3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(2):579-588. doi:10.7150/jca.63991 This issue Cite
Research Paper
Intravenous Delivery of RNA Encoding Anti-PD-1 Human Monoclonal Antibody for Treating Intestinal Cancer
1. Department of Laboratory Medicine, Dongtai People's Hospital & Affiliated Dongtai Hospital of Nantong University, Yancheng 224200, P.R. China.
2. Department of Laboratory Medicine, Shanghai Tongji Hospital, School of Medcine, Tongji University, Shanghai 200065, P.R. China.
3. Department of Pathology, Tinghu People's Hospital of Yancheng City, Yancheng 224001, P.R. China.
4. Department of Laboratory Medicine, Tinghu People's Hospital of Yancheng City, Yancheng 224001, P.R. China.
* The authors contributed equally to this work.
Abstract
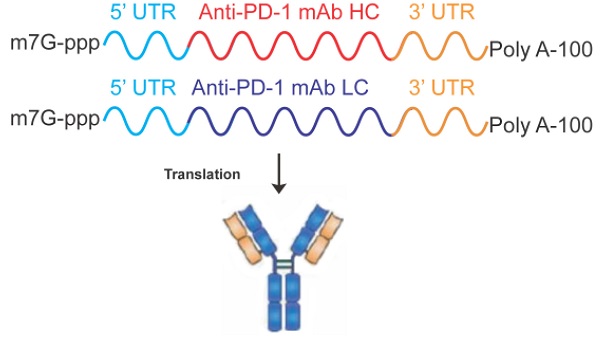
Recently, antibody-based therapeutic agents are becoming most leading biologics for treating many diseases, especially for cancer. However, large-scale application of antibody drugs is still hampered by high cost and complex technical process. Endogenous expression of proteins or antibodies can be achieved by applying in vitro transcription (IVT) technique to produce mRNA and then deliver into body, which supplies opportunity to avoid many disadvantages in antibody production as well as clinical applications. Here, we designed the IVT-mRNA encoding the Pembrolizumab, as a commercial anti-PD-1 monoclonal antibody (mAb). The in vitro functional properties and in vivo antitumor activities of the Pembrolizumab expressed from mRNA were both assessed. Maximized expression level of the Pembrolizumab from IVT-mRNA was achieved via optimizing the usage of signal peptide and molar ratio of heavy/light chain. Then the mRNA was further formulated by lipid nanoparticle (LNP), which enable efficient in vivo delivery and protect mRNA from degradation. Intravenously delivered the single dose of mRNA-LNPs in mice resulted in duration of serum Pembrolizumab level over 25 μg/mL more than 35 days. Pharmacokinetic study exhibited significantly enhanced drug exposure of mRNA-encoded mAbs compared with direct injection of Pembrolizumab at same dose. Chronic treatment of the tumor-bearing mice with LNP-encapsulated Pembrolizumab mRNA effectively downregulated the growth of intestinal tumors and improved the animal survival. In brief, our present research demonstrated that the application of LNP-encapsulated IVT-mRNA can change the human body into a protein drug manufacturing site to express full-size mAbs for treating cancer and hold potential to be a novel alternative to protein-based therapies.
Keywords: mRNA, In vitro transcription, Lipid nanoparticle, Anti-PD-1 antibody, Protein replacement therapy

 Global reach, higher impact
Global reach, higher impact