3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(16):5053-5065. doi:10.7150/jca.57752 This issue Cite
Research Paper
miR-30a/SOX4 Double Negative Feedback Loop is modulated by Disulfiram and regulates EMT and Stem Cell-like properties in Breast Cancer
Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
*These authors contributed equally to this work.
Abstract
Background: Both epithelial-to-mesenchymal transition (EMT) and cancer stem cells play important roles in development and progression of breast cancer. MicroRNA (miR)-30 family members have been reported to be associated with the regulation of EMT and stem cell phenotypes, however, the underlying molecular mechanisms are not well understood.
Methods: miR-30a stable transfectants of breast cancer cell lines were created using a lentiviral system. Bioinformatics analysis was performed to explore miR-30a target genes and SOX4 was selected and identified by dual luciferase reporter assay. The effects of miR-30a and target gene SOX4 on EMT and CSC phenotypes in breast cancer were explored in vitro and in vivo.
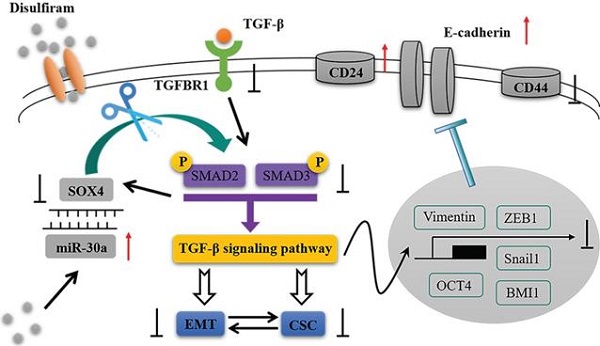
Results: Overexpression of miR-30a in breast cancer cells inhibited EMT and CSC phenotypes by targeting SOX4. Luciferase reporter assay confirmed that miR-30a directly targeted 3'UTR of SOX4, and formed a double-negative feedback loop with SOX4. Functional experiments demonstrated that knockdown of SOX4 suppressed EMT and CSC phenotypes of breast cancer cells through TGF-β/SMAD pathway, which was consistent with the inhibitory effects by overexpression of miR-30a. Additionally, we found disulfiram can upregulate miR-30a expression, and high miR-30a expression was associated with a good prognosis in breast cancer patients through TCGA database.
Conclusion: Our findings suggest a novel double-negative loop between miR-30a and SOX4 mediated regulation of EMT and CSC features in breast cancer through TGF-β/SMAD pathway, highlighting a novel therapeutic target for breast cancer.
Keywords: breast cancer, epithelial-mesenchymal transition, cancer stem cell, miR-30a, SOX4

 Global reach, higher impact
Global reach, higher impact