Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(5):1685-1694. doi:10.7150/jca.69278 This issue Cite
Review
Tumor-derived exosomes in hypoxic microenvironment: release mechanism, biological function and clinical application
1. Department of Burn and Plastic Surgery-Hand Surgery, First People's Hospital of Changshu City, Changshu Hospital Affiliated to Soochow University, Soochow, China.
2. College of Pharmaceutical Science, Soochow University, Soochow, China.
3. Department of General Surgery, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China.
4. Department of General Surgery, First People's Hospital of Changshu City, Changshu Hospital Affiliated to Soochow University, Soochow, China.
Received 2021-11-20; Accepted 2022-1-26; Published 2022-3-14
Abstract
Hypoxia is a key feature of solid tumors and is related to disease aggressiveness and adverse outcomes. It is recognized that the two-way communication between cancer cells and their microenvironment is critical to cancer progression. Increasing evidences show that the cellular communication and crosstalk between tumor cells and their microenvironment is not limited to secreted molecules, but also includes exosomes secreted by tumor cells. Exosomes are nano-scale extracellular vesicles (30-100 nm in diameter), which carry the molecular characteristics and cargo of the source cell, participating in intercellular communication through autocrine, paracrine and near-crine pathways. Recent studies have shown that cancer cells produce more exosomes under hypoxic conditions than normoxia conditions. The secretion and function of exosomes could be influenced by hypoxia in various types of cancer. Therefore, in this review, we summarize and discuss the latest research on the physiological mechanism of hypoxia regulating the secretion of exosomes, and the involvement of hypoxic exosomes in cancer progression and immune escape processes, and expounds the potential for targeting hypoxia-induced exosomes for cancer therapy strategies.
Keywords: Hypoxic microenvironment, Cancer, Exosomes, Biological functions, Cancer therapy
Background
The initial research on hypoxia of cancers mostly comes from the judgment of the oxygen tension of cancer cells. In 1964, Cater used oxygen cathode technique to detect the difference of oxygen tension in cancer tissue [1]. After that, in 1977 Vaupel named “Hypoxia in neoplastic tissue” for the first time and proved experimentally that with the increase in cancer volume, hypoxemia will appear in the cancer tissue, which may cause glucose depletion and cell lysis and necrosis [2].
Current research believes that cancer cells are exposed to a continuous medium of oxygen concentration, consisting of three tissue areas: normoxic zone, hypoxic zone, and necrotic zone [3]. Normal oxygen cells are located near functional blood vessels and have the typical ability to survive and proliferate. Hypoxia usually occurs in solid cancers about 100 μm away from functional blood vessels [4]. Cells about 150 μm away from blood vessels may be necrotic at very low oxygen concentrations (PO2≤1%) [5]. Later, researchers find that cancer hypoxia has different characteristics that can be divided into acute hypoxia or chronic hypoxia [6, 7]. The acute hypoxia, also known as ischemic hypoxia, is usually a structural and functional abnormality caused by the transient cancer disorder vasculature and structure [7, 8]. Chronic hypoxia limited hypoxia is due to the imbalance between the supply and demand of oxygen as the distance of cancer growth and diffusion increases, and the rapid expansion of the cancer leads to insufficient oxygen in cancer tissue 70-150 μm from the open blood vessel [6]. Acute and chronic hypoxia are associated with poor prognosis and cancer aggressive phenotype [8, 9].
The expression of most factors involved in the response of tumor cells to hypoxia is mainly regulated by hypoxia-inducible factor-1 (HIF-1). However, several other HIF-1 independent pathways, such as phospholipid protein trikinase (PI3K)-Akt, mammalian target rapamycin (mTOR), Wnt/β-caterine, mitochondrial activated protein kinase (MAPK), nuclear Factor-β and NADPH oxidase (NOX) are also involved in the adaptation of cancer cells under hypoxic conditions [10]. It is recognized that the two-way communication between cancer cells and their microenvironment is critical to cancer progression. More and more evidences show that the cellular communication and crosstalk between hypoxic tumor cells and their microenvironment is not limited to secreted molecules, but also includes exosomes secreted by hypoxic tumor cells [11].
Exosomes are nano-scale extracellular vesicles (30-100 nm in diameter), which are the extracellular form of intraluminal vesicles (ILVs) secreted after multivesicular endosomes (MVEs) is injected into the plasma membrane [12]. Exosomes released by different types of tumor cells carry the molecular characteristics and cargo of the source cell, and participate in intercellular communication through autocrine, paracrine and near-crine pathways [13, 14]. The results of recent studies clearly show that the hypoxic environment strongly regulates the biogenesis of exosomes and participates in the progression of cancer. Therefore, in this review, we summarize and discuss the latest research on the physiological mechanism of hypoxia regulating the secretion of exosomes, and the involvement of hypoxic exosomes in cancer progression and immune escape processes, and expounds the potential for targeting hypoxia-induce exosomes for cancer therapy strategies.
Response and adaptation to hypoxia in cancers
Hypoxia-induced changes in the proteome stimulate cancer growth, invasion, and metastasis by promoting adaptation and survival in harsh nutritionally deficient environments [8]. At the molecular level, the adaptation of cancer cells to hypoxic stress is mainly regulated by HIF, which is a transcription factor that accumulates when cellular oxygen levels decrease [15, 16].
HIFs are members of the Arnt sim transcription factor superfamily, which consists of an heterodimer of oxygen sensitive α subunit and a constitutively expressed β subunit (HIF1 β) [17]. At present, it has been found that there are three isoforms of HIF-1α, HIF-2α and HIF-3α [18]. Under normal oxygen, HIFα protein was rapidly hydroxylated by a group of prolyl hydroxylase domain (PHD) enzymes, resulting in rapid degradation of HIF α [19]. In the second mode of HIF α regulation, HIF α asparagine residues inactivate HIF α transcription activity by blocking the interaction of transcription cofactor cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) binding protein (CBP) and histone acetyltransferase P300 (P300 hat) with HIF α through the inhibitory factor of HIF1 (FIH1; also known as HIF1AN), thereby blocking transcription [20]. On the contrary, in the process of hypoxia, HIF α subunit did not hydroxylate, PHDs and FIH1 reduced the rate of HIFα protein hydroxylation, resulting in protein stabilization and CBP-p300 co-activation complex enhanced transcription activation, and increased HIFα level and HIF target gene expression activation [21].
The biology of exosomes
Exosomes are ILVs formed by the inward budding of the endosomal membrane during the maturation of MVEs, which are nanoscale extracellular vesicles (30-100nm in diameter) secreted after the infusion of MVE with cell membrane. In the mid-1990s, it was reported that exosomes are secreted by B lymphocytes [22] and dendritic cells [23] with potential functions related to immune regulation and were considered to be vehicles in anti-tumor immune responses. Exosome secretion has now expanded to many different cell types, and its effect on cell-to-cell communication under normal and pathological conditions has now been fully demonstrated [24].
Biogenesis of exosomes
Exosomes are generated in a process that involves double invagination of the plasma membrane. The first invagination of the plasma membrane forms the de novo formation of an early-sorting endosome (ESE), through the endocytosis of cell-surface proteins and soluble proteins associated with the extracellular milieu. ESE could merge with the endoplasmic reticulum (ER), trans-Golgi network (TGN), and may directly merge with a preexisting ESE in some cases [25-28]. The further maturation of ESEs forms late-sorting endosomes (LSEs), and the invagination of the plasma membrane of LSEs forms ILVs that are ultimately secreted as exosomes with a size range of about 40 to 160 nm in diameter. In the end, the fate of MVEs is to be dissolved by fusion with autophagosomes or lysosomes, or to release exosomes by fusion with the plasma membrane [29].
Machineries involved in the biogenesis of exosomes
Exosomes are generated within the endosomal system as ILVs during the process of maturation into MVE, a process that involves particular sorting machineries. At first, cargoes are segregated on the microdomains of the limiting membrane of MVE by these machineries, and then this microdomains inward budding and fission to form the small membrane vesicles containing sequestered cytosol [26].
In this process, the sorting of transmembrane cargoes into exosomes largely depends on the sorting machineries of endosomes. The discovery of endosomal sorting complexes required for transport (ESCRT) was a major breakthrough [30]. Exosomes can also be formed in an ESCRT independent manner. Additonally, some other mechanisms also play an important role in promoting exosomes biogenesis. For example, chaperone heat shock 70kDa protein (HSP70) and heat shock homologous 71kDa protein (HSC71) can participate in the co-sorting of loading cytoplasmic proteins into ILVs [31]. Some other transmembrane cargoes, such as glycosylphosphatidylinositol (GPI) anchored proteins, which probably enter exosomes due to their affinity for lipid domains and lipid rafts, directly participating in the generation of ILVs by affecting membrane-mediated biophysical properties. Additionally, the effects of ubiquitination [32] and farnesylation [33] have also been reported, but the concrete mechanisms are still not unclear.
Apart from proteins, exosomes can also load nucleic acids, including RNA (mRNA, miRNA, lncRNA) and DNA. Importantly, the selective loading of miRNA depends on its specific motif. Heterogeneous ribonucleoprotein A2B1(hnRNPA2B1) is a commonly expressed RNA-binding protein that controls the transport and subcellular localization of specific mRNA in neurons [34]. The glycosylation of hnRNPA2B1 can recognize and bind to specific motifs on miRNA, thereby regulating miRNA entry into exosomes [35].
The release of exosomes
Transport of MVEs
After the formation of MVEs, MVEs have two fates. One is to be transported to lysosomes or autophagosomes to be degraded. The other one is to release exosomes after fusion with the plasma membrane. However, the results of these two methods are quite different. There is a potential balance mechanism between these two pathways. The establishment of this balance will undoubtedly affect the function of the cell, and its mechanism details have yet to be explored.
In general, intracellular transport involves organelles and cytoskeleton (actin and microtubules), related molecular motors (Dynein, kinetin and inosine) and molecular switches (small GTPases) [36, 37]. Among them, small GTPases play a vital role in the transportation of MVEs. The Rab protein family is the largest subfamily in the small GTPases family. Of the 93 small GTPases members in Arabidopsis, 57 belong to the Rab subfamily [38]. Different Rab proteins are located at specific locations on the cytoplasmic side of the plasma membrane, mainly using as molecular switches to regulate complex vesicle transport and microtubule system activity to regulate the transport of eukaryotic cell vesicle and protein. There are two different conformations between which Rab protein switches: GTP-bound active form and the other GDP-bound inactive form. In the activated form of GTP-bound, Rab can recruit some specific effector proteins to regulate vesicle formation and actin- and tubulin-dependent vesicle movement [39]. Rab6 was initially considered to be a key participant in the retrograde transport of microtubules, controlling the transport of early vesicles from early endosomes through Golgi to TGN and from Golgi to the endoplasmic reticulum [40]. It seems to be involved in a complex network of protein-protein interactions.
In addition, Rab27a and Rab27b and their respective effectors, synaptotagmin-like protein-4 and exophilin-5, are also essential for exosome secretion [41]. Rab27b regulates the movement of MVEs torwards the plasma membrane, and both of these Rab27 isoforms can play a role in the step after MVEs are transported, which is the docking at the plasma membrane to promote fusion, and then increasing exosome secretion. Rearrangement of the sub-membrane actin cytoskeleton is a step that is common in all mechanisms involved in vesicle secretion. Naturally, the role of Rab27a in the docking of MVEs is also related to it [42]. Additionally, KIBRA may act as an adaptor-like protein and stabilizer for Rab27a to prevent the degradation of Rab27a by ubiquitination, thereby promoting the secretion of exosomes [43]. Rab27a can also control the secretion of secreted lysosomes (so-called lysosome-associated organelles), suggesting that MVE with exosome secretion capacity may be considered as a specialized compartment rather than a simple MVE subtype [44]. Researchers also found that Rab11 uses Munc13-4 (Rab binding protein) as an effector to regulate the release of exosomes by regulating the docking of MVEs to the plasma membrane [45]; Rab35 is localized in oligodendrocytes in a GTP-dependent manner to increases the density of vesicles, which may increase the secretion of exosomes through its docking or binding function [46].
These studies show that Rab subtypes are not constitutively expressed in all cell types, which means that each cell type can adapt to its own secretion mechanism for exosome secretion. Some other Rab subtypes, including Rab5, Rab7, and Rab22a, have also been reported in the role of MVEs transport.
Fusion of MVEs with the plasma membrane
The final step in exosome secretion requires the fusion of MVEs with the plasma membrane to release exosomes as ILVs. This process may be mediated by members of the soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) [47] protein and synapse binding protein family [48]. It is known that the SNARE complex involved in conventional lysosomal exocytosis is composed of vesicle-associated membrane protein 7 (VAMP7) on the lysosome, syntaxin 7 on the plasma membrane and lysosomal regulatory protein synapse binding protein 7. This complex is involved in exosome secretion [49] of certain cells (human leukemia cell line K562, but not in other cells (MDCK cells) [50]. Exosome secretion process has been shown to be regulated by Ca2+ in several cell types, which may play a role in the activation of SNARE complex [51]. Interestingly, synaptosome-related protein 23 (SNAP23) can regulate both exosome secretion [52] and lysosomal related organelles [53], which further strengthens the concept that MVE is a specialized secretory organelle. In addition, syntaxin 6 [54] in prostate cancer; synaptic homologue Ykt6 [55] in Drosophila; syntaxin 5 [56] in C. elegans; and syntaxin 3 in human cytomegalovirus [47] all play a regulatory role in the secretion of exosomes, which again certificates that exosome regulatory factors may show diversity due to the differences in organisms, cell types, or MVE subtypes.
The role of hypoxia in the regulation of exosome secretion
The role of hypoxia in the sorting process
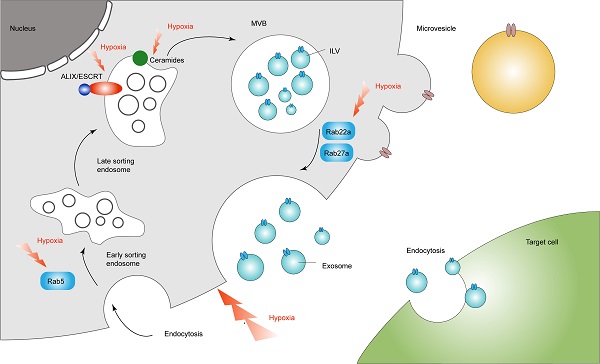
A variety of signaling molecules in the microenvironment can affect the level of ceramide. Hypoxia and ischemia can activate various signaling molecules to induce an increase in ceramide levels, which may be achieved by reducing the activity of ceramide enzymes [57]. Accordingly, hypoxia can promote the biogenesis of ILVs, increasing the secretion of exosomes. In addition to the sorting mechanism and RNA binding protein, selective loading of miRNA in exosomes is also regulated by the external microenvironment. It has been reported that the level of miRNA detected in exosomes under hypoxia was significantly higher than that under normoxia, suggesting that hypoxia may have an effect on the distribution of miRNAs in exosomes, and this process may be carried out in a HIF-dependent manner [58]. Among various cell types, microRNA-210 is considered to be one of the most stable and most important up-regulated miRNAs in response to hypoxia. The HRE on the proximal promoter of miR-210 can directly bind to HIF-1α and be regulated by it [59]. In ovarian cancer, HIF can induce the release of exosomes rich in various miRNAs, including miR-21-3p, miR-125b-5p and miR-181d-5p [60]. Similarly, under hypoxic conditions, miR-135b and miR-21 in exosomes secreted by melanoma and PANC cells also showed an increase [61, 62]. Therefore, hypoxia may affect the distribution of miRNA in exosome by depending on HIF. However, the specific mechanisms still need to be further explored.
All in all, the biogenesis of exosomes is certainly complex; it depends on the cargoes and cell type.
The role of hypoxia in the transport of MVEs
It has been shown that hypoxia can regulate the expression of Rab7 and Rab27a by inducing the expression of STAT3, thereby promoting the release of ovarian cancer cell exosomes [63]; HIF can activate the transcription genes of Rab22a and small GTPase Rab22a expression mediates the release of breast cancer cell exosomes [64]. Moreover, Rab5 has a possible mechanism of increased exosome secretion mediated in hypoxic PCa cells. It may regulate the transport of clathrin-coated vesicles from the cell membrane to early endosomes and the fusion of homotypic early endosomes, regulating the release of exosomes, and Rab5 accumulates more in the perinuclear area under hypoxic conditions [65]. It means that Rab5 can regulate the possibility of exosome secretion in a hypoxic environment. Besides, hypoxia can also affect the expression of RHO-related protein kinase (ROCK) to regulate the rearrangement of the actin cytoskeleton, which in turn affects the release process of exosomes [66].
In addition to promoting the secretion of exosomes by regulating the transport process of MVEs, hypoxia has also been shown to promote autophagy [67]. Therefore, we can speculate that the balance mechanism between the degradation of MVEs and the release of exosomes still exists in the hypoxic external environment, which need to be explored further.
Hypoxia in the regulation of exosome secretion. Hypoxia regulates exosomal secretion by affecting processes such as Rab5, Rab22a, Rab27a, ceremides and ALIX/ESCRT.
The role of hypoxia-induced exosomes involved in cancer biology
| Source cells | Regulatory factors | Biological function | Mechanism | Ref |
|---|---|---|---|---|
| Hepatocellular carcinoma | Exosomal miR-1273f | Increase angiogenesis | Downregulate its target LHX6 | [69] |
| Hepatocellular carcinoma | Exosomal miR-23a/b | Increase angiogenesis | Target the von Hippel-Lindau/hypoxia-inducible factor axis | [70] |
| Hepatocellular carcinoma | Exosomal miR-155 | Increase angiogenesis | / | [71] |
| Oral Squamous Cell Carcinoma | Exosomal miR-21 | Increase the migration and invasion | HIF-1 and HIF-2 increase the expression of miR-21 | [58] |
| Bladder cancer | Exosomal lncRNA-UCA1 | Increase the migration and invasion | LncRNA-UCA1 promotes tumor progression though EMT | [72] |
| Oral Squamous Cell Carcinoma | Exosomal miR-21 | Regulate immune response | Target PTEN/PD-L1 axis | [78] |
| Nasopharyngeal carcinoma | Exosomal miR-24-3p | Regulate immune response | Downregulate its target FGF11 | [79] |
| Pancreatic cancer | Exosomal miR-301a-3p | Regulate immune response | Target PTEN/PI3K axis | [84] |
| Lung cancer | Exosomal miR-103a | Regulate immune response | Target AKT/STAT3 axis | [85] |
| Epithelial ovarian cancer | Exosomal miR-940 | Regulate immune response | / | [86] |
| Lung cancer | Exosomal TGF-β | Regulate immune response | Decrease the expression of NKG2D | [91] |
As mentioned in the review, we can recognize the regulatory role of hypoxia in the process of exosome secretion (Figure 1). This gives us a new direction for targeted treatment of tumors through the regulation of exosomes through the hypoxic microenvironment.
The role of hypoxia-induced exosomes involved in tumor microenvironment
Increasing studies have found that exosomes are involved in the formation and metastasis of cancer blood vessels, and lead to the immune escape of cancer by regulating the differentiation of immune cells (Table 1).
Hypoxia-induced exosomes promote cancer angiogenesis and metastasis
According to conventional opinion, cells enter the bloodstream from the primary tumor. They then implant to distant organs like the lungs, where the cells grow into metastatic tumors. Another idea is that the primary tumor sends out exosomes, which prepare the tumor cell to take root at a distance and recruit nourish incoming tumor cell. It is reported that lncRNA-ROR can promote the growth of hepatocellular carcinoma cells under hypoxic stress [68]. Similarly, exosomes, as mediators of cell-to-cell communication, have also been reported to be involved in the angiogenesis and metastasis of cancer under hypoxic stress. For example, exosomes miR-1273f and miR-23a/b of hypoxic HCC cells promote the aggressive phenotype of cancer cells and drive the growth and development of HCC [69, 70]; Interestingly, exosomes released from HCC under hypoxia have also been found to be able to enrich miR-155. Exosomes increase miR-155 in HUVECs, thereby promoting the formation of blood vessels [71]. The renewal of blood vessels contributes to tumor metastasis. Therefore, we further explored the impact of exosomes released by tumors on tumor metastasis in the hypoxic microenvironment.
A recent study showed that hypoxic oral squamous cell carcinoma (OSCC) promotes the secretion of exosomes through HIF-1 and HIF-2, and the miR-21 carried by exosomes can promote the migration and invasion of other oral squamous cell carcinoma cells [58]. Meanwhile, the researchers observed the internalization process of bladder cancer cells with exosomes by fluorescently labeling exosomes. At the same time, they detected a significant high expression of lncRNA-UCA1 in cells with internalized exosomes. Further in vitro experiments confirmed that exosomes promote the EMT process, which indicating that hypoxia exosome lncRNA-UCA1 of bladder cancer cells promoted the invasive phenotype. Importantly, in vivo experiments also confirmed that the exosomes released by hypoxic bladder cancer cells can promote the proliferation of bladder cancer more significantly than that of normoxic bladder cancer cells [72].
Hypoxia-induced exosomes influence cancer immune escape
Increasing evidences show that tumor-derived exosomes can induce T cell apoptosis, reduce NK cell activity, inhibit IFN-γ-dependent type II macrophage expression, and change the differentiation of monocytes to increase the number of bone marrow-derived suppressor cells (MSDC), which reduces immune surveillance and thus causes immune escape from tumors [72-74].
T cells
With the deepening in our understanding of the pathogenesis and characteristics of cancer, the importance of the immune system in tumor progression has been generally recognized. A large number of studies have shown that the exhaustion of CD8+ T cells and the expansion of Tregs in tumor-infiltrating lymphocytes play an important role in tumor progression and immune escape [75]. It is reported that exosomes derived from gastric cancer cells change CD8+T cell gene expression, thereby inducing CD8+T cell apoptosis [76]. A recent study also found that exosomal miR-208b related with Oxaliplatin resistance promotes Tregs expansion in colorectal cancer [77]. It is worth noting that the role of exosomes released by cancer in the regulation of T cell differentiation under hypoxic microenvironment cannot be ignored. Given that hypoxia is a common feature of solid tumors and alter tumor exosome levels, Ling Li et al. found that tumor exosomes alter the proliferation and cytotoxicity of T cells in a way that relies on HSP70 but not dendritic cells. Further studies have found that exosomal miR-21 from OSCC enhance the inhibitory effect of MDSCs on T cells through the PTEN/PD-L1 axis, thereby causing cancer progression [78]. Additionally, Ye et al [79]. isolated exosomes from nasopharyngeal carcinoma (NPC) cell lines and patient sera, or control NP69 cells and healthy donor sera and found that miR-24-3p was markedly enriched in cancer derived exosomes. The study revealed that exosomes derived from tumor hypoxic cells mediate the transfer of miR-24-3p from tumor cells to T cells, inhibiting the proliferation of Th1 and Th17, while inducing the differentiation of Tregs. The future challenge will be to combine bioengineering technology to realize the application of exosomes released by hypoxic tumors in immunotherapy.
Macrophages
Since macrophages are involved in innate immunity, they are an integral part of the tumor microenvironment. Tumor-associated macrophages (TAM) play a major role in cancer-related inflammation and constitute an important regulator of tumorigenesis [80]. According to different phenotypes, TAMs are usually divided into two categories: M1-like and M2-like [81]. M1-TAM is mainly involved in the inhibition of tumor growth [82]. In contrast, M2-TAMs mainly exert immunosuppressive function and promote tumor growth [83]. A recent study showed that pancreatic cancer cell derived hypoxia exosomes express miR-301a-3p can polarize macrophages through the PTEN/PI3K signaling pathway. The polarization of macrophages leads to enhanced metastasis potential of pancreatic cancer cells in vitro and in vivo. Therefore, this study considered exosome miR-301a-3p as a new target for mediating tumor immune avoidance [84]. In addition, extracellular vesicles from hypoxic lung cancer also increased the M2 polarization of macrophages through the transfer of miR-103a. The mechanism of this process that hypoxic exosome miR-103a increases the activation of AKT and STAT3 and expression of angiogenic factors [85]. Interestingly, another study found that hypoxia induces macrophage polarization involving the expression of exosomes in epithelial ovarian cancer [86]. Specifically, the study showed that SKOv3 cells and their secreted exosomes under hypoxic conditions expressed much more miR-940 than cells and exosomes under normoxic conditions. Importantly, the researchers also found that cancer-derived exosomes in a hypoxic microenvironment can significantly increase the expression of M2-Like macrophage marker proteins, which suggested that exosomes derived from epithelial ovarian cancer could deliver miR-940 to macrophages, thereby inducing macrophages to an M2-like phenotype. The above studies indicate that under hypoxic microenvironment, cancer promotes the polarization of macrophages to M2 by secreting exosomes, thereby shaping an inhibitory tumor microenvironment.
Natural killer cells
In recent years, the role of the innate immune system in anti-tumor therapy has received increasing attention due to its possible role in the early stages of tumor development [87]. As a subset of intact congenital lymphoid cells, NK cells are thought to be capable of killing primary tumor cells and metastatic cells by producing natural toxicity and performing functions similar to CD8+ cytotoxic T cells [88]. Interestingly, some studies have also found that NK cells can be regulated by exosomes. It is reported that exosomes secreted by HCC can carry circUHRF1, inhibit miR-449c-5p through the sponge effect, thereby up-regulating the expression of T cell immunoglobulin and mucin domain 3 (TIM-3). Tim-3 exerts its immunosuppressive effect, causing the immune escape of HCC [89]. Coincidentally, Szczepanski et al. found that exosomes from acute myeloid leukemia can down-regulate the expression of NK cell activation receptors, especially NKG2D. This process is induced by tumor-derived exosomes carrying the TGF-β [90]. It is worthy of our attention that hypoxic stress can also participate in the regulation of NK cells by exosomes. Burchem et al. cultivated lung cancer cells by establishing a hypoxic microenvironment in vitro, and found that hypoxia promoted the secretion of MVs-TGF-β by lung cancer cells, thereby impairing NK-mediated cytotoxicity and NK cell function [91]. Further research also found that the impairment of NK-mediated cytotoxicity by hypoxic tumor-derived MVs involves a decrease in NKG2D induced by TGF-β. This mechanism is consistent with the study by Szczepanski et al under normoxia. This result indicates that the hypoxic microenvironment mainly plays a role in promoting the release of extracellular vesicles, and exerts an inhibitory effect on NK cells through the increase of extracellular vesicles.
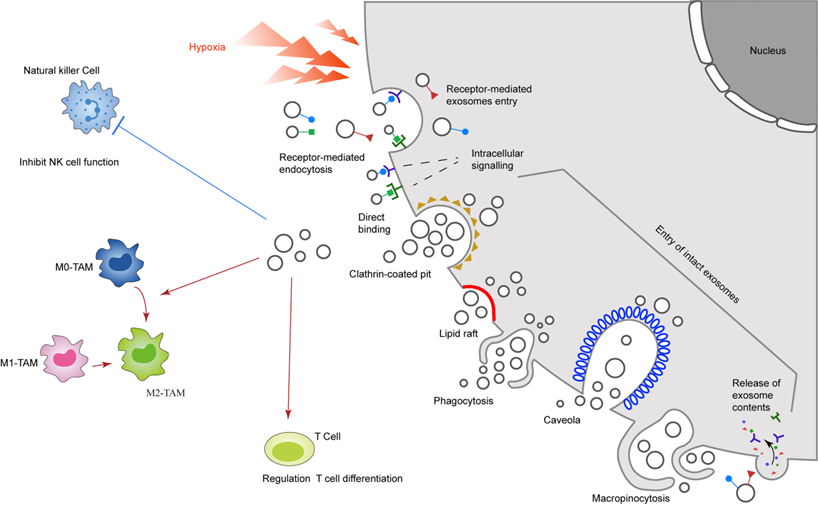
In short, exosomes can act as biologically active vesicles, regulating the functions of different types of immune cells by participating in more than one molecular pathway responsible for the genetic changes of recipient cells (Figure 2).
The potential application of hypoxia-exosomes in cancer treatment
In view of the important role of exosomes in regulating the immune microenvironment, depleting exosomes or blocking the uptake of exosomes may become a new cancer immunotherapy [25]. In recent years, a new device that can remove blood components below 200 nm, including tumor-derived exosomes that interact with the fixed affinity agent of the device, called the Aethlon ADAPT™ system, has been successfully applied to hepatitis C for the first time Virus patients [92]. Studies have found that hypoxia-induced exosomal circZNF91 can competitively bind miR-23b-3p when delivered to normoxic pancreatic cancer cells, thereby eliminating the inhibition of miR-23b-3p on the expression of deacetylase Sirtuin1 (SIRT1). Therefore, up-regulated SIRT1 enhances the deacetylation-dependent stability of HIF-1α protein, and promotes the Gemcitabine resistance of normoxic pancreatic cancer cells [93]. In addition, the study also found that hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma [94]. It could be speculated that if the Aethlon ADAPT™ system were used to eliminate exosomal circZNF91 or miR-340-5p, it may improve the efficacy of Gitacitabine and radiotherapy. In addition, the development of inhibitors for the exosome release pathway is also a promising treatment strategy. For example, histone deacetylase 6 (HDAC6), a tumor suppressor, restores the phagocytosis of macrophages by inhibiting the exosomal miRNA let-7i-5p, thereby inhibiting tumor progression [95]. However, these studies are currently only based on animal experiments, and there are no studies on patients. In the future, further clinical trials are needed to explore the clinical application of exosomes.
Conclusions and perspectives
The regulation of the release and function of exosomes by hypoxia is now considered a new and exciting field in cancer research. Revealing the mechanism of hypoxia regulating exosomes secretion and the biological functions of exosomes will help to find targets for intervention in exosomes secretion and strategies to treat cancer. Current studies have found that hypoxia regulates the secretion of exosomes by regulating the sorting process and transport of MVEs. This suggests that in the future, researchers can target these two processes to develop drugs for exosomal secretion, so as to suppress tumors at an early stage.
Hypoxia-induced exosomes influence cancer immune system. The solid red line represents the promoting effect, and the dotted blue line represents the inhibitory effect. Hypoxia promotes the release of exosomes from cancer cells, thereby inhibiting the function of NK cells and inducing the differentiation of macrophages and T cells.
In addition, the above studies have shown that exosomes play a role in angiogenesis and tumor metastasis, and can cause tumor immune escape by regulating the differentiation of immune cells. This helps us understand the mechanism of hypoxic exosomes transfer and target cell selection, thereby improving the prospects of exosomal therapeutic targeting and its development as a therapeutic delivery vehicle. However, the technology for exosome targeting is not yet fully mature and needs to be further explored. As these unknown factors are gradually revealed, we believe that exosomes will become an important tool for cancer treatment in the near future.
Abbreviations
HIF-1: Hypoxia-inducible factor-1; PI3K: Phospholipid protein trikinase; MAPK: mitochondrial activated protein kinase; NOX: NADPH oxidase; PHD: Prolyl hydroxylase domain; VHL: Von Hippel Lindau; cAMP: cyclic adenosine monophosphate; CREB: cAMP response element binding protein; ILVs: intraluminal vesicles; MVE: multivesicular endosomes; ESE: early-sorting endosome; ER: endoplasmic reticulum; TGN: trans-Golgi network; ESCRT: endosomal sorting complexes required for transport; ALIX: ALG-2 interacting protein X; LBPA: lysobisphosphatidic acid; S1P: Sphingosine1-Phosphate; SNARE: soluble N-ethylmaleimide sensitive factor attachment protein receptor; HSP70: heat shock 70kDa protein; HSC71: heat shock homologous 71kDa protein; GPI: glycosylphosphatidylinositol; HnRNPA2B1: Heterogeneous ribonucleoprotein A2B1; SNAP23: Synaptosome-related protein 23; ROCK: RHO-related protein kinase; MSDC: marrow-derived suppressor cells; MSDC: marrow-derived suppressor cells; TAM: Tumor-associated macrophages; TIM-3: T cell immunoglobulin and mucin domain 3; NPC: nasopharyngeal carcinoma; SIRT1: Sirtuin1; HDAC6: Histone deacetylase 6; Gi: G protein; CBP: cAMP response element binding protein binding protein; VAMP7: vesicle-associated membrane protein 7.
Acknowledgements
Funding
This work was supported by Suzhou Youth Science and Technology Program (KJXW2021067).
Author Contributions
Da Qian wrote and conceived the manuscript, Yaoyao Xie and Mingyao Huang participated in the revision of the manuscript, Jianfeng Gu conceived the manuscript.
Availability of data and materials
The datasets are available from the corresponding author on reasonable request.
Consent for publication
All of the authors are aware of and agree to the content of the paper and their being listed as a co-author of the paper.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cater DB. Oxygen Tension in Neoplastic Tissues. Tumori. 1964;50:435-44
2. Vaupel P. Hypoxia in neoplastic tissue. Microvasc Res. 1977;13:399-408
3. Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR. Hypoxia-Modified Cancer Cell Metabolism. Front Cell Dev Biol. 2019;7:4
4. Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177-82
5. Tomes L, Emberley E, Niu Y, Troup S, Pastorek J, Strange K. et al. Necrosis and hypoxia in invasive breast carcinoma. Breast Cancer Res Treat. 2003;81:61-9
6. Hockel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol. 2001;28:36-41
7. Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol. 2001;18:243-59
8. Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl 5):4-9
9. Williams KJ, Cowen RL, Stratford IJ. Hypoxia and oxidative stress. Tumour hypoxia-therapeutic considerations. Breast Cancer Res. 2001;3:328-31
10. Kumar A, Deep G. Hypoxia in tumor microenvironment regulates exosome biogenesis: Molecular mechanisms and translational opportunities. Cancer Lett. 2020;479:23-30
11. Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl). 2013;91:431-7
12. Huang M, Peng X, Yang L, Yang S, Li X, Tang S. et al. Non-coding RNA derived from extracellular vesicles in cancer immune escape: Biological functions and potential clinical applications. Cancer Lett. 2021;501:234-46
13. Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126:1216-23
14. Whiteside TL, Diergaarde B, Hong CS. Tumor-Derived Exosomes (TEX) and Their Role in Immuno-Oncology. Int J Mol Sci. 2021;22:6234
15. Schito L, Rey S. Hypoxic pathobiology of breast cancer metastasis. Biochim Biophys Acta Rev Cancer. 2017;1868:239-45
16. Wolff M, Kosyna FK, Dunst J, Jelkmann W, Depping R. Impact of hypoxia inducible factors on estrogen receptor expression in breast cancer cells. Arch Biochem Biophys. 2017;613:23-30
17. Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447-54
18. Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115-28
19. Haase VH. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int. 2017;21(Suppl 1):S110-S24
20. Lisy K, Peet DJ. Turn me on: regulating HIF transcriptional activity. Cell Death Differ. 2008;15:642-9
21. Huang M, Yang L, Peng X, Wei S, Fan Q, Yang S. et al. Autonomous glucose metabolic reprogramming of tumour cells under hypoxia: opportunities for targeted therapy. J Exp Clin Cancer Res. 2020;39:185
22. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ. et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161-72
23. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594-600
24. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-89
25. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208-15
26. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28
27. McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18:52
28. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17
29. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977
30. Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20:4-11
31. Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J. et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309-18
32. Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis. 2005;35:398-403
33. Luhtala N, Aslanian A, Yates JR 3rd, Hunter T. Secreted Glioblastoma Nanovesicles Contain Intracellular Signaling Proteins and Active Ras Incorporated in a Farnesylation-dependent Manner. J Biol Chem. 2017;292:611-28
34. Levesque K, Halvorsen M, Abrahamyan L, Chatel-Chaix L, Poupon V, Gordon H. et al. Trafficking of HIV-1 RNA is mediated by heterogeneous nuclear ribonucleoprotein A2 expression and impacts on viral assembly. Traffic. 2006;7:1177-93
35. Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980
36. Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153-66
37. Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671-82
38. Song S, Cong W, Zhou S, Shi Y, Dai W, Zhang H. et al. Small GTPases: Structure, biological function and its interaction with nanoparticles. Asian J Pharm Sci. 2019;14:30-9
39. Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2:REVIEWS3007
40. Darchen F, Goud B. Multiple aspects of Rab protein action in the secretory pathway: focus on Rab3 and Rab6. Biochimie. 2000;82:375-84
41. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19-30 sup pp 1-13
42. Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M. et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214:197-213
43. Song L, Tang S, Han X, Jiang Z, Dong L, Liu C. et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun. 2019;10:1639
44. Marks MS, Heijnen HF, Raposo G. Lysosome-related organelles: unusual compartments become mainstream. Curr Opin Cell Biol. 2013;25:495-505
45. Messenger SW, Woo SS, Sun Z, Martin TFJ. A Ca(2+)-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J Cell Biol. 2018;217:2877-90
46. Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA. et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223-32
47. Giovannone AJ, Reales E, Bhattaram P, Fraile-Ramos A, Weimbs T. Monoubiquitination of syntaxin 3 leads to retrieval from the basolateral plasma membrane and facilitates cargo recruitment to exosomes. Mol Biol Cell. 2017;28:2843-53
48. Jahn R, Scheller RH. SNAREs-engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631-43
49. Fader CM, Sanchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793:1901-16
50. Proux-Gillardeaux V, Raposo G, Irinopoulou T, Galli T. Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biol Cell. 2007;99:261-71
51. Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131-43
52. Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H. et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun. 2017;8:14041
53. Puri N, Roche PA. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci U S A. 2008;105:2580-5
54. Peak TC, Panigrahi GK, Praharaj PP, Su Y, Shi L, Chyr J. et al. Syntaxin 6-mediated exosome secretion regulates enzalutamide resistance in prostate cancer. Mol Carcinog. 2020;59:62-72
55. Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036-45
56. Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M. et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211:27-37
57. Novgorodov SA, Gudz TI. Ceramide and mitochondria in ischemia/reperfusion. J Cardiovasc Pharmacol. 2009;53:198-208
58. Li L, Li C, Wang S, Wang Z, Jiang J, Wang W. et al. Exosomes Derived from Hypoxic Oral Squamous Cell Carcinoma Cells Deliver miR-21 to Normoxic Cells to Elicit a Prometastatic Phenotype. Cancer Res. 2016;76:1770-80
59. Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK. et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35:856-67
60. Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80-91
61. Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748-57
62. Mace TA, Collins AL, Wojcik SE, Croce CM, Lesinski GB, Bloomston M. Hypoxia induces the overexpression of microRNA-21 in pancreatic cancer cells. J Surg Res. 2013;184:855-60
63. Dorayappan KDP, Wanner R, Wallbillich JJ, Saini U, Zingarelli R, Suarez AA. et al. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37:3806-21
64. Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ. et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111:E3234-42
65. Panigrahi GK, Praharaj PP, Peak TC, Long J, Singh R, Rhim JS. et al. Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells. Sci Rep. 2018;8:3853
66. Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2001;25:628-35
67. Feng X, Zhang H, Meng L, Song H, Zhou Q, Qu C. et al. Hypoxia-induced acetylation of PAK1 enhances autophagy and promotes brain tumorigenesis via phosphorylating ATG5. Autophagy. 2021;17:723-42
68. Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci. 2014;127:1585-94
69. Yu Y, Min Z, Zhou Z, Linhong M, Tao R, Yan L. et al. Hypoxia-induced exosomes promote hepatocellular carcinoma proliferation and metastasis via miR-1273f transfer. Exp Cell Res. 2019;385:111649
70. Liu Y, Tan J, Ou S, Chen J, Chen L. Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis. J Physiol Biochem. 2019;75:391-401
71. Matsuura Y, Wada H, Eguchi H, Gotoh K, Kobayashi S, Kinoshita M. et al. Exosomal miR-155 Derived from Hepatocellular Carcinoma Cells Under Hypoxia Promotes Angiogenesis in Endothelial Cells. Dig Dis Sci. 2019;64:792-802
72. Xue M, Chen W, Xiang A, Wang R, Chen H, Pan J. et al. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer. 2017;16:143
73. Poutsiaka DD, Schroder EW, Taylor DD, Levy EM, Black PH. Membrane vesicles shed by murine melanoma cells selectively inhibit the expression of Ia antigen by macrophages. J Immunol. 1985;134:138-44
74. Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P. et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303-16
75. Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X. et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169:1342-56 e16
76. Liu J, Wu S, Zheng X, Zheng P, Fu Y, Wu C. et al. Immune suppressed tumor microenvironment by exosomes derived from gastric cancer cells via modulating immune functions. Sci Rep. 2020;10:14749
77. Ning T, Li J, He Y, Zhang H, Wang X, Deng T. et al. Exosomal miR-208b related with oxaliplatin resistance promotes Treg expansion in colorectal cancer. Mol Ther. 2021;29:2723-2736
78. Li L, Cao B, Liang X, Lu S, Luo H, Wang Z. et al. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral gammadelta T cell equilibrium via tumor-derived exosomes. Oncogene. 2019;38:2830-43
79. Ye SB, Zhang H, Cai TT, Liu YN, Ni JJ, He J. et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J Pathol. 2016;240:329-40
80. Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33-40
81. Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front Oncol. 2020;10:188
82. Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520-9
83. Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209-12
84. Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T. et al. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kgamma to Promote Pancreatic Cancer Metastasis. Cancer Res. 2018;78:4586-98
85. Hsu YL, Hung JY, Chang WA, Jian SF, Lin YS, Pan YC. et al. Hypoxic Lung-Cancer-Derived Extracellular Vesicle MicroRNA-103a Increases the Oncogenic Effects of Macrophages by Targeting PTEN. Mol Ther. 2018;26:568-81
86. Chen X, Ying X, Wang X, Wu X, Zhu Q, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep. 2017;38:522-8
87. Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19:120
88. Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18:671-88
89. Zhang PF, Gao C, Huang XY, Lu JC, Guo XJ, Shi GM. et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer. 2020;19:110
90. Wen SW, Sceneay J, Lima LG, Wong CS, Becker M, Krumeich S. et al. The Biodistribution and Immune Suppressive Effects of Breast Cancer-Derived Exosomes. Cancer Res. 2016;76:6816-27
91. Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E. et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-beta and miR23a transfer. Oncoimmunology. 2016;5:e1062968
92. Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134
93. Zeng Z, Zhao Y, Chen Q, Zhu S, Niu Y, Ye Z. et al. Hypoxic exosomal HIF-1alpha-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene. 2021;40:5505-5517
94. Chen F, Xu B, Li J, Yang X, Gu J, Yao X. et al. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J Exp Clin Cancer Res. 2021;40:38
95. Yang HD, Kim HS, Kim SY, Na MJ, Yang G, Eun JW. et al. HDAC6 Suppresses Let-7i-5p to Elicit TSP1/CD47-Mediated Anti-Tumorigenesis and Phagocytosis of Hepatocellular Carcinoma. Hepatology. 2019;70:1262-79
Author contact
![]() Corresponding author: Jianfeng Gu, Email: jscsgjfcn
Corresponding author: Jianfeng Gu, Email: jscsgjfcn

 Global reach, higher impact
Global reach, higher impact