Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(7):2171-2178. doi:10.7150/jca.70894 This issue Cite
Research Paper
The survival impact of radiotherapy on synchronous metastatic rectal cancer: metastatic site can serve for radiotherapy-decision
1. Department of General Surgery, Xiangya Hospital, Central South University, Changsha, China.
2. National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
3. Department of General Visceral and Thoracic Surgery, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
4. Department of Thoracic surgery, Fujian Provincial Hospital, Fuzhou, China.
5. Department of Cardiology, Xiangya Hospital, Central South University, Changsha, China.
Received 2022-1-10; Accepted 2022-3-19; Published 2022-4-4
Abstract
Purpose: The metastatic site seems to represent a malignancy with a different biological characteristic. Radiotherapy, as a successful, well-tolerated, cost-effective and time-efficient intervention, is able to provide clear benefits for the treatment of locally advanced rectal cancer and has become an essential component of palliative oncology care. The real-world effect of radiotherapy on the survival outcomes of metastatic rectal cancer (mRC) patients might do exist and was worth exploring.
Patients and methods: Data were extracted from the Surveillance, Epidemiology, and End Results (SEER) database in this retrospective analysis. The statistical methods included Pearson's chi-square test, Log-rank test, Cox regression model and propensity score matching (PSM).
Results: The multivariable Cox regression displayed that radiotherapy may not be used as a prognostic factor for mRC (p=0.057). However, radiotherapy may be associated with the prognosis if the metastatic site was excluded from the multivariate analysis (p<0.001). Radiotherapy seemed to fail to improve OS before PSM (p<0.001) and after PSM without the metastatic site as a matching factor (p<0.001). Nevertheless, there was no significant survival difference between radiotherapy and non-radiotherapy cohort after PSM with the metastatic site as a matching factor (p=0.057). All of M1a rectal cancer patients appear to obtain survival benefit from radiotherapy without the impact of PSM (p<0.001). Notwithstanding, radiotherapy was associated with improved OS of patients with rectal liver-limited metastasis (p=0.023) and did not appear to provide survival benefit for rectal lung-limited (p=0.386) and other-limited metastasis (p=0.385). Both of M1b mRC with and without liver metastasis did not seem to obtain survival benefit from radiotherapy.
Conclusions: Carefully selected data from the SEER database suggested that radiotherapy appears to improve overall survival only in patients with rectal liver-limited metastasis.
Keywords: rectal cancer, radiotherapy, metastatic site, overall survival, SEER database
Introduction
Colorectal cancer (CRC) is ranked as the top third malignancy in males and the second in females [1], and includes approximately 30%-50% rectal cancer (RC) [2]. Metastasis is considered as the main cause of high mortality among rectal cancer patients [3]. About 15-20% of RC exhibited distant metastasis at the time of diagnosis [4]. Currently, the advancements in diagnostics, surgical techniques, new oncologic drugs and radiotherapy have significantly improved prognosis of rectal cancer, including prolonged survival outcomes of metastatic rectal cancer (mRC) [5].
Several previous studies reported that the metastatic site is an important prognostic factor for synchronous metastatic colorectal cancer [6, 7]. More important, the metastatic site seems to represent a malignancy with a different biological characteristics [7]. However, many studies analyzed metastatic rectal cancer as a whole without considering the metastatic site [8, 9], which may provide an inaccurate conclusion. Radiotherapy, as a successful, well-tolerated, cost-effective and time-efficient intervention, is able to provide clear benefits for the treatment of stage II/III rectal cancer and has become an essential component of palliative oncology care [8, 10, 11]. However, it is not yet clear about the effect of radiotherapy on survival in the treatment of mRC. The real-world effect of radiotherapy on the survival outcomes of mRC patients might do exist and was worth exploring.
This study herein took advantage of the large patient population of the Surveillance, Epidemiology, and End Results (SEER) database to comprehensively examine the impact of radiotherapy on survival outcomes of mRC based on the metastatic site. These data can inform rectal oncologists in counseling patients with stage IV rectal cancer with synchronous metastatic disease seeking prognostic information when weighing radiotherapy decisions.
Material and methods
Patients Screening
Data were extracted from the SEER linked database in this retrospective analysis. The SEER Program of the National Cancer Institute is an authoritative source of information on cancer incidence and survival in the United States (U.S.) that is updated annually. The rectal adenocarcinoma patients (ICD-O-3: 8140, 8144, 8145, 8201, 8210, 8211, 8213, 8220, 8221, 8253, 8255, 8260, 8261, 8262, 8263, 8310, 8323, 8480, 8481, 8490) with distant metastasis was collected from the period 2010-2016, 12,487 patients in total. Exclusion criteria: the diagnosed at autopsy or death certificate (n=8); Survival months is 0 (n=850); M1NOS, T0 and blank(s) in AJCC stage (n=736); the metastatic status of liver, lung, bone and brain is unknown or N/A (n=486); the final study sample contained 10,407 patients (Figure 1).
The flow diagram.
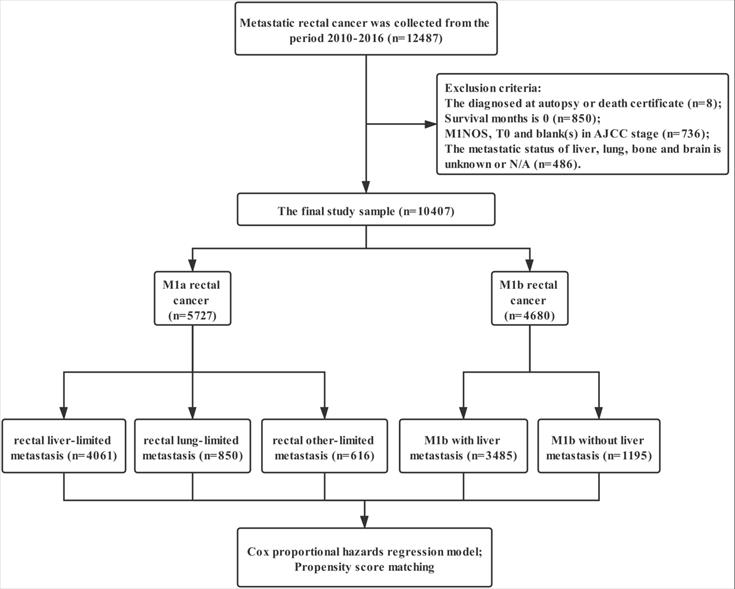
For each patient, the following data was acquired: insurance, age at diagnosis, marital status, gender, race, grade, histological type, T stage, N stage, regional nodes examined (RNE), CEA, surgery for primary tumor, metastatic site, radiotherapy and chemotherapy. We defined surgery with RNE ≥12 as standard proctectomy and that with RNE <12/NOS as simplified proctectomy. The definition of rectal liver-limited and lung-limited metastasis is M1a rectal cancer with liver and lung metastases at the time of diagnosis. Rectal other-limited metastasis included M1a rectal cancer with bone, brain and unknown site metastasis.
Statistical Analysis
Intergroup comparisons were analyzed using Pearson's chi-square test. Log-rank test was used to compare overall survival (OS) between different groups. A hazard ratio (HR) and a 95% confidence interval (CI) were evaluated by a univariate and multivariate Cox proportional hazards regression model. Univariate analysis of variables with significant differences was included in the Cox regression model for multivariate analysis. In order to eliminate the influence of other variables, we conducted a 1:1 propensity score matching (PSM). Statistical analyses were performed with IBM SPSS statistics trial ver. 25.0 (IBM, Armonk, NY, USA). All reported p-values lower than 0.05 were considered significant.
Results
Patient Characteristics
The characteristics of patients with metastatic rectal cancer enrolled from the SEER database were summarized in Table 1. The total population included 5727 cases (55.03%) of M1a rectal cancer (liver-limited: 4061, 39.02%; lung-limited: 850; other-limited: 816, 7.84%) and 4680 patients (44.97%) with M1b rectal cancer (with liver metastasis: 3485, 33.49%; without liver metastasis: 1195, 11.48%). 3540 patients (34.02%) with metastatic rectal cancer received radiotherapy and non-radiotherapy group contained 6867 cases (65.98%) in this study. Metastatic rectal cancer patients with T3-4 and N+ tend to receive radiotherapy. Interestingly, there was no significant difference regarding metastatic site between radiotherapy and non-radiotherapy group (p=0.129). However, the proportion of patients with rectal liver-limited metastasis receiving radiotherapy (1301/4061, 32.04%) was lower than that of those with rectal lung-limited (390/850, 45.88%) and other-limited metastasis (464/816, 56.86%), and the rate of M1b rectal cancer patients with liver metastasis (944/3485, 27.09%) was also lower than that of those without liver metastasis (441/1195, 36.90%).
Characteristics of metastatic rectal cancer
| Characteristics | Total (n=10407) | Non-radiotherapy (n=6867) | Radiotherapy (n=3540) | p-value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Insurance | 0.693 | ||||||
| Yes | 9708 | 93.28% | 6401 | 93.21% | 3307 | 93.42% | |
| No/NOS | 699 | 6.72% | 466 | 6.79% | 233 | 6.58% | |
| Gender | 0.651 | ||||||
| Female | 4003 | 38.46% | 2652 | 38.62% | 1351 | 38.16% | |
| Male | 6404 | 61.54% | 4215 | 61.38% | 2189 | 61.84% | |
| Age (years) | <0.001 | ||||||
| ≤ 65 | 6578 | 63.21% | 4166 | 60.67% | 2412 | 68.14% | |
| > 65 | 3829 | 36.79% | 2701 | 39.33% | 1128 | 31.86% | |
| Marital status | 0.001 | ||||||
| Married | 5237 | 50.32% | 3378 | 49.19% | 1859 | 52.51% | |
| Unmarried/NOS | 5170 | 49.68% | 3489 | 50.81% | 1681 | 47.49% | |
| Race | 0.394 | ||||||
| White | 8170 | 78.50% | 5374 | 78.26% | 2796 | 78.98% | |
| Non-white | 2237 | 21.50% | 1493 | 21.74% | 744 | 21.02% | |
| Pathologic grade | 0.004 | ||||||
| Grade I/II | 7397 | 71.08% | 4161 | 60.59% | 2236 | 63.16% | |
| Grade III/IV | 1694 | 16.28% | 1122 | 16.34% | 572 | 16.16% | |
| Unknown | 2316 | 22.25% | 1584 | 23.07% | 732 | 20.68% | |
| Histologic type | 0.956 | ||||||
| Adenocarcinomas | 9829 | 94.45% | 6485 | 94.44% | 3344 | 94.46% | |
| MCC/SRCC | 578 | 5.55% | 382 | 5.56% | 196 | 5.54% | |
| T staging | <0.001 | ||||||
| T1-2 | 1487 | 14.29% | 1053 | 15.33% | 434 | 12.26% | |
| T3-4 | 5138 | 49.37% | 3068 | 44.68% | 2070 | 58.47% | |
| Tx | 3782 | 36.34% | 2746 | 39.99% | 1036 | 29.27% | |
| N staging | 0.660 | ||||||
| N0 | 3599 | 34.58% | 2501 | 36.42% | 1098 | 31.02% | |
| N+ | 5630 | 54.10% | 3476 | 50.62% | 2154 | 60.85% | |
| Nx | 1178 | 11.32% | 890 | 12.96% | 288 | 8.14% | |
| Surgery | <0.001 | ||||||
| Standard Proctectomy | 2744 | 26.37% | 1786 | 26.01% | 958 | 27.06% | |
| Simplified Proctectomy | 1037 | 9.96% | 529 | 7.70% | 508 | 14.35% | |
| Non-proctectomy | 6626 | 63.67% | 4552 | 66.29% | 2074 | 58.59% | |
| Chemotherapy | <0.001 | ||||||
| Yes | 8175 | 78.55% | 4980 | 72.52% | 3195 | 90.25% | |
| No | 2232 | 21.45% | 1887 | 27.48% | 345 | 9.75% | |
| CEA | <0.001 | ||||||
| Negative | 1372 | 13.18% | 785 | 11.43% | 587 | 16.58% | |
| Positive | 6245 | 60.01% | 4210 | 61.31% | 2035 | 57.49% | |
| NOS | 2790 | 26.81% | 1872 | 27.26% | 918 | 25.93% | |
| Metastatic site | 0.129 | ||||||
| M1a: Liver-limited | 4061 | 39.02% | 2760 | 40.19% | 1301 | 36.75% | |
| M1a: Lung-limited | 850 | 8.17% | 460 | 6.70% | 390 | 11.02% | |
| M1a: Other-limited | 816 | 7.84% | 352 | 5.13% | 464 | 13.11% | |
| M1b with liver metastasis | 3485 | 33.49% | 2541 | 37.00% | 944 | 26.67% | |
| M1b without liver metastasis | 1195 | 11.48% | 754 | 10.98% | 441 | 12.46% | |
MCC: mucinous cell carcinoma; SRCC: signet ring cell carcinoma; NOS: Not otherwise specified.
The effect of radiotherapy on the total population
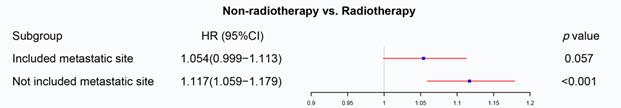
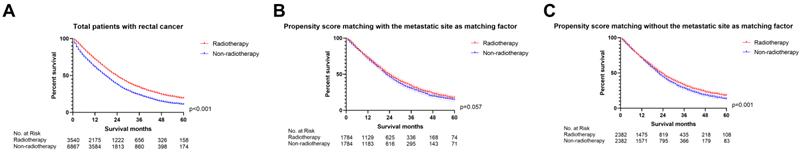
We firstly applied univariable and multivariable Cox regression analysis to explore the effect of radiotherapy on metastatic rectal cancer patients (Table S1). Radiotherapy, age, marital status, race, pathologic grade, histologic type, T staging, N staging, surgery, chemotherapy, CEA and metastatic site were significant for overall survival in the univariable Cox regression model and brought into multivariable analysis. The multivariable Cox regression displayed that radiotherapy cannot be used as a prognostic factor for mRC (p=0.057, Figure 2). However, radiotherapy became an important prognostic factor if the metastatic site was excluded from the multivariate analysis (p<0.001, Figure 2). The metastatic site seems to be an important factor affecting the sensitivity of mRC to radiotherapy. Then PSM was utilized to verify the results of the multivariable Cox regression analysis. Characteristics of mRC patients after PSM with or without the metastatic site as a matching factor were showed in Table S2. Radiotherapy was able to improve OS before PSM (p<0.001, Figure 3A) and after PSM without the metastatic site as a matching factor (p<0.001, Figure 3C). However, there was no significant survival difference between radiotherapy and non-radiotherapy cohort after PSM with the metastatic site as a matching factor (p=0.057, Figure 3B). Therefore, we decided to analyze the effect of radiotherapy on mRC based on metastatic site.
The forest plot was used to display the role of radiotherapy in the multivariable Cox regression. Radiotherapy cannot be used as a prognostic factor for mRC (p=0.057), but became an important prognostic factor if the metastatic site was excluded from the multivariate analysis (p<0.001). (The results were extracted from Table S1.)
The survival curves showed that (A) radiotherapy was able to improve OS before PSM (p<0.001); (B) there was no significant survival difference between radiotherapy and non-radiotherapy cohort after PSM with the metastatic site as a matching factor (p=0.057); (C) radiotherapy can improve OS after PSM without the metastatic site as a matching factor (p<0.001). (The results of PSM were summarized in Table S2.)
The forest plot displayed the effect of radiotherapy on M1a rectal cancer. Radiotherapy can be used as a prognostic factor for rectal liver-limited metastasis (p=0.007) but failed to improve survival for rectal lung-limited (p=0.060) and other-limited metastasis (p=0.596). (The results were extracted from Table S3.)
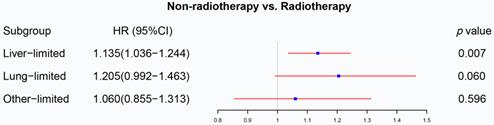
The effect of radiotherapy on M1a metastatic rectal cancer
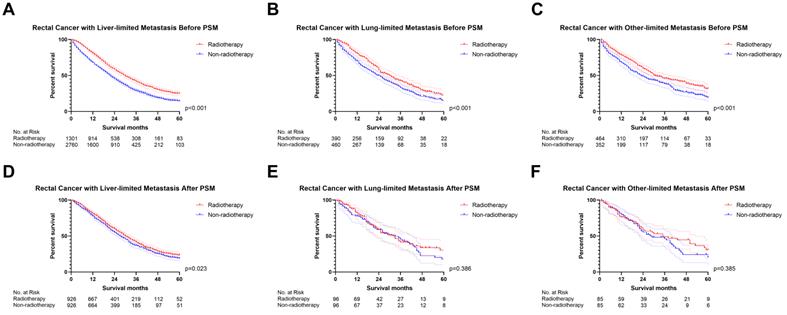
We initially analyzed the effect of radiotherapy on rectal cancer patients with one site or organ and classified those into liver-limited, lung-limited and other-limited mRC. The results of the Cox regression model are displayed in Table S3. The multivariable Cox regression analysis confirmed that radiotherapy can be used as a prognostic factor for rectal liver-limited metastasis (p=0.007, Figure 4) but failed to improve survival for rectal lung-limited (p=0.060, Figure 4) and other-limited metastasis (p=0.596, Figure 4), which then was further confirmed by log-rank survival analysis after PSM. Table S4 displayed the characteristics of patients with M1a mRC before and after PSM. All of the three groups can obtain survival benefit from radiotherapy without the impact of PSM (p<0.001, Figure 5A-C). However, radiotherapy was able to improve OS of patients with rectal liver-limited metastasis (p=0.023, Figure 5D) and cannot provide survival benefit for rectal lung-limited (p=0.386, Figure 5E) and other-limited metastasis (p=0.385, Figure 5F).
The effect of radiotherapy on M1b metastatic rectal cancer
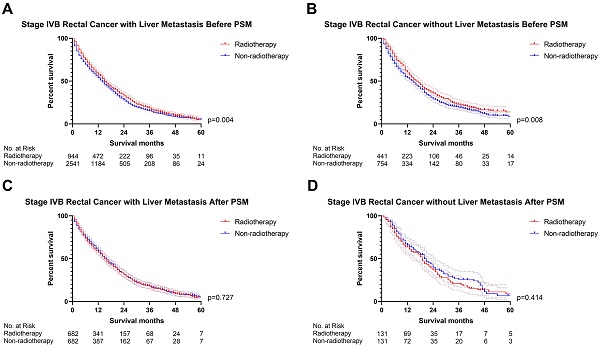
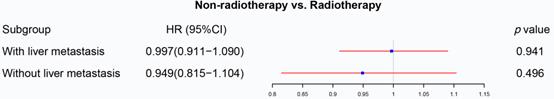
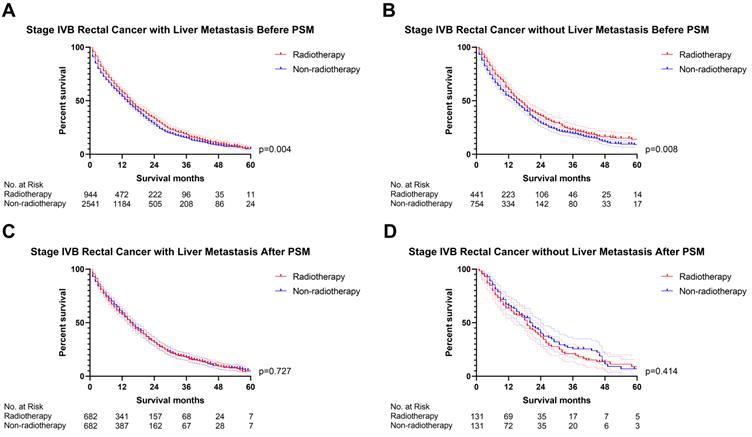
The previous section proved that radiotherapy can only improve the prognosis of liver-limited mRC. Therefore, we divided the patients with M1b metastatic rectal cancer into ones with and without liver metastasis. Cox regression analysis (Table S5) confirmed that radiotherapy was not able to significantly affect OS of M1b mRC patients with (p=0.941, Figure 6) and without liver metastasis (p=0.496, Figure 6). The results of PSM were consistent with the Cox regression models (Before PSM: p=0.004 in M1b mRC patients with liver metastasis, Figure 7A; p=0.008 in M1b mRC patients without liver metastasis, Figure 7B; After PSM: p=0.727 in M1b mRC patients with liver metastasis, Figure 7C; p=0.414 in M1b mRC patients without liver metastasis, Figure 7D) (Table S6). Collectively, both of M1b mRC with and without liver metastasis cannot obtain survival benefit from radiotherapy.
The survival curves demonstrated that (A) rectal liver-limited metastasis (B) rectal lung-limited metastasis and (C) rectal other-limited metastasis can obtain survival benefit from radiotherapy before PSM (p<0.001); (D) radiotherapy was able to improve OS of patients with rectal liver-limited metastasis (p=0.023) after PSM; (E) radiotherapy cannot provide survival benefit for rectal lung-limited (p=0.386) and (F) other-limited metastasis (p=0.385, Figure 1F) after PSM. (The results of PSM were summarized in Table S4.)
The forest plot illustrated the effect of radiotherapy on M1b rectal cancer. Radiotherapy was not able to significantly affect OS of M1b mRC patients with (p=0.941) and without liver metastasis (p=0.496). (The results were extracted from Table S5.)
The survival curves indicated that (A) M1b rectal cancer with liver metastasis (p=0.004) and (B) M1b rectal cancer without liver metastasis (p=0.008) can obtain survival benefit from radiotherapy before PSM; However, radiotherapy cannot provide survival benefit for (C) M1b rectal cancer with liver metastasis (p=0.727) and (D) M1b rectal cancer without liver metastasis (p=0.414) after PSM. (The results of PSM were summarized in Table S6.)
Discussion
To the best of our knowledge, this study was the first study to specifically investigate the effect of radiotherapy on metastatic rectal cancer patients based on the metastatic site. Somatic mutations and microsatellite instability status of the primary neoplasm have been previously indicated to impact patterns of colorectal metastasis [7, 12-14], which indicated that the molecular phenotype of the primary tumor is one of the key factors in determining the metastatic site in rectal cancer. Meanwhile, a large number of studies reported that the radiosensitivity of rectal cancer was related to the molecular phenotype [15-18]. Hence, the metastatic site may be used as a factor in radiotherapy decisions for mRC.
Currently, radiotherapy is the recommended treatment option for patients with synchronous mRC according the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines [19]. A previous study using the SEER database demonstrated that radiotherapy was associated with a significant survival advantage in mRC without considering the metastatic site [8], which was consistent with our preliminary results. Actually, radiotherapy cannot provide survival benefit to mRC when the Cox regression analysis and PSM included the metastatic site. Therefore, the metastatic site may be an important factor affecting the sensitivity of mRC to radiotherapy. Moreover, a retrospective clinical study containing 89 synchronous rectal liver metastasis patients suggested that radiotherapy could significantly reduce the pelvic failure rate, but regrettably failed to explore the relationship between radiotherapy and overall survival [20]. These preliminary evidences prompted us to further explore the role of radiotherapy on mRC according to the metastatic site. The final results confirmed that radiotherapy can only improve OS of patients with rectal liver-limited metastasis in this study.
The underlying mechanisms driving patterns of rectal metastasis are somewhat unclear. However, the metastatic site can be used as an indicator of the metastatic pattern of rectal cancer. Clinical evidence indicated that venous drainage of the colorectum into the portal system likely influences the pattern of metastatic spread first to the liver, and then to the lungs through the systemic circulation [21, 22]. The high frequency of lung metastasis has been attributed to the potential hematogenous spread of distal rectal cancer through the inferior iliac veins and the inferior vena cava [22]. Bone metastasis typically occurs via hematogenous dissemination [23]. The metastasis pattern of rectal cancer with distant lymph node metastasis may be unique. Unfortunately, the SEER database does not provide detail information regarding distant lymph node metastasis. One feature of rectal liver-limited is that the metastatic pathway does not involve the systemic circulatory system, which, contrarily, is involved in the rectal metastasis to lung, bone, brain. Meanwhile, we explored the effect of radiotherapy on M1b mRC with liver metastasis, that metastatic mechanism may involve both of portal system and systemic circulation. Although the mechanism is unclear, radiotherapy may not provide survival benefits to mRC patients with metastatic pathway involving systemic circulation.
Our previous research explored the prognostic factors of colorectal liver-limited and lung-limited metastasis, that were inconsistent between the two groups [24, 25]. Similarly, T staging can be used as a prognostic factor for rectal lung-limited metastasis but not for rectal liver-limited metastasis in this study (Table S3). On the contrary, N staging was associated with OS of rectal liver-limited metastasis and failed to affect OS of rectal lung-limited metastasis (Table S3). Moreover, both of T and N staging were not related to OS of rectal other-limited metastasis in the multivariable Cox regression analysis. These results reflected, to some extent, that the different metastasis sites were caused by nonidentical metastasis pathways in rectal cancer, which need to be blocked by distinct treatments. Altogether, effective treatment methods need to be explored to improve the prognosis of mRC by targeted blocking the metastatic pathways.
This study taking advantage of the large patient population of the SEER database is able to provide credible evidence regarding radiotherapy options and promote individualized treatment for mRC. First of all, our study can well encourage patients with rectal liver-limited to receive radiotherapy. Oncologists and patients did not realize the positive effect of radiotherapy on rectal liver-limited metastasis, which was the main reason for the disappointing low percentage of rectal liver-limited metastases patients receiving radiotherapy (32.04% in this study). In addition, this research can prompt oncologists to explore unique treatment strategies suitable for rectal cancer with different metastatic sites. In fact, current treatment strategies for mRC are mostly based on the experiences from rectal liver metastases [26-28]. However, such strategies may not improve the prognosis of all mRC patients, such as the role of radiotherapy in this study. Meanwhile, the different results between with and without the metastatic site as an analysis factor reminded rectal cancer scholars that the metastatic site cannot be ignored in the research of metastatic rectal cancer. However, we still need to explore the molecular mechanism regarding that rectal liver-limited metastasis can benefit from radiotherapy, and radiotherapy resistance in patients with rectal cancer metastasis to other sites, which is of great significance for us to understand the molecular mechanism of rectal cancer radiotherapy sensitivity. The exploration of these molecular mechanisms is also helpful for us to predict the most likely metastasis sites of locoregional rectal cancer, so as to formulate targeted treatment strategies.
This study has certain limitations. As a non-random retrospective study, selection bias and confounding factors inevitably existed in this study. Even though PSM analysis was used in this study to remedy these defects, there were still some unrecognized confounders and some known confounders that could not be controlled. For example, the degree of tumor invasion, the distance from the tumor to the anus, surgical complications and recovery will affect the decision-making of radiotherapy. However, these variables are currently not directly available from the SEER database, so they can only be controlled indirectly. In addition, we did not deeply discuss the effect of radiotherapy on rectal bone and brain metastasis due to the limitation of the number of cases. And we failed to analysis mRC with distant lymph node metastasis since the SEER database only recorded four sites of metastasis at diagnosis. Moreover, SEER database lacks some important data, such as ECOG score, surgical details (resectable status, surgical margin), chemotherapy (whether 5-FU based) and radiotherapy details (target design, technology and dose), which is undoubtedly one of the shortcomings of this study. Now with the advent of precision therapy, genomic data also have great clinical reference value for guiding prognosis and treatment, but this is not recorded in the SEER database. These missing variables are critical to prognosis and need to be discussed in future studies. At last, this study only accessed retrospective data and need to be further verified by prospective research in the future.
Conclusion
The metastatic site might serve for radiotherapy-decision in patients with synchronous metastatic rectal cancer. Radiotherapy appears to improve overall survival only in patients with rectal liver-limited metastasis. These findings are likely to inform rectal oncologists in counseling patients with stage IV rectal cancer with synchronous metastatic disease seeking prognostic information when weighing radiotherapy decisions.
Supplementary Material
Supplementary tables.
Acknowledgements
The author, DW, gratefully acknowledges financial support from China Scholarship Council.
Funding
This study was supported by the Nature Scientific Foundation of China (Grant No.81702956); the Strategy-Oriented Special Project of Central South University in China (Grant No. ZLXD2017003); the Natural Science Foundation of Hunan Province (Grant No.2020JJ4903 and 2020JJ5920); and The Colorectal cancer medical seed research fund project named “Effect and mechanism of YAP1 on EGFR resistance in K-ras wild-type metastatic colorectal cancer” from the Beijing Bethune Public Welfare Foundation.
Ethics approval
This study was approved by the Ethics Committee of the Xiangya Hospital of Central South University. Details can be found in the copy of the approval document provided.
Consent for publication
Patients' informed consent was waived because of the retrospective nature of the study design.
Consent to participate
Informed consent is waived as SEER is a de-identified, publicly available cancer database.
Data availability statement
These data were derived from the Surveillance, Epidemiology and End Results (SEER) database (https://seer.cancer.gov/) and identified using the SEER*Stat software (Version 8.3.5) (https://seer.cancer.gov/seerstat/).
Author contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by YL and WL. LZ downloaded and screened the data from SEER database. All authors participated in analyzing the data. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394-424
2. Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM. et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA surgery. 2015;150:17-22
3. Roth ES, Fetzer DT, Barron BJ, Joseph UA, Gayed IW, Wan DQ. Does colon cancer ever metastasize to bone first? A temporal analysis of colorectal cancer progression. BMC cancer. 2009;9:274
4. Goodman KA, Milgrom SA, Herman JM, Abdel-Wahab M, Azad N, Blackstock AW. et al. ACR Appropriateness Criteria® rectal cancer: metastatic disease at presentation. Oncology (Williston Park, NY). 2014;28:867-71 76, 78
5. Guren MG, Kørner H, Pfeffer F, Myklebust T, Eriksen MT, Edna TH. et al. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993-2010. Acta oncologica (Stockholm, Sweden). 2015;54:1714-22
6. Rumpold H, Kirchweger P, Niedersüß-Beke D, Falch D, Wundsam H, Metz-Gercek S. et al. Prognostic value of metastatic pattern in colorectal cancer: a multicenter retrospective analysis in a real-life cohort. Acta oncologica (Stockholm, Sweden). 2021;60:180-6
7. Cavallaro P, Bordeianou L, Stafford C, Clark J, Berger D, Cusack J. et al. Impact of Single-organ Metastasis to the Liver or Lung and Genetic Mutation Status on Prognosis in Stage IV Colorectal Cancer. Clinical colorectal cancer. 2020;19:e8-e17
8. Liu Q, Shan Z, Luo D, Cai S, Li Q, Li X. Palliative beam radiotherapy offered real-world survival benefit to metastatic rectal cancer: A large US population-based and propensity score-matched study. Journal of Cancer. 2019;10:1216-25
9. Luo D, Liu Q, Zhu J, Ma Y, Cai S, Li Q. et al. Survival Benefit of Preoperative Versus Postoperative Radiotherapy in Metastatic Rectal Cancer Treated With Definitive Surgical Resection of Primary Tumor: A Population Based, Propensity Score-Matched Study. Journal of Cancer. 2019;10:1307-12
10. Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:2913-9
11. Ellsworth S, Smith T, Lutz S. Radiation oncologists, mortality, and treatment choices. International journal of radiation oncology, biology, physics. 2013;87:437-9
12. Cejas P, López-Gómez M, Aguayo C, Madero R, de Castro Carpeño J, Belda-Iniesta C. et al. KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PloS one. 2009;4:e8199
13. Pereira AA, Rego JF, Morris V, Overman MJ, Eng C, Garrett CR. et al. Association between KRAS mutation and lung metastasis in advanced colorectal cancer. British journal of cancer. 2015;112:424-8
14. Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH. et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623-32
15. Ha Thi HT, Duong HQ, Hong S. Emerging roles of non-coding RNAs in the response of rectal cancer to radiotherapy (Review). International journal of oncology. 2021;58:344-58
16. Yokoi K, Yamashita K, Ishii S, Tanaka T, Nishizawa N, Tsutsui A. et al. Comprehensive molecular exploration identified promoter DNA methylation of the CRBP1 gene as a determinant of radiation sensitivity in rectal cancer. British journal of cancer. 2017;116:1046-56
17. Huerta S, Gao X, Dineen S, Kapur P, Saha D, Meyer J. Role of p53, Bax, p21, and DNA-PKcs in radiation sensitivity of HCT-116 cells and xenografts. Surgery. 2013;154:143-51
18. Ferrandon S, DeVecchio J, Duraes L, Chouhan H, Karagkounis G, Davenport J. et al. CoA Synthase (COASY) Mediates Radiation Resistance via PI3K Signaling in Rectal Cancer. Cancer research. 2020;80:334-46
19. NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Rectal Cancer, Version 6.2020. 2020
20. Kim JW, Kim YB, Kim NK, Min BS, Shin SJ, Ahn JB. et al. The role of adjuvant pelvic radiotherapy in rectal cancer with synchronous liver metastasis: a retrospective study. Radiation oncology (London, England). 2010;5:75
21. Tan KK, Lopes Gde L Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2009;13:642-8
22. Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J. et al. Clinical patterns of metastasis. Cancer metastasis reviews. 2006;25:221-32
23. Kimura T. Multidisciplinary Approach for Bone Metastasis: A Review. Cancers. 2018;10:156
24. Li Y, Zhou Z, Liu D, Zhou M, Tan F, Liu W. et al. Predictive and Prognostic Factors of Synchronous Colorectal Lung-Limited Metastasis. Gastroenterology research and practice. 2020;2020:6131485
25. Li Y, Liu W, Zhao L, Güngör C, Xu Y, Song X. et al. Nomograms predicting Overall Survival and Cancer-specific Survival for Synchronous Colorectal Liver-limited Metastasis. Journal of Cancer. 2020;11:6213-25
26. Tejani MA, ter Veer A, Milne D, Ottesen R, Bekaii-Saab T, Benson AB 3rd. et al. Systemic therapy for advanced appendiceal adenocarcinoma: an analysis from the NCCN Oncology Outcomes Database for colorectal cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2014;12:1123-30
27. Riemsma RP, Bala MM, Wolff R, Kleijnen J. Transarterial (chemo)embolisation versus no intervention or placebo intervention for liver metastases. The Cochrane database of systematic reviews. 2013: CD009498.
28. Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL. et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. British journal of cancer. 2010;103:324-31
Author contact
![]() Corresponding author: Wenxue Liu, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China; Department of Cardiology, Xiangya Hospital, Central South University, Changsha, China; E-mail: liuwenxue25net.
Corresponding author: Wenxue Liu, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China; Department of Cardiology, Xiangya Hospital, Central South University, Changsha, China; E-mail: liuwenxue25net.

 Global reach, higher impact
Global reach, higher impact