Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(6):1706-1712. doi:10.7150/jca.63509 This issue Cite
Research Paper
Expression of Prostate-specific Membrane Antigen (PSMA) in Papillary Renal Cell Carcinoma - Overview and Report on a Large Multicenter Cohort
1. Dept. of Medical Oncology, National Center of Tumor Diseases, University Hospital Heidelberg, 69120 Heidelberg, Germany
2. Institute of Pathology, University Hospital Erlangen-Nuernberg, Friedrich Alexander University (FAU), 91054 Erlangen, Germany
3. Department of Urology, University Hospital Münster, 48149 Münster, Germany
4. Department of Urology, University Hospital Göttingen, 37075 Göttingen, Germany
5. Institute of Pathology, University Hospital Göttingen, 37075 Göttingen, Germany
6. Department of Urology and Pediatric Urology, University Hospital Saarland (UKS), 66421 Homburg, Germany
7. Department of Urology, University Hospital Marburg, 35037 Marburg, Germany
8. Department of Urology, University Hospital Munich, 81337 Munich, Germany
9. Department of Urology, University Hospital Heidelberg, 69120 Heidelberg, Germany
10. Institute of Pathology, University Hospital Mainz, 55131 Mainz, Germany
11. Department of Urology and Pediatric Urology, University Hospital Erlangen, 91058 Erlangen, Germany
12. Department of Urology, Marien-Hospital Herne, Ruhr University Bochum, 44625 Herne, Germany
13. Department of Urology, University Hospital Mainz, 55131 Mainz, Germany
14. Department of Urology, University Hospital Frankfurt, 60590 Frankfurt/Main, Germany
15. Department of Urology, University Hospital Regensburg, 93053 Regensburg, Germany
16. Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Hannover Medical School, 30625 Hannover, Germany
17. Present address: Institute of Urology, Prosper-Hospital GmbH, 45659 Recklinghausen, Germany
18. Present address: Urological Group and Clinic Derouet/Pönicke/Becker, Boxberg Centre, 66538 Neunkirchen, Germany
19. Present address: Department of Urology, Asklepios Clinics Altona, 22763 Hamburg, Germany
20. Present address: Institute of Pathology/Gerhard-Domagk Institute, University Hospital Münster, 48149 Münster, Germany
21. Present address: Department of Urology and Pediatric Urology, University Hospital Mainz, 55131 Mainz, Germany
22. Present address: Department of Gynecology, University Hospital Mainz, 55131 Mainz, Germany
23. Present address: Department of Urology, Kreiskliniken Altötting-Burghausen, 84489 Burghausen, Germany
24. Present address: Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Hannover Medical School, Hannover, Germany
†These authors contributed equally to this work.
‡Member of the IAGN (Interdisciplinary working group renal cell cancer, DKG)
§Member of the ICOG-CCC-H (Interdisciplinary CoOperative Immune Oncology Group, Comprehensive Cancer Center, Hannover, Germany).
Received 2021-6-3; Accepted 2022-1-28; Published 2022-3-14
Abstract
Prostate specific membrane antigen (PSMA) is an emerging diagnostic and therapeutic target in prostate cancer. 68Ga-PSMA-labeled hybrid imaging is used for the detection of prostate primary tumors and metastases. Therapeutic applications such as Lutetium-177 PSMA radionuclide therapy or bispecific antibodies that target PSMA are currently under investigation within clinical trials. The expression of PSMA, however, is not specific to prostate-tissue. It has been described in the neovascular endothelium of different types of cancer such as breast cancer, and clear cell renal cell carcinoma (ccRCC). The aim of this study was to analyze PSMA expression in papillary RCC (pRCC) type 1 and type 2, the most common non-ccRCC subtypes, and to evaluate the potential of PSMA-targeted imaging and treatment in pRCC. Formalin-fixed, paraffin-embedded tissue samples of primary tumors were analyzed for PSMA expression by immunohistochemistry. Out of n=374 pRCC specimens from the multicenter PANZAR consortium, n=197 pRCC type 1 and n=110 type 2 specimens were eligible for analysis and correlated with clinical data. In pRCC type 1 PSMA staining was positive in 4 of 197 (2.0%) samples whereas none (0/110) of the pRCC type 2 samples were positive for PSMA in this large cohort of pRCC patients. No significant PSMA expression was detected in pRCC. Reflecting current clinical evaluation of PMSA expression in RCC do not encourage further analysis in papillary subtypes.
Keywords: prostate specific membrane antigen, papillary renal cell carcinoma, kidney cancer
Introduction
Papillary renal cell carcinoma (pRCC) is the second most common type of RCC according to the current (2016) classification of the World Health organization (WHO) and attributes to approximately 10-15% of RCC cases (1, 2). It is divided into two subtypes, type 1 and type 2. Type 2 tumors are associated with unfavorable clinical outcomes compared to type 1 pRCC (3-5). Comprehensive molecular characterization revealed that alterations in the MET (mesenchymal-epithelial transition) gene are a common feature of type 1 pRCC whereas type 2 pRCC is a heterogenous disease with many subtypes. In pRCC type 2 mutations in SETD2, fumarat hydratase (FH) and CKDN2A silencing as well as TFE3 fusions have been described (6).
Prostate specific membrane antigen (PSMA; glutamate carboxypeptidase II) is a type 2 transmembrane glycoprotein that consists of three parts: an 18 amino acid intracellular part, an 24 amino acid transmembrane part, and an 707 amino acid external part (7). PSMA is located on the short arm of chromosome 11 and has in vitro neuropeptidase and folate hydrolase activity (8). Initially, PSMA was considered to be exclusively expressed in prostatic tissue. An upregulation of PSMA in prostate cancer specimens can be observed and there is growing evidence that androgen deprivation drives this increase of PSMA (9). The extent of immunohistochemical PSMA expression in prostate cancer tissue is correlated to tumor uptake on 68Ga-PSMA positron emission tomography (PET)/ computer tomography (CT) (10). 68Ga-PSMA-labeled hybrid imaging is nowadays widely used in Europe and Australia to detect recurrent prostate cancer as it outperforms conventional imaging techniques (11, 12). Although not currently approved, PSMA directed radioligand therapies with Lutetium-177 (177Lu-PSMA-617; 177Lu-PSMA-I&T) or Actinium-225 (225Ac-PSMA-617) or others are under investigation within clinical trials and are also used off-label for treatment of refractory metastatic prostate cancer (13-19).
Despite its name PSMA is not specific to prostate tissue. Physiologic expression was described in salivary glands, proximal renal tubules, brain, and small intestine tissues (20, 21). PSMA expression has been found in the neovascular endothelium of several types of cancer, e.g. breast cancer, colorectal cancer, non-small cell lung carcinoma, and RCC (21-24). Among RCC samples, PSMA expression has been studied in larger cohorts of clear cell RCC (ccRCC). Only small sample sizes of non-ccRCC were included in those analyses and results were inconclusive. Within ccRCC PSMA expression was found to be increased in vena cava tumor thrombi compared to renal tumor mass suggesting a potential mechanism for progression and malignant neovascularization (25).
Small case series have reported promising results of PET/CT in RCC patients using different PSMA directed radiotracers potentially outperforming conventional imaging modalities (26-29). Clinical trials are underway exploring the use of 68Ga-PSMA-labeled hybrid imaging for patients with PSMA positive tumors other than prostate cancer (Table 1).
Overview of prospective clinical trials (diagnostic) targeting Prostate Specific Membrane Antigen (PSMA) in Renal Cell Carcinoma.
| NCT identifier | Tumor entity | Tracer, mode of imaging | Trial phase | Status |
|---|---|---|---|---|
| NCT03427476 | Metastatic RCC | 18F-CTT1057 PET/CT or MRT | 1 | Completed |
| NCT02687139 | Clear cell RCC | 18F-DCFPyL PET/CT | 1 | Completed, (30) |
| NCT03387514 | Metastatic clear cell RCC | 18F-DCFPyL PET/CT | 2 | Recruiting |
| NCT03073395 | Metastatic RCC | 68Ga-P16-093 PET/CT | 1 | Recruiting |
| NCT02978586 | breast cancer, lung cancer, and other tumor types know to express “PSMA” | 68Ga-PSMA PET/CT | - | Recruiting |
| NCT03453528 | Advanced/metastatic solid tumors | 68Ga-PSMA PET/CT | - | Recruiting |
| NCT03073395 | Metastatic RCC | 68Ga-P16-093 PET/CT | 1 | Recruiting |
| NCT03841760 | PSMA-expressing non-prostate tumor | 18F-DCFPyL PET/CT or 68Ga-PSMA-11 PET/CT | 2 | Recruiting |
| NCT04147494 | RCC, solid cancer | 68Ga-FAPI-46 PET/CT or 68Ga-PSMA PET/CT | 1 | Recruiting |
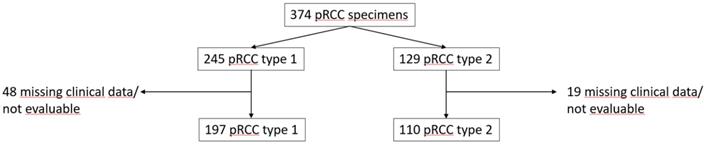
Consortium diagram of PANZAR cohort.
The aim of our investigation was to determine the expression of PSMA in pRCC type 1 and 2 and to evaluate PSMA as a potential diagnostic or therapeutic target in these RCC subtypes.
Material and Methods
Patient cohort
Routine kidney surgery due to kidney tumor was performed between 1994 and 2007. A total of 374 pRCC type 1 and 2 specimens, 245 (65.5%) type 1 and 129 (34.5%) type 2, from the multicentric PANZAR consortium were analyzed (Figure 1).
Primary tumor samples were perceived from the PANZAR contributing partners (in alphabetical order: Erlangen, Heidelberg, Herne, Homburg, Mainz, Mannheim, Marburg, Münster, LMU Munich, TU Munich and Regensburg). Detailed clinical data of the cohort have been previously published (31). For the analysis of PSMA expression samples of n=307 patients were available. Papillary subtype and pathological TNM staging were determined by an experienced uropathologist (AH). For each case, the papillary subtype was defined according to 2004 WHO tumor classification, and pathological TNM was performed according to the 2002 TNM classification. Grading was performed according to ISUP and WHO. Papillary type 1, or type 2 RCC were defined as previously reported (32). Patients and pathological data were accessed retrospectively. The study was performed according to the standards established in the Declaration of Helsinki and in concordance with each local ethic committee recommendations.
Tissue preparation and Immunohistochemistry
One representative area of the pRCC tumors was selected to construct tissue microarrays. PSMA expression was determined by immunohistochemistry (IHC). 2 μm TMA slides were stained for PSMA (Anti-PSMA, clone 3E6, M3620, DAKO, dilution 1:20) with a fully automated Dako Autostainer (Dako, Agilent pathology systems). The antibody is established in routine pathological diagnostic. Antigen retrieval was accomplished at pH=7.2. The bound antibody was visualized with an HRP-conjugated secondary antibody (Dako) and the diaminobenzidine chromogen (Dako). Sections were briefly rinsed in tap water, counterstained with Mayer's Hematoxylin solution and then mounted. All stained tissue samples were assessed in a blind way by pathologists (FE, AH). The evaluation was performed under a Leitz ARISTOPLAN light microscope (Leica Microsystems, Germany) with a x10 eyepiece, a 22-mm field of view and x40 objective lens (Plan FLUOTAR x40/0.70). All microarrays were stained in the same batch, and positive and negative controls were included according to the antibody manufacturer's instructions. Prostate cancer tissue was used as positive control. The immunohistochemical results were reported as staining intensity and percentage of positively staining cells following the immunoreactive score. The staining reaction was classified as positive (>/=1%).
Statistical Analyses
Descriptive statistics were performed with SPSS v25 (Armonk, NY, USA). Two-sided p-values below 0.005 were considered statistically significant.
Results
Tissue microarrays of n=307 patients with pRCC type 1 (n=197 (64.2%)) and type 2 (n=110 (35.8%)) were eligible for analysis. Clinical and pathological parameters of the analyzed cohort are displayed in Table 2.
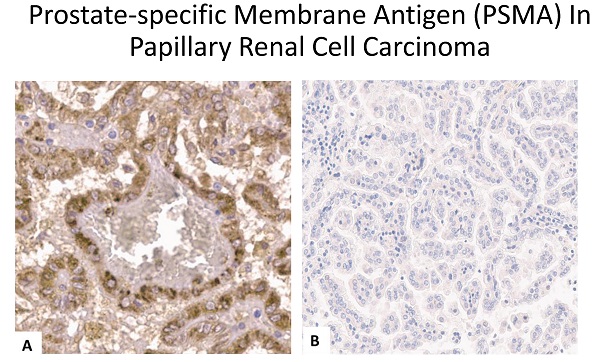
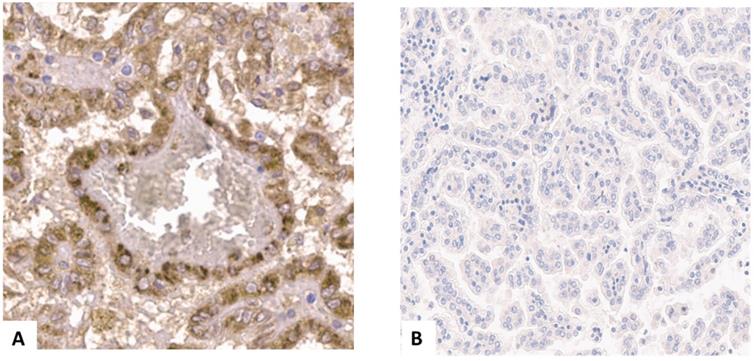
In pRCC type 1 PSMA staining was positive in 4/197 (2.0%) of specimens, and in none (0.0%) of type 2 specimens (Figure 2A and 2B). In these four samples intensity of PSMA staining was strong in almost 100% of the area. PSMA staining was detected primarily in the cell membrane and cytoplasm of tumor cells. Furthermore, a weak staining reaction was detected in single endothelial cells of the neovasculature in these cases. Therefore, we used a binary cutoff system with positive or negative staining reaction.
In all four PSMA positive tumors stage was pT1 cN0 cM0 and grading was G2. No significant association between clinicopathological parameters nor follow up parameters and PSMA positivity were observed (Table 2, outcome parameters not shown).
(A) Positive prostate specific membrane antigen (PSMA) staining in a papillary renal cell carcinoma type 1 specimen. (B) negative PSMA staining in a papillary renal cell carcinoma type 1 specimen.
Clinical and pathological parameters of the analyzed cohort.
| Variable | pRCC Type 1 PSMA- n=193 (98.0%) | pRCC Type 1 PSMA+ n= 4 (2.0%) | p-value | pRCC Type 2 PSMA- n=110 (100.0%) | pRCC Type 2 PSMA+ n=0 (0%) | p-value |
|---|---|---|---|---|---|---|
| Age [years] median (range) | 63.0 (15-86) | 66.5 (57-77) | p=.400 | 66.0 (18-85) | ||
| NE (n) | 34 | 0 | 26 | |||
| Sex | p=.816 | |||||
| Male, n (%) | 126 (65.3) | 3 (75.0) | 64 (58.2) | |||
| Female, n (%) | 32 (16.6) | 1 (25.0) | 20 (18.2) | |||
| NE, n (%) | 35 (18.1) | 0 (0.0) | 26 (23.6) | |||
| TNM Stage | p=.382 | |||||
| pT1, n (%) | 106 (54.9) | 2 (50.0) | 37 (33.6) | |||
| pT2, n (%) | 35 (18.1) | 2 (50.0) | 14 (12.7) | |||
| pT3, n (%) | 17 (8.8) | 0 (0.0) | 30 (27.3) | |||
| pT4, n (%) | 0 (0.0) | 0 (0.0) | 1 (0.9) | |||
| NE, n (%) | 35 (18.1) | 0 (0.0) | 28 (25.5) | |||
| Grade | p=.367 | |||||
| G1, n (%) | 45 (23.3) | 0 (0.0) | 9 (8.2) | |||
| G2, n (%) | 107 (55.4) | 4 (100.0) | 52 (47.3) | |||
| G3, n (%) | 4 (2.1) | 0 (0.0) | 19 (17.3) | |||
| G4, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| NE, n (%) | 37 (19.2) | 0 (0.0) | 30 (27.3) | |||
| Lymph node metastasis # | p=.751 | |||||
| N-, n (%) | 187 (94.9) | 4 (100.0) | 93 (84.5) | |||
| N+, n (%) | 6 (3.1) | 0 (0.0) | 17 (15.5) | |||
| NE, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Distant metastasis# | p=.819 | |||||
| M-, n (%) | 153 (79.3) | 4 (100.0) | 65 (59.1) | |||
| M+, n (%) | 2 (1.0) | 0 (0.0) | 14 (12.7) | |||
| NE, n (%) | 38 (19.7) | 0 (0.0) | 31 (28.2) | |||
| Locally Advanced disease | p=.471 | |||||
| T1/T2 N0M0, n (%) | 138 (71.5) | 4 (100.0) | 48 (43.6) | |||
| T3/4 and/or N+ and/or M+, n (%) | 18 (9.3) | 0 (0.0) | 31 (28.2) | |||
| NE, n (%) | 37 (19.2) | 0 (0.0) | 31 (28.2) |
# at time of renal surgery. Legend: N- = lymph node status unknown or tumor cells absent from regional lymph nodes, N+ = regional lymph node metastasis present, NE = not evaluable.
Discussion
PSMA expression by immunohistochemistry has been explored for many non-prostatic tumor types and among them RCC. Diagnostic, as well as therapeutic trials are ongoing in RCC addressing PSMA as target. In non-malignant renal tissue PSMA is found in the proximal renal tubules (20, 23). Upregulation of PSMA in the neovasculature of ccRCC samples has been described whereas the overall expression level in RCC is low (21, 22, 33). Since biology of ccRCC and non-ccRCC is different, data on PSMA expression in non-ccRCC, like pRCC is important to evaluate, to address the potential role of PSMA in this subtype of RCC.
Data on PSMA expression in pRCC has been reported, however reported sample sizes are considerably small, and results are inconclusive. Spatz et al. report on 257 RCC samples of all subtypes analyzing PSMA expression in the vasculature of renal primary tumors, wherein 22 (8.6%) papillary subtypes were included (24). Herein, in particular PSMA expression was found in 82% of the ccRCC specimen and only in 13.6% of the pRCC specimen (n=3; weakly positive (n=2), strongly positive (n=1)). An association with PSMA expression and clinicopathological risk parameters was primarily found for ccRCC only, not in non-ccRCC. Al-Ahamdie et al. investigated 75 nephrectomy specimens, among them 15 pRCC samples (22). Whereas ccRCC specimen showed a diffuse (24/30, 80%) and mostly a strong (25/30, 83%) PSMA staining pattern, in pRCC specimen no diffuse PMSA expression and only focal expression (11/15, 73%) with minimal strong expression (5/15, 33%) was identified. Among all RCC subtypes analyzed ccRCC showed the most, non-ccRCC lesser and pRCC the least intense and extensive PSMA expression. No association of clinicopathological parameters and PSMA expression was found in pRCC, neither in type 1 nor 2. Kinoshita et al. analyzed 18 renal tumor samples. While 11 samples were PSMA negative, they found a moderate staining intensity in 2 of 2 pRCC and 2 of 9 ccRCC samples and a weak PSMA staining in 3 of 9 ccRCC samples (21). Baccala et al found that positive PSMA staining was detectable in 76.2% of ccRCC, 31.2% of chromophobe RCC, 52.6% of oncocytoma, and 0% of PRCC samples (23). Evangelista et al give an overview on PSMA PET imaging in RCC (34) that has been used in small case series or proof-of-concept trials. While for ccRCC results indicate that further usage should be explored no statement can be made regarding pRCC due to small sample size.
PANZAR is the largest pRCC cohort reported to date. To elucidate whether PSMA might play a role in pRCC, due to the heterogenous data about this histological subset we analyzed PSMA expression on tissue microarrays of 197 patients with pRCC type 1 and 110 patients with pRCC type 2. Our analysis showed a rare expression in pRCC type 1 and no PSMA expression in pRCC type 2 samples. Accordingly, no association with clinicopathological parameter could be observed. Our results are not consistent with all previously published data. However, in concordance to prior reports e.g. (23), we could strongly underline a limited impact of PSMA expression in pRCC. Differences in amount of positivity among the different analyses remain elusive, but limitations of all analyzes were the same: methodology of IHC, different ages of specimen cohorts, as well as their retrospective character. A further limitation is the use of tissue microarrays, because only a small part of tumor can be analyzed. This small section often shows only several neovasculature vessels, which leads to a limited significance. However, the tissue micro-arrays (TMAs) of the PANZAR cohort have successfully been used in several former analyses and we consider those samples as representative for pRCC. Also, thickness of slides of the TMA used in our analysis is within standard range (2-7 µm). Analysis of several samples per tumor specimen would have been possible. Considering missing clinical relevance in the case of heterogenous expression (i.e. missing target for application of theranostic/ therapeutic approaches) we decided against further analyses.
Furthermore, PSMA is an unspecific antigen. It is expressed in prostate tissue and, to a lesser extent, in peripheral and central nervous system, small intestinal and salivary gland tissues. Cytoplasmatic and, to a lesser degree, membranous PSMA expression has been recently documented in 11% of analyzed urinary bladder adenocarcinomas (35). The lack of specificity could explain the positive staining reaction in several cases.
What to consider from our findings from bench side to bed side? Case series on 68Ga-PSMA targeted PET imaging in RCC patients showed encouraging results for ccRCC in detecting metastatic lesions that were not revealed in conventional imaging ((30, 36); Reviews: (37, 38)). In pRCC patients, however, 68GA- PSMA-PET-PET CT did not detect additional metastases compared to conventional imaging modalities (39). SUVmax in pRCC was also reported to be lower than in other RCC types. As sample sizes were small (number of pRCC patients between 1-3 patients) results were discussed not to be representative (39). The missing expression of PSMA as reported for the PANZAR cohort, however, explains that finding well. Larger trials to investigate PSMA hybrid imaging in RCC should probably exclude pRCC.
Therapeutic applications targeting PSMA have found entrance into the treatment of prostate cancer and are currently investigated for other cancer entities (40). Besides Lutetium-177-PSMA or Actinium-225-PSMA radionuclide therapy bispecific antibodies that target PSMA have been investigated in prostate cancer (41). PSMA targeted CAR-T cell therapy might offer an additional treatment option for PSMA positive tumors and probably will enter clinic in the near future (42). However, as for imaging we do not consider PSMA directed therapeutics promising for patients suffering from pRCC.
Conclusions
No relevant PSMA expression and consequently no relevant clinicopathological association with PMSA was detected in the pRCC PANZAR consortium cohort. Based on our data, a prognostic value of PMSA in pRCC is unlikely. Further on, due to missing PSMA expression in pRCC our findings point out that neither in pRCC type 1 nor type 2 PSMA targeted PET imaging or PMSA targeted therapeutical approaches seem to be reasonable. Whether PSMA targeted PET imaging and treatment modalities might expand the diagnostic and therapeutic landscape in other RCC subtypes needs to be further elucidated.
Abbreviations
ccRCC: clear cell renal cell carcinoma; CT: computer tomography; PET: positron emission tomography; pRCC: papillary renal cell carcinoma; PSMA: prostate specific membrane antigen; RCC: renal cell carcinoma; SUV: standardized uptake value.
Acknowledgements
This work was supported by a Ferdinand Eisenberger grant of the Deutsche Gesellschaft für Urologie (German Society of Urology), ID StS1/FE-13 (Sandra Steffens).
Funding
For the publication fee we acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding programme “Open Access Publikationskosten” as well as by Heidelberg University.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author Contributions
Conceptualization, Stefanie Zschäbitz, Franziska Erlmeier, Philipp Ivanyi and Sandra Steffens; Data curation, Franziska Erlmeier and Sandra Steffens; Formal analysis, Stefanie Zschäbitz, Franziska Erlmeier, Abbas Agaimy, Yvonne Mondorf, Philipp Ivanyi and Sandra Steffens; Funding acquisition, Sandra Steffens; Investigation, Stefanie Zschäbitz, Franziska Erlmeier and Sandra Steffens; Methodology, Franziska Erlmeier, Christine Stöhr, Iris Polifka, Abbas Agaimy, Arndt Hartmann and Sandra Steffens; Resources, Edwin Herrmann, Lutz Trojan, Frank Becker, Christian Wülfing, Peter Barth, Michael Stöckle, Michael Staehler, Christian Stief, Axel Haferkamp, Markus Hohenfellner, Bernd Wullich, Joachim Noldus, Walburgis Brenner, Frederik Roos, Bernhard Walter, Wolfgang Otto, Maximilian Burger, Andres Schrader, Arndt Hartmann and Sandra Steffens; Supervision, Sandra Steffens; Validation, Franziska Erlmeier and Sandra Steffens; Visualization, Stefanie Zschäbitz and Franziska Erlmeier; Writing - original draft, Stefanie Zschäbitz, Franziska Erlmeier, Philipp Ivanyi and Sandra Steffens; Writing - review & editing, Stefanie Zschäbitz, Franziska Erlmeier, Christine Stöhr, Edwin Herrmann, Iris Polifka, Abbas Agaimy, Lutz Trojan, Philipp Ströbel, Frank Becker, Christian Wülfing, Peter Barth, Michael Stöckle, Michael Staehler, Christian Stief, Axel Haferkamp, Markus Hohenfellner, Stephan Macher-Göppinger, Bernd Wullich, Joachim Noldus, Walburgis Brenner, Frederik Roos, Bernhard Walter, Wolfgang Otto, Maximilian Burger, Andres Schrader, Yvonne Mondorf, Arndt Hartmann, Philipp Ivanyi and Sandra Steffens. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Moch H. [The WHO/ISUP grading system for renal carcinoma]. Pathologe. 2016;37(4):355-60
2. Williamson SR, Gill AJ, Argani P, Chen YB. et al. Report From the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers: III: Molecular Pathology of Kidney Cancer. Am J Surg Pathol. 2020;44(7):e47-e65
3. Deng J, Li L, Xia H. et al. A comparison of the prognosis of papillary and clear cell renal cell carcinoma: Evidence from a meta-analysis. Medicine (Baltimore). 2019;98(27):e16309
4. Pignot G, Elie C, Conquy S. et al. Survival analysis of 130 patients with papillary renal cell carcinoma: prognostic utility of type 1 and type 2 subclassification. Urology. 2007;69(2):230-5
5. Wagener N, Edelmann D, Benner A. et al. Outcome of papillary versus clear cell renal cell carcinoma varies significantly in non-metastatic disease. PLoS One. 2017;12(9):e0184173
6. Linehan WM, Spellman PT, Ricketts CJ. et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med. 2016;374(2):135-45
7. Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(Suppl 10):S13-8
8. Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci U S A. 1996;93(2):749-53
9. Rosar F, Dewes S, Ries M. et al. New insights in the paradigm of upregulation of tumoral PSMA expression by androgen receptor blockade: Enzalutamide induces PSMA upregulation in castration-resistant prostate cancer even in patients having previously progressed on enzalutamide. Eur J Nucl Med Mol Imaging. 2020;47(3):687-94
10. Burger I, Ruschoff J, Hermanns T, et al. Extent of immunohistochemical PSMA-expression in primary prostate cancer shows a high correlation with tumor uptake on 68 Ga-PSMA-11-PET.: Journal of Nuclear Medicine.2020; 61 (suppl. 1):1263
11. Parker C, Castro E, Fizazi K. et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119-34
12. van Leeuwen PJ, Stricker P, Hruby G. et al. (68) Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117(5):732-9
13. Kratochwil C, Haberkorn U, Giesel FL. Ac-PSMA-617 for Therapy of Prostate Cancer. Semin Nucl Med. 2020;50(2):133-40
14. Kratochwil C, Fendler WP, Eiber M, B. et al. EANM procedure guidelines for radionuclide therapy with. Eur J Nucl Med Mol Imaging. 2019;46(12):2536-44
15. Dhiantravan N, Emmett L, Joshua AM. et al. UpFrontPSMA: A Randomised Phase 2 Study of Sequential. BJU Int. 2021;128(3):331-342
16. Emmett L, Crumbaker M, Ho B. et al. Results of a Prospective Phase 2 Pilot Trial of. Clin Genitourin Cancer. 2019;17(1):15-22
17. Hofman MS, Emmett L, Sandhu S. et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021 397/10276):797-804
18. Sartor O, Morris MJ, Messman R, Kraus BJ. VISION: An international, prospective, open-label, multicenter, randomized phase III study of 177Lu-PSMA-617 in the treatment of patients with progressive PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2020 (Suppl. 6)
19. Sartor O, de Bono J, Chi Kim N. et al. Lutetium-177-PSMA-617 for Metastatic Castration Resistant Prostate Cancer. New Engl J Med. 2021;385(12):1091-110
20. Silver DA, Pellicer I, Fair WR. et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3(1):81-5
21. Kinoshita Y, Kuratsukuri K, Landas S. et al. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J Surg. 2006;30(4):628-36
22. Al-Ahmadie HA, Olgac S, Gregor PD. et al. Expression of prostate-specific membrane antigen in renal cortical tumors. Mod Pathol. 2008;21(6):727-32
23. Baccala A, Sercia L, Li J. et al. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology. 2007;70(2):385-90
24. Spatz S, Tolkach Y, Jung K. et al. Comprehensive Evaluation of Prostate Specific Membrane Antigen Expression in the Vasculature of Renal Tumors: Implications for Imaging Studies and Prognostic Role. J Urol. 2018;199(2):370-7
25. Morgantetti G, Ng KL, Samaratunga H. et al. Prostate specific membrane antigen (PSMA) expression in vena cava tumour thrombi of clear cell renal cell carcinoma suggests a role for PSMA-driven tumour neoangiogenesis. Transl Androl Urol. 2019;8(Suppl 2):S147-S55
26. Sawicki LM, Buchbender C, Boos J. et al. Diagnostic potential of PET/CT using a. Eur J Nucl Med Mol Imaging. 2017;44(1):102-7
27. Salas Fragomeni RA, Amir T, Sheikhbahaei S. et al. Imaging of Nonprostate Cancers Using PSMA-Targeted Radiotracers: Rationale, Current State of the Field, and a Call to Arms. J Nucl Med. 2018;59(6):871-7
28. Pozzessere C, Bassanelli M, Ceribelli A. et al. Renal Cell Carcinoma: the Oncologist Asks, Can PSMA PET/CT Answer? Curr Urol Rep. 2019;20(11):68
29. Tariq A, Kwok M, Pearce A. et al. The role of dual tracer PSMA and FDG PET/CT in renal cell carcinoma (RCC) compared to conventional imaging: A multi-institutional case series with intra-individual comparison. Urol Oncol. 2021 Dec 8;S1078-1439(21)00502-0
30. Meyer AR, Carducci MA, Denmeade SR. et al. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted. Ann Nucl Med. 2019;33(8):617-23
31. Herrmann E, Trojan L, Becker F, Wülfing C, Schrader AJ, Barth P. et al. Prognostic factors of papillary renal cell carcinoma: results from a multi-institutional series after pathological review. J Urol. 2010;183(2):460-6
32. Polifka I, Agaimy A, Herrmann E. et al. High proliferation rate and TNM stage but not histomorphological subtype are independent prognostic markers for overall survival in papillary renal cell carcinoma. Hum Pathol. 2019;83:212-23
33. Chang SS, Reuter VE, Heston WD. et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59(13):3192-8
34. Evangelista L, Basso U, Maruzzo M. et al. The Role of Radiolabeled Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography for the Evaluation of Renal Cancer. Eur Urol Focus. 2020;6(1):146-50
35. Lane Z, Hansel DE, Epstein JI. Immunohistochemical expression of prostatic antigens in adenocarcinoma and villous adenoma of the urinary bladder. Am J Surg Pathol. 2008;32(9):1322-6
36. Rhee H, Blazak J, Tham CM. et al. Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016;6(1):76
37. Ahn T, Roberts MJ, Abduljabar A. et al. A Review of Prostate-Specific Membrane Antigen (PSMA) Positron Emission Tomography (PET) in Renal Cell Carcinoma (RCC). Mol Imaging Biol. 2019;21(5):799-807
38. Van de Wiele C, Sathekge M, de Spiegeleer B. et al. PSMA-Targeting Positron Emission Agents for Imaging Solid Tumors Other Than Non-Prostate Carcinoma: A Systematic Review. Int J Mol Sci. 2019 20(19)
39. Yin Y, Campbell SP, Markowski MC. et al. Inconsistent Detection of Sites of Metastatic Non-Clear Cell Renal Cell Carcinoma with PSMA-Targeted [Mol Imaging Biol. 2019;21(3):567-73.
40. Zekri L, Vogt F, Osburg L. et al. An IgG-based bispecific antibody for improved dual targeting in PSMA-positive cancer. EMBO Mol Med. 2021;13(2):e11902
41. Hummel H-D, Käufer P, Grünlich C. et al. Phase 1 study of pasotuxizumab (BAY 2010112), a PSMA-targeting Bispecific T cell Engager (BiTE) immunotherapy for metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2019 p. 5034-5034
42. Narayan V, Gladney W, Plesa G. et al. A phase I clinical trial of PSMA-directed/TGFβ-insensitive CAR-T cells in metastatic castration-resistant prostate cancer. J Clin Oncol. 2019 37 (Suppl. 7)
Author contact
![]() Corresponding author: Email: Stefanie.zschaebitzuni-heidelberg.de. Phone: +49(0)6121-56-35950, Fax: +49(0)6121-56-7265
Corresponding author: Email: Stefanie.zschaebitzuni-heidelberg.de. Phone: +49(0)6121-56-35950, Fax: +49(0)6121-56-7265

 Global reach, higher impact
Global reach, higher impact