, previous studies found that flavonoids had stronger anti-cancer effects with low toxicity which make them can serve as an ideal candidate for chemotherapeutic adjuvant [18]. Galangin is a natural flavonoid product that extracted from the root of galangal and exhibits various anti-cancer effects against multiple tumor types (e.g. lung cancer, colon cancer and breast cancer) [19-22], rising a high possibility of galangin as an anti-cancer adjuvant agent. Although it has been reported that galangin suppressed CCA cells growth and metastasis [19], the underlying mechanism and whether it suitable serve as an adjuvant agent in anti-CCA treatment still largely unknown.
MicroRNAs (miRNAs) are a class of non-coding, single-stranded, small RNAs and play a crucial role in regulating gene expression at post-transcriptional level. It is well established that dysregulated miRNAs are important regulators involved in controlling various biological processes in tumor cells, such as proliferation, apoptosis, invasion, metastasis and others [23-27]. Moreover, miRNAs not only contribute importantly to mediate multiple chemotherapy drugs' anti-cancer effects, but also involve in chemotherapeutic drug resistance [28-30]. Herein, we find that miR-455-5p targets protein phosphatase 1 regulatory subunit 12A (PPP1R12A) and represses mitogen-activated protein kinase (MAPK) and PI3K/AKT pathway activation, thereby regulating CCA cells growth and metastasis. Moreover, using in vitro and tumor xenograft approaches, we show that miR-455-5p mediates galangin's anti-cancer effects on CCA. Our results establish an essential role of miR-455-5p in CCA development and galangin may provide a potential therapeutic adjuvant agent for anti-CCA treatment.
Methods
MiR-455-5p and protein phosphatase 1 regulatory subunit 12A expression data collection
We explored miR‐455-5p and protein phosphatase 1 regulatory subunit 12A (PPP1R12A) expression in CCA samples in TCGA Data Portal from ENCORI Pan-Cancer Analysis Platform (http://starbase.sysu.edu.cn/panCancer.php). Meanwhile, we also use this web server to analyze the correlation between miR-455-5p and PPP1R12A expression.
CCA cell line culture and transfection
Two human CCA cell lines (TFK-1 and HCCC9810) and HEK 293T cells (CRL-3216) were obtained from the American Type Culture Collection (USA). TFK-1 and HCCC9810 cells were grown in RPMI-1640 medium (C11875500BT, Gibco, USA) supplemented with 10% fetal bovine serum (10270106, Gibco, USA) and antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin) (P1400, Solarbio, China) in a humidified 5% CO2 incubator at 37°C. HEK 293T cells were cultured in DMEM supplemented 10% FBS and 1% penicillin and streptomycin. Cells passaged less than 8 times were used for all experiments. TFK-1 and HCCC9810 cells were plated at 5, 000 cells/well on 96-well plate, 120, 000 cells/well on 12-well plate or 250, 000 cells/well on 6-well plate, grown to 70%-80% confluency, and treated with galangin (282200, Sigma, USA) for 24 hours. For transfection experiments, cells were transfected with 100 nM of MiRNA non-specific mimic control (NS-m) (miR1N0000001-1-5, Ribobio, China), miR-455-5p mimic (455-m) (miR1N0003150-1-5, Ribobio, China), miRNA non-specific inhibitor (NS-i) (miR2N0000001-1-5, Ribobio, China), miR-455-5p inhibitor (455-i) (miR20003150-1-5, Ribobio, China), siRNA negative control (si-NC) (siN0000002-1-5, Ribobio, China) or PPP1R12A siRNA (si-PPP1R12A) (stB0004345B-1-5, Ribobio, China) and Lipofectamine 2000 transfection reagent from Invitrogen (11668019, USA) was used for transfection according to the manufacturer's instructions. After 14-16 hours of incubation, cells were replaced with fresh culture medium and incubated with another 12 hours before harvested for western blot and real-time PCR analysis. In some experiments, transfected cells were treated with galangin (150 μM) for 24 hours before used for further analysis.
Cell proliferation assay
BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 488 (C0071S, Beyotime, China) was used for cell proliferation analysis following the manufacturer's instructions. Briefly, TFK-1 and HCCC9810 cells were plated on 96-well plates at a density of 5, 000 cell/well and transfected miRNA mimic, miRNA inhibitor or siRNA with or without galangin (150 μM). Pre-warmed EDU-containing medium (20 μM) was added and incubated for 3 hours. Then cells were fixed with 4% paraformaldehyde for 15 min and followed by 0.3% triton-X 100 incubation at room temperature for 15 minutes. After 3 times wash with PBS, prepared Click System Solution was added into each well and incubated at 37 °C for 30 min in darkness. Then cells were washed with PBS for 3 times and incubated with Hoechst 33342 (0.5 μg/ml) at room temperature for 10 minutes. The numbers of Edu positive cells per view were observed under a fluorescence microscope and quantified from randomly acquired images.
Cell apoptosis assay
TFK-1 and HCCC9810 cells were plated on 6-well plates at a density of 150, 000 cells/well and after indicated treatment. The apoptosis assay was performed using the Annexin V-FITC Apoptosis Detection Kit (C1062M, Beyotime, China) following the manufacturer's protocols. Cells were stained with Annexin V-FITC and propidium iodide (PI) in the dark. After washing with PBS, the apoptosis assay was examined by a flow cytometer (BD FACSCCanto II). Data were analyzed with FlowJo software (TreeStar, Inc. Ashland, OR).
CCK-8 assay
TFK-1 and HCCC9810 cells were plated on 96-well plates at a density of 50, 000 cells/well and treated with different doses of galangin (12.5, 25, 50, 75, 100, 150, 200, 300, 400 μM) for 24 hours. After treatment, 10 μl CCK-8 (CK04, DOJIDO) was added into each well and incubated for 2 hours. The absorbance was measured at 450 nm using an ELISA plate reader (DTX880, Beckman).
Cell migration and invasion assays
The 8 μm Transwell chamber (3422, Corning, USA) was used for the experiment. Before the start of the experiment, the diluted matrigel (354166, Corning, USA) (1:8) was used to spread in the upper chamber, and placed in the incubator for 2 hours (Migration experiment without this step). MiRNA mimic, miRNA inhibitor or si-RNA transfected TFK-1 and HCCC9810 cells with or without galangin treatment were adjusted to a density of 15, 000/200 μl using a serum-free medium and seeded in the upper chamber. 600μl of medium containing 10% FBS was added to the lower chamber. After 24 h, cells were fixed with 4% paraformaldehyde for 15 min and 0.1% crystal violet solution (G1062, Solarbio, China) for 15 min. Then wipe off the uninjured cells in the upper chamber and pictures were taken under a light microscope (Nikon, Japan).
Dual-luciferase reporter assay
The wild-type (WT) and mutant-type (Mut) 3'-untranslated regions (UTRs) of PPP1R12A, which contain predicted binding sites for miR-455-5p, were synthesized and cloned to pmirGLO Dual-Luciferase miRNA Target expression vector (E1330, Promega, USA) by Vigene Biosciences (China). HEK 239T cells (150, 000 cells/well) were plated on 12-well plate, grown to 70% confluency, co-transfected with 250 ng WT or Mut luciferase reporter constructs, 10 ng Renilla plasmid (E2231, Promega, USA) and 100 nM 455-m or NS-m using lipofectamine 2000 according to the manufacturer's protocols. 24 hours after transfection, the cell lysates were collected and the luciferase activities were measured using the Dual-Glo Luciferase Assay System (E2920, Promega, USA) according to the manufacturer's instructions. Values were measured in a fluorescence reader (Veritas 9100-002, Turner Biosystems, USA). Each reading of luciferase activity was normalized to the Renilla activity.
Real-time PCR
Tumor tissues were homogenized using TissueLyser II (Qiagen) according to manufacturer' instructions. Total RNA from treated TFK-1 and HCCC9810 cells or tumor tissues were isolated using Trizol reagent (9109, Takara, Japan) according to the manufacturer's instructions. 500 ng total RNA was reverse-transcribed to generate cDNA using the PrimeScript RT reagent kit (RR036A, Takara, Japan) according to the manufactures instruction. Real-time PCR analysis of PPP1R12A mRNA expression was performed using the TB Green Premix EX Tag kit (RR820A, Tarkara, Japan) with the Light Cycler 480 real-time PCR system (Rhoche) following the manufacturer's instruction. The primer sequences involved in the experiment were as follows: PPP1R12A, forward, GACAAAACCCCTGGCTTCTG; reverse, AGCTGCCCGTCTTTCTAAGT; GAPDH, forward, CTGCACCACCAACTGCTTAG, reverse, AGGTCCACCACTGACACGTT. Specific primers including miR-455-5p (MQPS0001464-1-100) and U6 (MQPS0000002-1-100) were purchased from RiboBio. The expression of targeted genes was analyzed using the 2‑ΔΔCq method.
Western blot analysis
Tumor tissues were homogenized using TissueLyser II (Qiagen) according to manufacturer' instructions. Protein from tissues and CCA Cells were isolated using RIPA buffer (P0013B, Beyotime, China) with phosphatase inhibitor (ST505, Beyotime, China). 20 μg of protein from each sample used for the 10% SDS-PAGE and then transferred to PVDF membrane (IPVH00010, Millipore, Germany). After transfer and blocking, membranes were incubated with following antibodies, Bax (1:1000, 60267, proteintech, China), Bcl-2 (1:1000, 60178, proteintech, China), caspase-3 (1:1000, 14220, CST, USA), Cleaved-Caspase3 (1:1000, 9664, CST, USA), MMP-9 (1:1000, 3852, CST, USA), Vimentin (1:1000, 3932, CST, USA), PPP1R12A (1:1000, ab32519, Abcam, UK), phospho-PI3K (1:1000, AP0854, Abclonal, China), phospho-Akt (1:1000, 4060, CST, USA), phospho-p38 (1:1000, 4511, CST, USA), phospho-JNK (1:1000, 9255, CST, USA), phospho-ERK1/2 (1:1000, 4370, CST, USA), and GAPDH (1:1000, ab8245, Abcam, UK). The membranes were washed using TBST and then incubated with correspondent secondary antibody. DAB Horseradish Peroxidase Color Development Kit was used for development (P0018S, Beyotime, China). The statistical gray value of each strip, and then calculate the relative expression.
Subcutaneous xenograft animal model and Treatment
All animal experiments were approved by the Ethics Committee for Laboratory Animals of The Third Xiangya Hospital, Central South University, Hunan, China, and followed the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Testing, and Education by the New York Academy of Sciences, Ad Hoc Animal Research Committee. Four-weeks old Balb/c male nude mice were purchased from Hunan SJA Laboratory Animal Co. in China. TFK-1 cells (5 × 106) were resuspended in 100 μl PBS and subcutaneously injected into the right flank of mice. When the tumor sizes were above 50 mm3, mice were randomly divided into two groups (n = 6 mouse per group) and intragastric treated with galangin (80 mg/kg/day) combined with intratumoral injected with lipofectamine 2000 encapsulated 455-i or NS-i (5 nM/mouse) according to protocol previously described in [31]. The mice were sacrificed when they reach the experimental end point (day 28), and tumors were harvested for analysis.
Immunohistochemistry
Tumor tissues were fixed with neutral buffered 10% formalin solution (HT501128, Sigma), embedded in paraffin, cut into sections at 4 μm, and deparaffinized. Antigen retrieval was performed using sodium citrate buffer (10 nM sodium and 0.05% Tween 20 at pH 6.0) at 96oC for 30 minutes. Sections were incubated with anti-Ki67 (1:50, A2094, Abclonal) or PPP1R12A (1:50, 22117010AP, proteintech, China) for 120 minutes at room temperature. Primary antibodies binding to tissue sections was visualized using DAB Detection kit (ZLI-9017, Zsbio, China), and counterstained with hematoxylin. All images were captured using a Microscope VS120 Whole Slide Scanner (Olympus) and analyzed using the computer-assisted Image-Pro Plus software (Meida Cybernetics, Bethesda, MD). The quantification of Ki67, and PPP1R12A staining was performed as positive area percent. Data were analyzed in a blinded fashion by two independent observers.
Statistical analysis
All data are expressed as mean ± SEM. GraphPad 7.0 software package (GraphPad Software, Inc) was used for statistical analysis. Unpaired two-tailed student's t test was used to determine statistical significance between two groups normally distributed continuous variables. For multiple groups comparison, one-way ANOVA followed by Tukey multiple comparison analysis was used. For data without normal distribution, non-parametric Mann-Whitney U test or Kruskal-Wallis test was used. P < 0.05 was considered significant for all tests.
Results
Overexpression of miR-455-5p inhibits CCA cells proliferation and promotes apoptosis, migration and invasion
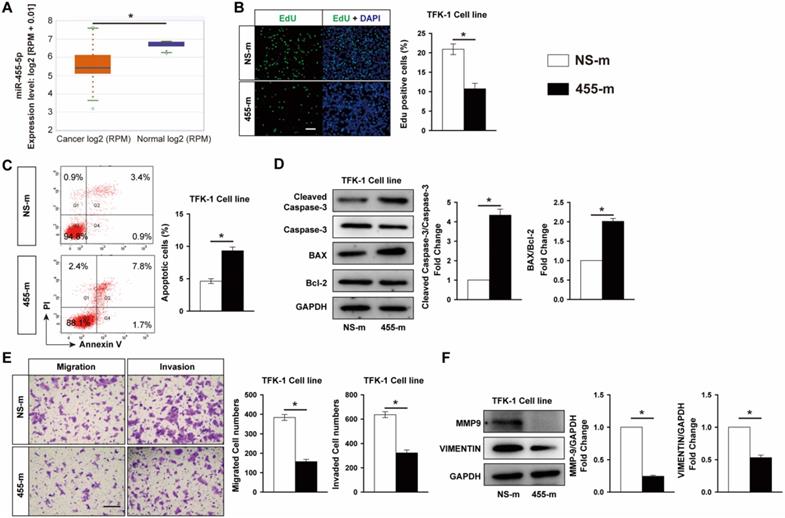
MiR-455-5p is an high conserved miRNA and have been shown to contribute importantly to multiple tumors development [25, 27, 32-35]. To investigate the role of miR-455-5p in CCA, we first performed an integrative analysis of CCA samples from The Cancer Genome Atlas (TCGA) database using ENCORI Pan-Cancer Analysis Platform. A total of 45 samples, including 9 normal and 36 CAA tissues, were extracted from database and the analysis revealed that the expression of miR-455-5p was significantly reduced in CAA tissues compared with normal tissue (Fig. 1A), indicating that miR-455-5p may contribute to CCA development. To assess the potential role of miR-455-5p in CCA development, we examined the effect of miR-455-5p on cell survival and metastasis in CCA cells by using gain-function experiments. As shown in Fig. 1B and Fig. S1A, compared with non-specific mimic control (NS-m) transfected CCA cell line, including bile duct carcinoma TFK-1 cells and intrahepatic bile duct carcinoma HCCC9810 cells, EdU staining assay exhibited that overexpression of miR-455-5p using miR-455-5p mimic (455-m) inhibited TFK-1 and HCCC9810 cells proliferation by 48.6% and 52.2%, respectively. We next investigated the effect of miR-455-5p on CCA cells apoptosis by flow cytometry (FACS) and western blot. In CCA cell lines transfected with miR-455-5p mimic, we observed a 2.02-fold induction of apoptotic cells in TFK-1 cells and 2.46-fold increase in HCCC9810 cells compared with cells transfected with NS-m (Fig. 1C and Fig. S1B). In agreement with this observation, overexpression of miR-455-5p increased the ratio of Bax to Bcl-2 and expression of cleaved caspase-3 at protein levels in both TFK-1 and HCCC9810 cell line (Fig. 1D and Fig. S1C). To further determine whether miR-455-5p also involved in metastasis, we performed transwell analysis to assess the effect of miR-455-5p on CCA cell migration and invasion. As shown in Fig. 1E, overexpression of miR-455-5p reduced TFK-1 cells migration by 59.1% and invasion by 49.2%. Similar phenotypic change was also observed in miR-455-5p transfected HCCC9810 cells (Fig. S1D). It is well established that epithelial-to-mesenchymal transition (EMT) and matrix metalloproteinases (MMPs; e.g. MMP9) play crucial roles in mediating tumor migration and invasion [36]. VIMENTIN is an important mesenchymal marker, we found that overexpression of miR-455-5p significantly reduced MMP-9 and VIMENTIN expression at protein levels in both HCCC9810 cells (Fig. 1F) and TFK-1 cells (Fig. S1E). The induction of miR-455-5p by miR-455-5p mimic transfection in both HCCC9810 and TFK-1 cells was confirmed by real-time PCR analysis (Fig. S2). Collectively, these data suggested that miR-455-5p is able to negatively affect CCA cells survival, apoptosis, migration and invasion.
MiR-455-5p is decreased in CCA cancer tissue samples and overexpression of miR-455-5p inhibits CCA cells proliferation, migration and invasion, but promotes apoptosis. (A) CCA samples (n=36) and normal tissues (n=9) in TCGA dataset from ENCORI Pan-Cancer Analysis Platform showed that miR-455-5p expression was significantly down‐regulated in tumor tissues. TFK-1 cells were transfected with miR‐455-5p mimics (455-m) or non-specific mimic control (NS-m) at 100 nM for 24h. (B) EdU analysis of cell proliferation, Scale bar, 20 μM. (C) FACS analysis of cell apoptosis. (D) Western blot analysis of cleaved caspase 3, Caspase 3, Bax and Bcl-2 protein expression. (E) Matrigel-coated Transwell analysis of migration and invasion, scale bar, 50 μm. (F) Western blot analysis of MMP9 and Vimentin protein expression. B to F, n = 3 independent experiments. Values were given as means ± SEM. *P < 0.05.
PPP1R12A is a functional target of miR-455-5p and inhibition of PPP1R12A expression suppresses CCA cells survival and metastasis
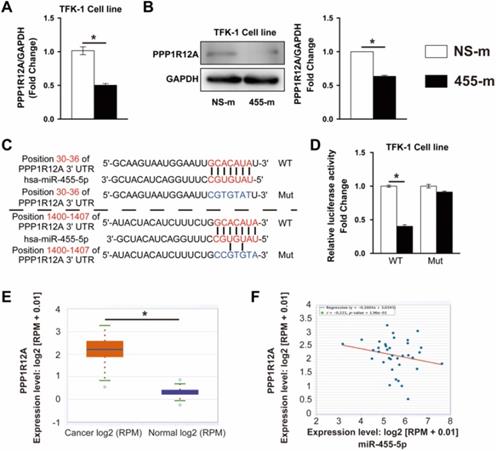
It is well established that miRNAs exert its function through regulating targeted gene expression at post-transcriptional level. To identify the potential target regulated by miR-455-5p, we performed bioinformatics assay using TargetScan [37] and miRWalk [38] algorithms and PPP1R12A, one of myosin phosphatase subunits controlling cell cycle and migration [25, 34, 39], was predicted to be a miR-455-5p target. In miR-455-5p-overexpressed TFK-1 cells, PPP1R12A expression was reduced by 51% at mRNA level and 40% at protein level (Fig. 2A and 2B). TargetScan algorithm indicated that 2 potential miR-455-5p-binding sites in the 3' UTR of PPP1R12A (Fig. 2C). Overexpression of miR-455-5p inhibited the activity of a luciferase reporter construct containing both PPP1R12A 3'UTR, while not the PPP1R12A construct with mutation sites at predicated site at 3'UTR region (Fig. 2D). Moreover, PPP1R12A expression and correlation analysis using the CCA samples from TCGA database exhibited that the expression of PPP1R12A was significantly increased in CCA cancer samples (Fig. 2E) and a negative correlation between miR-455-5p and PPP1R12A in CCA samples (Fig. 2F). Taken together, these data suggested that PPP1R12A may play an importantly role in CCA and miR-455-5p-mediated CCA cell survival and metastasis may through modulating PPP1R12A expression.
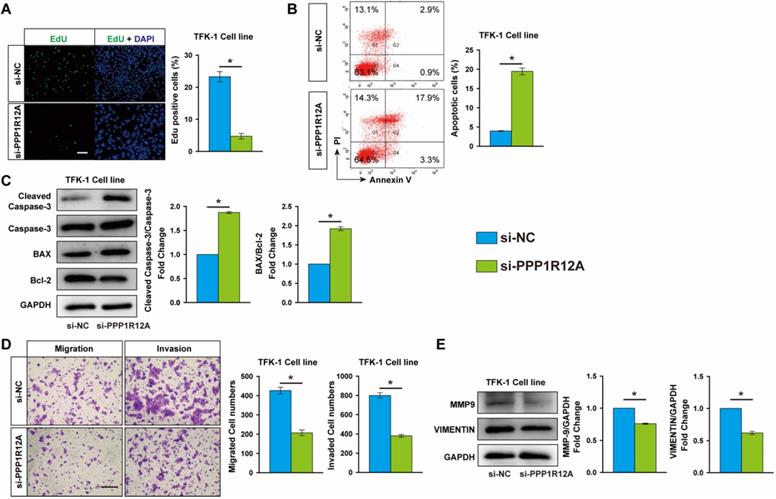
To assess the potential role of PPP1R12A in CCA, we examined the effect of PPP1R12A on CCA cells survival and metastasis by using loss-of-function experiments. EdU assay exhibited that Inhibition of PPP1R12A expression dramatically decreased TFK-1 and HCCC9810 cells proliferation by 79.7% and 72.3%, respectively (Fig. 3A and Fig. S3A). Moreover, cell apoptosis measurement by FACS using Annexin V and PI double staining showed that inhibition of PPP1R12A expression promoted both TFK-1 and HCCC9810 cells apoptosis by 4.9-fold and 2.33-fold, respectively (Fig. 3B and Fig. S3B). Consistent with these results, in TFK-1 and HCCC9810 cells transfected with PPP1R12A siRNA exhibited a significantly increased ratio of Bax to Bcl-2 and cleaved caspase-3 expression at protein levels compared with siRNA negative control transfected cells (Fig. 3C and Fig. S3C). Furthermore, Transwell assay showed that silencing PPP1R12A significantly decreased the numbers of migrated and invaded cells compared with siRNA negative control transfected HCCC9810 and TFK-1 cells (Fig. 3D and Fig. S3D). Consistently, the expression of MMP9 and VINMENTIN at protein levels in both PPP1R12A siRNA-transfected HCCC9810 and TFK-1 cells were significantly decreased compared with those cells transfected with siRNA negative control (Fig. 3E and Fig. S3E). The efficiency of PPP1R12A siRNA in TFK-1 and HCCC9810 cells was confirmed by Real-time PCR and western blot (Fig. S4A and S4B). In summary, these data (Fig. 2, Fig. 3 and Fig. S3) demonstrated that inhibition of PPP1R12A expression suppresses CCA cells survival and metastasis and may mediate the anti-tumor effects of miR-455-5p.
PPP1R12A is a direct target of miR-455-5p and negatively correlated with miR-455-5p expression in CCA tissue samples. TFK-1 cells were transfected with miR‐455-5p mimic (455-m) or non-specific mimic control (NS-m) at 100 nM for 24h. (A) Real-time PCR analysis of PPP1R12A mRNA expression, n = 3 independent experiments. (B) Western blot analysis of PPP1R12A protein expression, n = 3 independent experiments. (C) Bioinformatics analysis indicated potential 3'UTR position of miR‐455‐5p on PPP1R12A. (D) Luciferase reporter assay of PPP1R12A 3'-UTR activity in HEK 293T cells, n = 3 independent experiments. (E) CCA samples (n=36) and normal tissues (n=9) in TCGA dataset from ENCORI Pan-Cancer Analysis Platform showed that PPP1R12A expression was significantly up‐regulated in tumor tissues. (F) The expression of miR‐455-5p in CCA tissues was negatively correlated with the level of PPP1R12A mRNA. Values are given as means ± SEM. *P < 0.05.
MiR-455-5p expression is induced by galangin treatment and mediates the anti-tumor effects of galangin on CCA
Galangin is a natural flavonoid product extracted from the root of galangal and exhibits multiple anticancer effects against various tumors without serious side effects. We found that galangin treatment significantly decreased TFK-1 cells viability in a dose-dependent manner (Fig. S5). Unexpectedly, galangin potently increased miR-455-5p expression by 5.3-fold in TKF-1 cells and 4.2-fold in HCCC9810 cells, respectively (Fig. 4A), suggesting miR-455-5p may mediate the anti-tumor effects of galangin on CCA cells. To test this hypothesis, miR-455-5p antagomir was used to inhibit miR-455-5p expression in both TFK-1 and HCCC9810 cells (Fig. S6). Edu assay demonstrated that galangin treatment significantly decreased TFK-1 Cell proliferation, but not in TFK-1 cells transfected with miR-455-5p inhibitor (Fig. 4B). Moreover, FACS showed that galangin treatment increased TKF-1 and HCCC9810 cells apoptosis by 8.3- and 6.8-fold, respectively (Fig. 4C, Fig. S7A). These phenotypic alterstions were further confirmed by western blot assay using apoptotic marker cleaved caspase-3, Bcl2 and Bax (Fig. 4D, Fig. S7B). Furthermore, in CCA cells treated with galanin exhibited less migrated and invaded cells (Fig. 4E, Fig. S7C). In agreement with these results, the protein level of MMP9 and Vimentin were lower in both TKF-1 and HCCC9810 cells treated with galanin (Fig. 4F, Fig. S7D). In contrast, these galanin-mediated anti-tumor effects on CCA cells were abrogated by inhibition of miR-455-5p expression (Fig. 4C to 4F, Fig. S6 and S7).
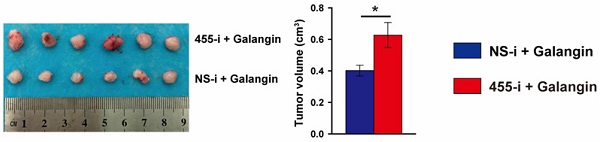
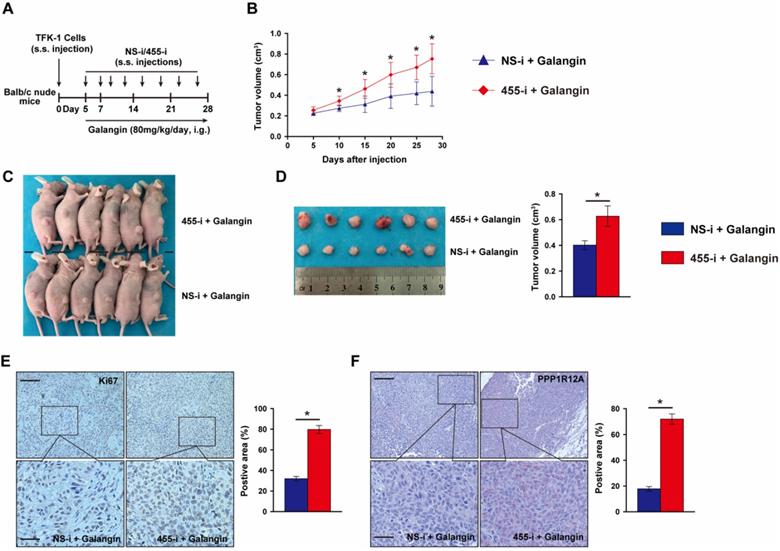
To further evaluate whether the anti-tumor effects of galangin on CCA depends on miR-455-5p expression and galangin could use for CCA therapeutics, we performed a tumor xenograft by subcutaneously injecting TFK-1 cells with miR-455-5p knockdown and galangin treatment (Fig. 5A). Compare to galangin treated Balb/c nude mice with NS-i injection, inhibition of miR-455-5p expression in mice received galangin treatment exhibited an expanded growth of TKF-1 cell xenograft (Fig. 5B to 5D, Fig. S8). Moreover, an increased proliferation marker Ki67 was observed in galangin treated mice with miR-455-5p inhibitor (Fig. 5E). Furthermore, PPP1R12A expression determined by immunohistochemistry staining was significant higher in galangin and 455-i treated Balb/c nude mice than those mice treatment with galangin and NS-i (Fig. 5F). Taken together, these findings suggest that the therapeutic effects of galangin on CCA and that miR-455-5p mediates the anti-tumor effects of galangin.
PPP1R12A knockdown suppresses CCA cells proliferation and promotes apoptosis. TFK-1 cells were transfected with siRNA negative control (si-NC) or PPP1R12A siRNA (si-PPP1R12A) at 100 nM for 24h. (A) EdU analysis of cell proliferation, Scale bar, 20 μM. (B) FACS analysis of cell apoptosis. (C) Western-blot analysis of cleaved caspase 3, Caspase 3, Bax and Bcl-2 expression at protein level. (D) Matrigel-coated Transwell analysis of migration and invasion, scale bar, 50 μm. (E) Western blot analysis of MMP9 and Vimentin protein expression. A to E, n = 3 independent experiments. Values are given as means ± SEM. *P < 0.05.
MiR-455-5p expression contributes importantly to galangin-mediated MAPK and PI3K/AKT pathway activity in CCA cells
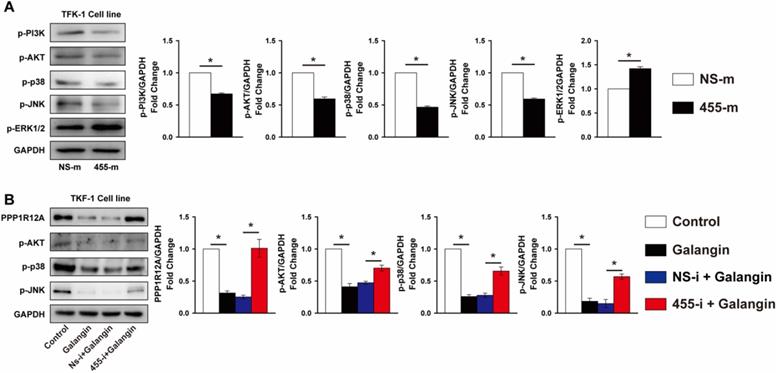
Previous studies demonstrated that the MAPK and PI3K/Akt signal pathway contributed greatly to tumor cells survival and metastasis [40, 41]. As shown in Fig. 6A, overexpression of miR-455-5p reduced the phosphorylation of p38 and JNK by 53% and 40% in TFK-1 cells, respectively, but increased ERK1/2 phosphorylation by 42% (Fig. 6A). Analogously, the phosphorylation of PI3K and AKT were also decreased by 33% and 40%, respectively, in TFK-1 cells transfected with miR-455-5p mimic compared to NS-m transfected cells (Fig. 6A). Similarly, galangin treatment significantly decreased phosphorylation of p38, JNK and AKT, as well as PPP1R12A expression (Fig. 6B). In contrast, inhibition of miR-455-5p expression abrogated these galangin-mediated phenotypic changes in TFK-1 cells (Fig. 6B). Collectively, these data indicate that miR-455-5p affects CCA cells survival and metastasis and mediates galangin's anti-tumor effects may through modulating PPP1R12A expression and MAPK and PI3K/AKT pathway.
Disscusion
In the present study, we provide evidence that the miR-455-5p expression was reduced in human CCA tissues and play critical roles in controlling CCA cells survival and metastasis. Moreover, we identified that PPP1R12A was a directly target of miR-455-5p and negative correlated with miR-455-5p expression in human CCA tissues, and PPP1R12A knockdown mimics miR-455-5p' effects on CCA cells. Furthermore, we found that galangin inhibited CCA growth both in vitro and in vivo, which was associated with increased miR-455-5p and decreased PPP1R12A expression. In support, using a loss-of-function approach, we demonstrated that inhibition of miR-455-5p expression abrogated galangin's anti-tumor effects. Finally, miR-455-5p overexpression and galangin treatment inhibited MAPK and PI3K/AKT pathway activity. Taken together, these data indicate that miR-455-5p play a critical role in controlling CCA growth and mediating the anti-tumor effects of galangin, at least in part, through targeting PPP1R12A and modulating MAPK and PI3K/AKT pathway.
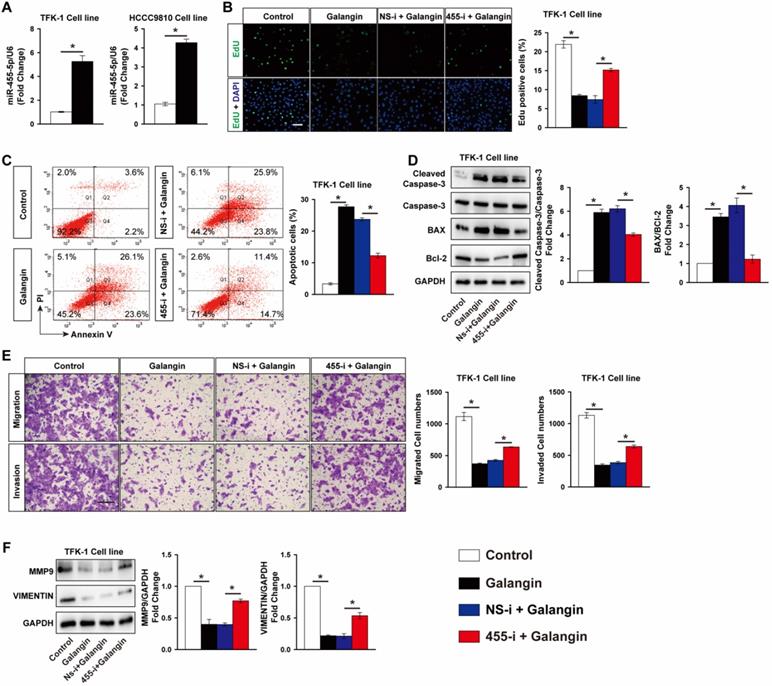
Inhibition of miR-455-5p abrogates galangin's anti-cancer effects on CCA cells. (A) Real-time PCR analysis of miR-455-5p expression in TFK-1 and HCCC9810 cells treated with galangin at 150 μM for 24h. TFK-1 cells were transfected with miR‐455-5p inhibitor (455-i) or non-specific inhibitor control (NS-i) at 100 nM for 24h followed by 150 μM galangin treatment for another 24h and harvested for (B) Edu analysis, Scale bar, 20 μM. (C) FACS analysis of apoptosis. (D) Western blot analysis of cleaved caspase 3, Caspase 3, Bax and Bcl-2 protein expression. (E) Matrigel-coated Transwell analysis of cell migration and invasion, scale bar, 50 μm. (F) Western blot analysis of MMP9 and Vimentin protein expression. A to F, n = 3 independent experiments. Values are given as means ± SEM. *P < 0.05.
Accumulating evidence demonstrated that miRNAs play an important role in tumor growth and metastasis by regulating protein expression at the post-transcriptional level [42]. For instance, miR-455-5p is an high conserved miRNA and play critical roles in multiple tumors development and progression [25, 27, 32-35, 39, 43]. Yet, the expression profile and role of miR-455-5p in CCA development and progression remains largely unknown. In the current study, we showed that the expression of miR-455-5p was reduced in CCA tissues compared to adjacent normal tissues. Previously studies identified that miRNAs enriched in a tissue- or cell-specific manner that can exact different biological functions by modulating large gene networks expression [44, 45]. This may explain why the expression of miR-455-5p was different to oral carcinoma and colon cancer. Findings from other groups suggested that miR-455-5p affected tumor growth largely dependent on modulating cell proliferation and apoptosis, an effect promoting tumor migration and invasion [39, 46, 47]. For example, overexpression of miR-455-5p suppressed prostate cancer cell proliferation and inhibited tumor growth by targeting C-C motif chemokine receptor 5 [47]. In colon cancer, miR-455-5p targeted galectin-9 expression, resulting in increased HT29 cells apoptosis and reduced proliferation [39]. In concordance with these observations, we demonstrated that miR-455-5p overexpression potently inhibited CCA cells proliferation, migration and invasion, whereas promoted apoptosis by using multiple complementary methods. Moreover, we demonstrated that PPP1R12A, a subunit of myosin phosphatase that play an critical role in regulating cell cycle and migration [25, 34, 39], was a new direct target of miR-455-5p and PPP1R12A expression was negatively correlated with miR-455-5p in CCA tissues. Indeed, previous studies found that PPP1R12A interacted with insulin receptor substrate 1-insulin-like growth factor 1 receptor (IRS1-IGF1R) complex and mediated the process of IRS1 dephosphorylation, promoting PI3K/AKT cascade activation and in turn led to tumor cell proliferation and tumor growth [43, 48]. In gastric cancer (GC) cells, silencing PPP1R12A inhibited GC cells proliferation by promoting cyclin D1 and c-myc expression to block the cell cycle [49]. Our findings also found that silencing PPP1R12A exhibited stronger anti-turmor effects on CCA cells that consistent with miR-455-5p overexpression. Taken together, these data suggest that miR-455-5p may use as a potential therapeutic target of CCA and the effects of miR-455-5p on suppressing CCA cells survival and metastasis, at least in part, through targeting PPP1R12A.
Accumulating evidence suggests that multiple signal pathways are playing critical roles in mediating cell proliferation, migration and invasion, the hallmarks of tumor growth and metastasis [50, 51]. Among those pathways, PI3K/AKT and MAPK pathways are probably the best known and interesting targets involved in preclinical and clinical trials for cancer treatment [52]. In the current study, we found that miR-455-5p overexpression significantly decreased PI3K/AKT phosphorylation in both HCCC9810 and TFK-1 cells, as well as p38 and JNK, two major MAPK downstream kinases, resulting in a pro-apoptotic phenotype. Together, these findings indicate that miR-455-5p inhibits MAPK and PI3K/AKT pathway activation, which in turn modulating CCA cells growth and metastasis.
MiR-455-5p knockdown abrogates Galangin-suppressed xenograft expansion in vivo. (A) Schematic illustration for the xenograftr modeling and galanign with non-specific inhibitor (NS-i) or miR-455-5p inhibitor (455-i) treatment. (B) The tumor growth curve over time of Balb/c nude mice mice treatment with galangin and NS-i or 455-i, respectively. (C) Tumor image of xenograft tissues after mice received galangin and NS-i or 455-i at day 28, respectively. (D) Tumor imaging and quantification of tumor volume. (E) Immunohistochemistry staining of Ki67 and quantification in xenograft tissues after mice received galangin and NS-i or 455-i, respectively. Scar bar 100 μM, insert 50 μM. (F) Immunohistochemistry staining of PPP1R12A expression and quantification in xenograft tissues after mice received galangin and NS-i or 455-i, respectively. Scar bar 100 μM, insert 25 μM. n = 6 mice per group. Values are given as means ± SEM. *P< 0.05.
miR-455-5p regulates PI3K/AKT and MAPK signal pathway activity and mediates galangin's effects. (A) Western blot analysis of phospho-PI3K, phopspho-AKT, phospho-p38, phospho-JNK and phospho-ERK1/2 expression at protein level in TFK-1 cells transfected with miR-455-5p mimic (455-m) or non-specific mimic control (NS-m) for 24h. (B) Western blot analysis of PPP1R12A, phospho-AKT, phospho-p38, phospho-JNK expression at protein level in TFK-1 cells transfected with miR-455-5p inhibitor (455-i) or non-specific inhibitor control (NS-i) for 24h, followed by galangin (150 μM) treatment for 24h. A and B, n = 3. Values are given as means ± SEM. *P < 0.05.
Although results from experimental and clinic studies demonstrate that gemcitabine and platinum is the first-line treatment of CCA patients [53, 54], the side effects and drug resistance remarkable limit those chemotherapy agents using in patients. Moreover, the median survival time in CCA patients by using those drugs is less than 8 to 12 months [7-9], making urgent clinical needed to develop novel therapeutic agents or discover appropriate adjuvant for CCA treatment. Accumulating studies identified that natural products, such as flavonoids, might be an eligible choice due to its stronger anti-tumor effects and low toxicity [18]. Galangin is a flavonoid derived from the root of galangal and exhibits various anti-tumor effects against multiple cancer types [20-22, 55, 56]. For instance, galangin inhibited glioma cells proliferation, metastasis, and angiogenesis by suppressing CD44 expression, a hall marker in glioma and associated with EMT [20]. Moreover, in retinoblastoma, galangin potently repressed cell progression and induced cell apoptosis through activating PTEN and caspase-3 pathways [55]. Furthermore, galangin induced gastric cancer cells apoptosis via regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase P [56]. In agreement with a recently published report [19], we found that galangin treatment potently induced CCA cells apoptosis and repressed proliferation and metastasis. Moreover, we added new evidence that, using both in vitro and in vivo approaches, the galangin inhibits CCA growth depended on miR-455-5p expression. Indeed, accumulating evidence suggested that galangin exhibits its anti-tumor effect not only act as a switch to regulate a specific, individual gene expression but rather to modulate expression of large gene networks (e.g. miR-21, H19, p53, CD44, and so on) [19, 20, 57, 58]. Collectively, these results suggest that galangin represents a high possibility as an adjuvant to treat CCA.
In summary, our findings demonstrate that miR-455-5p mediates galangin's anti-tumor effect and inhibits CCA cells proliferation, migration, and invasion, at least in part, by targeting PPP1R12A, an effect that decreases MAPK and PI3K/Akt pathway activation in CCA cells. Therefore, strategies targeting miR-455-5p can be serve as a potential therapeutic target for CCA and galangin may provide as novel treatment choice to improve chemotherapy efficiency.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This study was supported by the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (JY201703).
Ethical statement
This study was approved by the Ethics Committee for Human Research, Central South University and was conducted according to the approved guidelines. All animal experiments were approved by the Ethics Committee for Laboratory Animals of The Third Xiangya Hospital, Central South University, Hunan, China, and followed the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Testing, and Education by the New York Academy of Sciences, Ad Hoc Animal Research Committee.
Author Contributions
D.K. and M.J. designed this study. X.D., M.Z., Z.P. and Y.X. performed experiments, data analysis and produced figures. Z.Y. and Z.Z. performed data analysis and produced figures. X.D., D.K., and M.J. wrote the paper. All authors reviewed and approved the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Marin JJG, Lozano E, Briz O. et al. Molecular Bases of Chemoresistance in Cholangiocarcinoma. Curr Drug Targets. 2017;18(8):889-900
2. Simo KA, Halpin LE, McBrier NM. et al. Multimodality treatment of intrahepatic cholangiocarcinoma: A review. J Surg Oncol. 2016;113(1):62-83
3. Mathema VB, Na-Bangchang K. Current insights on cholangiocarcinoma research: a brief review. Asian Pac J Cancer Prev. 2015;16(4):1307-13
4. Rizvi S, Gores GJ. Liver capsule: Cholangiocarcinoma (CCA). Hepatology. 2016 63
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Ca A Cancer Journal for Clinicians. 2016;66(1):7-30
6. Gentilini A, Rombouts K, Galastri S. et al. Role of the stromal-derived factor-1 (SDF-1)-CXCR4 axis in the interaction between hepatic stellate cells and cholangiocarcinoma. Journal of Hepatology. 2012;57(4):813-20
7. Valle J, Wasan H, Palmer DH. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-81
8. Zhu AX. Future directions in the treatment of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29(2):355-61
9. Macias RI. Cholangiocarcinoma: Biology, Clinical Management, and Pharmacological Perspectives. ISRN Hepatol. 2014 2014828074
10. Park JO, Oh DY, Hsu C. et al. Gemcitabine Plus Cisplatin for Advanced Biliary Tract Cancer: A Systematic Review. Cancer Research & Treatment. 2015;47(3):343-61
11. Unno M. [Review of surgical treatment of perihilar cholangiocarcinoma: proper patient selection for combined vascular resection and reconstruction]. Nihon Geka Gakkai Zasshi. 2014;115(4):181-4
12. Lubezky N, Facciuto M, Harimoto N. et al. Surgical treatment of intrahepatic cholangiocarcinoma in the USA. Journal of Hepato-Biliary-Pancreatic Sciences. 2015;22(2):124-30
13. Tsuchikawa T, Hirano S, Okamura K. et al. Advances in the surgical treatment of hilar cholangiocarcinoma. Expert Review of Gastroenterology & Hepatology. 2015;9(3):369-74
14. Murakami Y, Yokoyama T, Takesue Y. et al. Long-term survival of peripheral intrahepatic cholangiocarcinoma with metastasis to the para-aortic lymph nodes. Surgery. 2000;127(1):105-106
15. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168-79
16. Jemal A, Siegel R, Ward E. et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71-96
17. Atanasov AG, Zotchev SB, Dirsch VM. et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021:200-16
18. Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20(2):187-210
19. Zou Y, Li R, Kuang D. et al. Galangin Inhibits Cholangiocarcinoma Cell Growth and Metastasis through Downregulation of MicroRNA-21 Expression. Biomed Res Int. 2020 20205846938
20. Chen D, Li D, Xu XB. et al. Galangin inhibits epithelial-mesenchymal transition and angiogenesis by downregulating CD44 in glioma. J Cancer. 2019;10(19):4499-508
21. Vukovic NL, Obradovic AD, Vukic MD. et al. Cytotoxic, proapoptotic and antioxidative potential of flavonoids isolated from propolis against colon (HCT-116) and breast (MDA-MB-231) cancer cell lines. Food Res Int. 2018:10671-80
22. Yu S, Gong LS, Li NF. et al. Galangin (GG) combined with cisplatin (DDP) to suppress human lung cancer by inhibition of STAT3-regulated NF-κB and Bcl-2/Bax signaling pathways. Biomed Pharmacother. 2018:97213-24
23. Belver L, de Yébenes VG, Ramiro AR. MicroRNAs prevent the generation of autoreactive antibodies. Immunity. 2010;33(5):713-22
24. Harrandah AM, Mora RA, Chan EKL. Emerging microRNAs in cancer diagnosis, progression, and immune surveillance. Cancer Lett. 2018:438126-132
25. Wang J, Wang Y, Sun D. et al. miR-455-5p promotes cell growth and invasion by targeting SOCO3 in non-small cell lung cancer. Oncotarget. 2017;8(70):114956-65
26. Liu Y, Tang Y, Li P. Inhibitory effect of microRNA-455-5p on biological functions of esophageal squamous cell carcinoma Eca109 cells via Rab31. Exp Ther Med. 2018;16(6):4959-66
27. Shoshan E, Mobley AK, Braeuer RR. et al. Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis. Nat Cell Biol. 2015;17(3):311-21
28. Kovalchuk O, Filkowski J, Meservy J. et al. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7(7):2152-9
29. Núñez-Olvera SI, Chávez-Munguía B, Del Rocío Terrones-Gurrola MC. et al. A novel protective role for microRNA-3135b in Golgi apparatus fragmentation induced by chemotherapy via GOLPH3/AKT1/mTOR axis in colorectal cancer cells. Sci Rep. 2020;10(1):10555
30. Dai F, Dai L, Zheng X. et al. Non-coding RNAs in drug resistance of head and neck cancers: A review. Biomed Pharmacother. 2020;127:110231
31. Sun X, He S, Wara AKM. et al. Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2014;114(1):32-40
32. Liu J, Zhang J, Li Y. et al. MiR-455-5p acts as a novel tumor suppressor in gastric cancer by down-regulating RAB18. Gene. 2016;592(2):308-15
33. Wang J, Lu Y, Zeng Y. et al. Expression profile and biological function of miR-455-5p in colorectal carcinoma. Oncol Lett. 2019;17(2):2131-40
34. Xing Q, Xie H, Zhu B. et al. MiR-455-5p Suppresses the Progression of Prostate Cancer by Targeting CCR5. 2019; 2019: 6394784.
35. Cheng CM, Shiah SG, Huang CC. et al. Up-regulation of miR-455-5p by the TGF-β-SMAD signalling axis promotes the proliferation of oral squamous cancer cells by targeting UBE2B. J Pathol. 2016;240(1):38-49
36. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97-110
37. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15-20
38. Sticht C, De La Torre C, Parveen A. et al. miRWalk: An online resource for prediction of microRNA binding sites. PLoS One. 2018;13(10):e0206239
39. Yang Q, Hou C, Huang D. et al. miR-455-5p functions as a potential oncogene by targeting galectin-9 in colon cancer. Oncol Lett. 2017;13(3):1958-64
40. Qureshi HA, Pearl JA, Anderson KA. et al. Fibroblast Growth Factor 19 Activates the Unfolded Protein Response and Mitogen-Activated Protein Kinase Phosphorylation in H-69 Cholangiocyte Cells. J Liver. 2014;3(3):158
41. Wang Y, Liang Y, Yang G. et al. Tetraspanin 1 promotes epithelial-to-mesenchymal transition and metastasis of cholangiocarcinoma via PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37(1):300
42. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857-66
43. Xin Y, Wang X, Meng K. et al. Identification of exosomal miR-455-5p and miR-1255a as therapeutic targets for breast cancer. Biosci Rep. 2020;40(1):BSR20190303
44. Cheng HS, Besla R, Li A. et al. Paradoxical Suppression of Atherosclerosis in the Absence of microRNA-146a. Circ Res. 2017;121(4):354-67
45. Virtue AT, McCright SJ, Wright JM. et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med. 2019;11(496):eaav1892
46. Liu Z, Zou D, Yang X. et al. Melatonin inhibits colon cancer RKO cell migration by downregulating Rho-associated protein kinase expression via the p38/MAPK signaling pathway. Mol Med Rep. 2017;16(6):9383-92
47. Xing Q, Xie H, Zhu B. et al. MiR-455-5p Suppresses the Progression of Prostate Cancer by Targeting CCR5. Biomed Res Int. 2019;2019:6394784
48. Rizvi S, Khan SA, Hallemeier CL. et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95-111
49. Patel VB, Zhabyeyev P, Chen X. et al. PI3Kalpha-regulated gelsolin activity is a critical determinant of cardiac cytoskeletal remodeling and heart disease. Nat Commun. 2018;9(1):5390
50. Zhang L, Huang D, Shao D. et al. Fenretinide inhibits the proliferation and migration of human liver cancer HepG2 cells by downregulating the activation of myosin light chain kinase through the p38-MAPK signaling pathway. Oncol Rep. 2018;40(1):518-26
51. Lin ZY, Chen G, Zhang YQ. et al. MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer. Mol Cancer. 2017;16(1):48
52. Heissler SM, Manstein DJ. Nonmuscle myosin-2: mix and match. Cell Mol Life Sci. 2013;70(1):1-21
53. Schweitzer N, Vogel A. Systemic therapy of cholangiocarcinoma: From chemotherapy to targeted therapies. Best Pract Res Clin Gastroenterol. 2015;29(2):345-53
54. Ramirez-Merino N, Aix SP, Cortes-Funes H. Chemotherapy for cholangiocarcinoma: An update. World J Gastrointest Oncol. 2013;5(7):171-6
55. Zou WW, Xu SP. Galangin inhibits the cell progression and induces cell apoptosis through activating PTEN and Caspase-3 pathways in retinoblastoma. Biomed Pharmacother. 2018:97851-63
56. Kim DA, Jeon YK, Nam MJ. Galangin induces apoptosis in gastric cancer cells via regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase P. Food Chem Toxicol. 2012;50(3-4):684-8
57. Zhong X, Huang S, Liu D. et al. Galangin promotes cell apoptosis through suppression of H19 expression in hepatocellular carcinoma cells. Cancer Med. 2020;9(15):5546-57
58. Huang H, Chen AY, Ye X. et al. Galangin, a Flavonoid from Lesser Galangal, Induced Apoptosis via p53-Dependent Pathway in Ovarian Cancer Cells. Molecules. 2020;25(7):1579
Author contact
![]() Corresponding authors: Dabin Kuang, E-mail: kdbedu.cn; and Minna Jiang, E-mail: 408957278com
Corresponding authors: Dabin Kuang, E-mail: kdbedu.cn; and Minna Jiang, E-mail: 408957278com

 Global reach, higher impact
Global reach, higher impact