3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2021; 12(21):6498-6506. doi:10.7150/jca.59251 This issue Cite
Research Paper
Safety and efficacy of fecal microbiota transplantation to treat and prevent recurrent Clostridioides difficile in cancer patients
1. Department of Internal Medicine, Baylor College of Medicine, Houston, TX, USA.
2. Department of Internal Medicine/Pediatrics, The University of Texas Health Science Center at Houston, Houston, TX.
3. Department of Hepatobiliary and Pancreatic Surgery, Zhongnan Hospital of Wuhan University, Wuhan, China.
4. Department of Gastroenterology, Hepatology, and Nutrition, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
5. Department of Biosciences, Rice University, Houston, Texas, USA.
6. Center for Infectious Diseases, School of Public Health, The University of Texas Health Science Center at Houston, Houston, TX, USA.
7. Department of Infectious Diseases, Infection Control, and Employee Health, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
*Co-first authors.
Abstract
Background: Cancer patients are at increased risk of recurrent Clostridioides difficile infection (rCDI) due to malignancy itself, cancer therapy, and frequent antibiotic use and have a lower response rate to standard oral antibiotics. There are limited data on the safety and efficacy of fecal microbiota transplantation (FMT) for treating rCDI in cancer patients. We aim to describe our experience of using FMT to treat rCDI at a tertiary cancer center.
Methods: We conducted a retrospective study of cancer patients who underwent FMT for rCDI at The University of Texas MD Anderson Cancer Center from June 2017 through January 2020. Baseline clinical data and risk factors related to rCDI and FMT were evaluated and compared between cancer types and between cases with remission and recurrence.
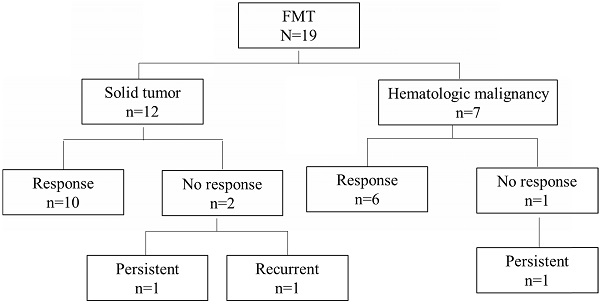
Results: A total of 19 patients were studied: 12 with solid malignancies and 7 with hematologic malignancies. Most patients had stage IV cancer, and 21% of patients were in cancer remission. On average, patients had 2 episodes of CDI and received 3 courses of antibiotics within 1 year before FMT. 84% of patients with rCDI responded to FMT. Compared with patients who had CDI remission following FMT, non-remission cases were more likely to have received antibiotics following FMT. There were no serious adverse events or mortality within 30 days associated with FMT.
Conclusions: FMT is safe, well-tolerated, and efficacious in treating rCDI in selected cancer patients. However, additional antibiotic use for complications from chemotherapy or immunosuppression negatively affected the efficacy of FMT in this population with advanced cancer.
Keywords: Recurrent Clostridioides difficile infection, fecal microbiota transplantation, FMT, cancer, malignancy

 Global reach, higher impact
Global reach, higher impact