Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(9):2751-2756. doi:10.7150/jca.69811 This issue Cite
Research Paper
Peroxiredoxins - Urinary Surveillance Biomarkers in Urothelial Cancer
1. ICMR-National Institute of Pathology, New Delhi, Safdarjung Hospital 110029, India.
2. Department of Urology, VMMC, Safdarjung Hospital 110029, India.
3. Dept of Laboratory Oncology, Dr. BRA-Institute Rotary Cancer Hospital, AIIMS, New Delhi, India.
4. Division of Cellular & Molecular Diagnostics, ICMR-National Institute of Cancer Prevention & Research, NOIDA, India.
Received 2021-12-7; Accepted 2022-3-17; Published 2022-6-13
Abstract
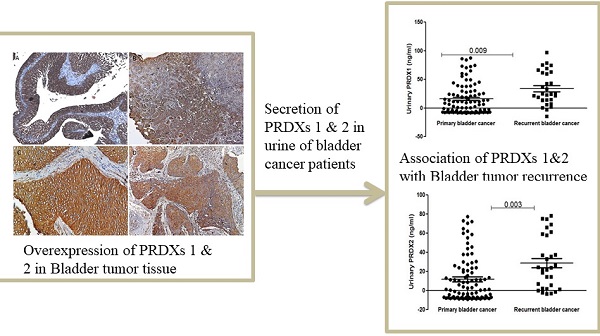
Introduction: Urinary bladder cancer ranks the fourth most common cancer in men worldwide. Peroxiredoxins (PRDXs) are antioxidant enzymes that play an important role in cell proliferation and apoptosis. In the present study, we investigated whether PRDX 1 and 2 can be used as a urinary biomarker for surveillance of recurrence in urothelial cancer.
Materials and Methods: PRDX1 and PRDX2 expression levels were examined in 119 bladder tumor specimens by immunohistochemistry and in 150 urine samples (case: 100; healthy controls: 50) using ELISA and their association with recurrence and survival of patients was evaluated.
Results: Immunohistochemistry on FFPE tissue showed that both PRDX1 and PRDX2 were positive in bladder tumors and expressed in the cytoplasm and membrane of tumor cells. A significant elevation of urinary PRDX1 and PRDX2 concentration was found in bladder cancer patients and recurrent cases compared to the urine of healthy controls and primary bladder cancer patients (p<0.001 & p<0.01) respectively. However, the concentration of both proteins was not found associated with survival.
Conclusion: Elevated urinary PRDX1 and PRDX2 in bladder cancer patients was found to be associated with recurrence and the estimation of urinary PRDX1 and PRDX2 during follow-up may help to extend the period between cystoscopies in patient follow-up.
Keywords: Bladder cancer, Urinary PRDX1, and PRDX2
Introduction
Urothelial carcinoma is the most prevalent genitourinary male cancer with the highest morbidity rate [1] Despite treatment, 70% of bladder tumors recur [2] and only lifelong cystoscopy helps in surveillance. The secreted or excreted byproduct of the tumor can be used as diagnostic or prognostic markers, and modify clinical management and follow-up. Numerous studies have assessed urinary biomarkers to detect possible biomarkers that could be predictive of recurrence [3-7]. Various molecular factors of cell proliferation, redox regulation, cell differentiation, etc. may promote tumor resistance against therapy and could be responsible for frequent recurrence of the tumor [8, 9].
Peroxiredoxins (PRDXs) are non-selenium-dependent glutathione peroxidases, 22 to 27 kDa, and scavenge peroxides, organic hydroperoxides, and peroxynitrite. They are ubiquitously expressed, thiol-dependent peroxidases, with a conserved cysteine residue. The most common six isoforms of PRDX (PRDX 1-6) are found in mammals and associated with proliferation, apoptosis and differentiation [10,11]. PRDXs have a cytoprotective antioxidant function and play a role in cellular processes involving redox homeostasis. The high expression of PRDX2 is reported to be associated with increased resistance to chemotherapeutic drugs and associated with a high proliferation rate and tumor recurrence [12,13]. Hence, alteration in expression of PRDXs in disease appears important to evaluate PRDXs as diagnostic or surveillance biomarkers. Therefore, in the present study, the expression levels of PRDX 1 and PRDX2 were evaluated in bladder cancer tissue and patients' urine, and their association with recurrence on follow-up was evaluated.
Material and Methods
Sample collection
This study included patients with bladder cancer presenting to the Outpatient Department of Urology, Safdarjung Hospital, New Delhi for four years. Samples used for this study were obtained with informed consent and with the approval of the Safdarjung hospital Ethics Committee (EC/SJH/VMMC/Project/I4/07-325). The patients who presented with hematuria to urology OPD were examined by cystoscopy and urine cytology. The patients who were positive for malignancy on urine cytology with cystoscopic lesion confirmed histopathologically were included in the study and samples were collected after written consent was obtained. Patients with histological grades pTa, metastatic disease, concurrent tumours and associated upper tract transitional cell carcinoma were excluded from the study. Controls included age and sex-matched healthy volunteers who did not have any other history of co-morbidity or fever in the 3 weeks before sample collection.
A total of 274 samples (119 tumor tissue, 5 normal mucosa and urine samples from 100 bladder cancer patients and 50 healthy controls) were included in this study. Demographic details of samples used are summarized in Table 1.
Demographic details of study cohort
| Immunohistochemistry | ELISA | |||
|---|---|---|---|---|
| Patients, n=119 (%) | Normal mucosa, n=5 (%) | Patients, n=100 (%) | Control, n=50 (%) | |
| Median Age | 58 | 54 | 58 | 55 |
| 1st to 3rd IQR | 53 to 68 | 51 to 60 | 49 to 56 | 45 to 61 |
| Gender | ||||
| Female | 16 (14) | 0 | 14 (14) | 12 (24) |
| Male | 103 (86) | 5 (100) | 86 (86) | 38 (76) |
| Grade | ||||
| LG | 57 (48) | -- | -- | -- |
| HG | 62 (52) | -- | -- | -- |
| Stage | ||||
| PT1 | 94 (78) | -- | -- | -- |
| PT2 | 25 (22) | -- | -- | -- |
| Recurrence | -- | -- | 41 (27) | -- |
IQR, interquartile range; F, Female; M, Male; LG, low grade; HG, High grade; pT1, Non-muscle invasive; pT2, Muscle invasive.
Immunohistochemistry
The slides with FFPE sections were placed in an incubator at 55 °C for 15 to 20 minutes. Slides were rehydrated and heat-induced antigen retrieval was performed in the TE buffer (Tris-EDTA buffer) in a water bath at 95 °C for 20 minutes. Endoperoxidase activity was blocked with 3% H2O2 and slides were incubated with primary antibody (PRDXI- 1:100 & PRDXII- 1:200; Thermo Scientific) at 4 °C overnight. Slides were incubated with HRP conjugated secondary antibody the next day. DAB was used as a chromogen, and sections were counter-stained with Haematoxylin. Slides were mounted with DPX and observed under the light microscope.
Urine ELISA (Enzyme-linked immunosorbent assay)
Collected urine samples were centrifuged at 4,000g for 5 min and the supernatant was collected for use. Enzyme-linked immunosorbent assay (ELISA) was used to quantify PRDX1 (Abcam, ab185983) and PRDX2 (R&D Systems, DY3489) concentration in urine. The assays were performed in duplicate. The protocol given in the manufacturers' instructions was followed and readings were obtained at 450nm.
Statistical analysis
A Chi-square test was performed to find the significant association between categorical variables. The Mann-Whitney test was used to evaluate the significance of differences in marker concentration between each group. The median concentration of urinary markers was taken as a cut-off for survival analysis. Kaplan Meier analysis was performed for discerning the difference in recurrence-free survival and significance was computed by log-rank. A probability less than 0.05 was considered significant. All statistical analyses were performed using the statistical package for the Statistical Package for social sciences (SPSS) software version 19 (SPSS, Chicago, IL, USA).
Results
Expression of PRDX1 and PRDX2 in Bladder tumor
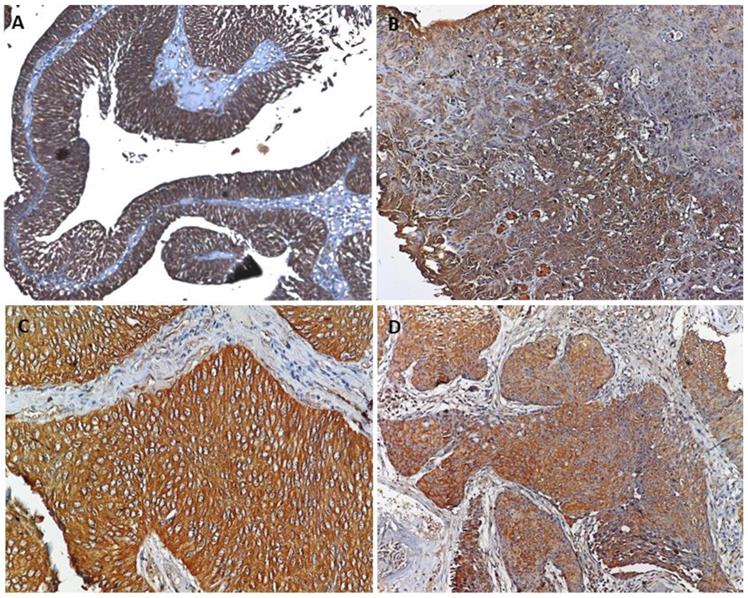
Formalin-fixed, paraffin-embedded (FFPE) tissue from 119 cases of urothelial cancer and 5 cases with normal mucosa was used to evaluate the expression and localization of the protein (PRDX1 and PRDX2) in the tumor by immunohistochemistry. These cases included LGpT1 (n=53), LGpT2 (n=4), HGpT1 (n=41) and HGpT2 (n=21) for the expression and localization of markers. Hematoxylin and eosin staining were performed on each tissue section to confirm the presence of tumors in the section. PRDX1 and PRDX2 were both found to be expressed in tumor cells and these proteins were absent in normal mucosa evaluated as control. Both proteins were expressed in cytoplasm and membrane in all subgroups of bladder tumors. The statistical test showed no significant difference in expression of the proteins between the grades and stage of the tumor (Figure 1).
A. Representative image of PRDX1 and PRDX2 showed cytoplasmic and membranous expression in (A & C) non-invasive bladder cancer and (B & D) invasive bladder cancer.
Estimation of PRDX1 and PRDX2 in urine
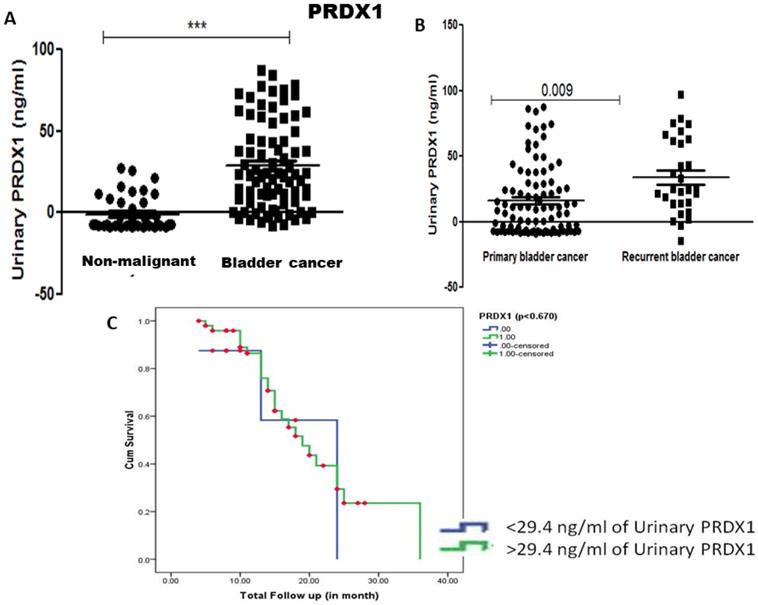
Levels of PRDX1 and PRDX2 protein were estimated in the urine of 100 cancer patients and 50 non-malignant controls by ELISA. The median concentration of urinary PRDX1 was 29.4 ng/ml and significant elevation of urinary PRDX1 was found in bladder cancer patients compared to urine from healthy controls (p<0.001). Though the median concentration of urinary PRDX1 was higher in the urine sample of recurrent bladder cancer patients compared to primary bladder cancer patients the difference was not statistically significant (Figure 2). Kaplan Meier survival analysis with median concentration (29.4 ng/ml) of urinary PRDX1 as cut-off value showed that the concentration of urinary PRDX1 was not associated with recurrence-free survival (Figure 2).
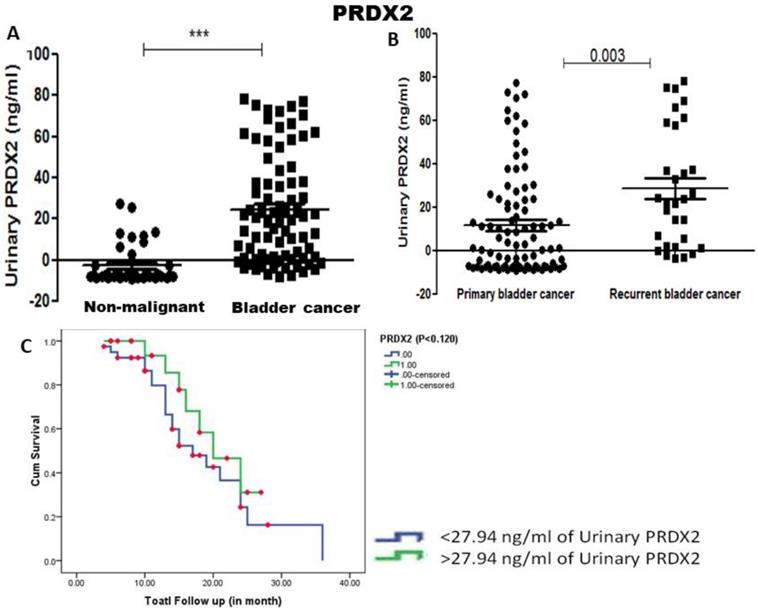
Urinary PRDX2 concentration was similarly found to be significantly more in bladder cancer patients compared to urine from healthy controls (p<0.001). Unlike PRDX1, significant elevation of urinary PRDX2 was found in recurrent bladder cancer patients compared to primary bladder cancer patients (p=0.003) (Figure 3). Median concentration 27.94 ng/ml of urinary PRDX2 was taken as cut-off value and Kaplan Meier survival analysis showed no association with recurrence-free survival (p=0.125) (Figure 3).
PRDX1 and 2 both act as diagnostic markers, but it is PRDX2 which is significantly increased in recurrence and can be used as a urinary surveillance marker in bladder cancer patients.
Discussion
Oxidative stress has been shown to be associated with prognosis in urinary bladder cancer [14]. Oxidative stress stimulates the expression of PRDXs and is regulated by transcriptional mechanisms [15]. Peroxiredoxins (PRDXs) are a member of the glutathione peroxidases family which destroys peroxides, organic hydroperoxides, and peroxynitrite [16]. Peroxiredoxin 1 (PRDX1) is an antioxidant enzyme and plays an important role in H2O2-mediated cell signaling [17]. PRDX1 inhibits the activation of oncogenes (c-Abl and c-myc, and PTEN) which is essential for its tumor-suppressive function [18]. Both PRDX 1&2 protect mitochondria and affect growth and differentiation by scavenging hydrogen peroxide in the mitochondria [19]. These proteins are overexpressed in malignancy [20,21,22] and may be a potential target for cancer therapy.
We found expression of PRDX1 was significantly increased in bladder cancer tumors when compared to normal mucosa and similar results are reported in esophagus squamous cell carcinoma [23,24] and colorectal carcinoma [25]. Urinary concentration of both PRDXs was found significantly elevated in patients but was not associated with the recurrence-free survival of the patient.
A similar study by Quanet et al. found that enhanced PRDX1 expression in bladder cancer tissue is strongly associated with development and progression but its expression did not correlate with disease-free survival in patients with bladder cancer [26]. Gao et al performed an analysis of the expression of peroxiredoxins across 33 different organ cancers from TCGA database and found that PRDX1 was associated with poor survival and PRDX2 with favorable survival [27] Overexpression of PRDX1 was found to be an independent poor prognostic factor for overall survival in hepatocarcinoma and the role of SUMO in carcinogenesis has been demonstrated [28]. Soini et al demonstrated that tissue expression of peroxiredoxins did not have an association with prognosis but that serum and urine concentration of 8OHdG, another oxidative enzyme, did have an association. They also suggested that elevated levels of PRDXs may be used as a target for therapy [14]. Our study did not show statistically significant association of either marker with survival but were both significantly increased in patients compared to controls and may function as surveillance markers.
A) Urinary concentration of PRDX1 was estimated in the urine sample and found significantly elevated in the urine of bladder cancer patient compared to non-malignant control (p-value < 0.001, calculated by Mann Whitney U test). B) Elevated urinary concentration of PRDX1 was found in the urine sample of recurrent bladder cancer patient compared to primary bladder cancer and C) Kaplan Meier analysis showed the concentration of PRDX1 (29.4 ng/ml) was not associated with recurrence-free survival (p=0.67).
A) Urinary concentration of PRDX2 was found significantly elevated in bladder cancer patients compared to non-malignant control (p-value < 0.001, calculated by Mann Whitney U test). B) Significant elevation in the concentration of urinary PRDX2 in recurrent bladder cancer compared to primary bladder cancer (p-value=0.003) and C) Kaplan Meier analysis showed lower concentration (<27.94 ng/ml) of PRDX2 associated with recurrence and poorer survival of bladder cancer patients (log-rank t-test, p=0.125).
The study is limited by the sample size. A larger cohort and ROC analysis would have given a more accurate cut-off. Literature review showed studies which demonstrated tissue expression of peroxiredoxins and association with survival was analyzed, but the evaluation of these as disease status markers in non-invasive samples has not been performed. The significantly elevated urinary concentrations of PRDX1 & 2 in bladder cancer patients compared to healthy individuals suggests that these are potential biomarkers and can be used for disease surveillance.
Conclusion
The high expression of both markers in tumour tissue, irrespective of grade and muscle invasion as well as their elevated concentrations in urine of recurrent cases makes these two PRDXs good urinary markers for follow-up of bladder cancer cases.
Acknowledgements
The authors acknowledge the financial support given for this work by the Indian Council of Medical Research.
Author Contributions
NK: contributed to the design, implementation of the research and took the lead in writing the manuscript; PT- helped in study design and providing resources; PV and AK- provided clinical sample and helped in manuscript writing; SH- helped in editing; UA conceived the original idea and final editing of the manuscript.
NK was supported by a Senior Research Fellowship granted by ICMR (Ref no. 3/2/2/233/14-NCD-III).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30
2. Sugano K, Kakizoe T. Genetic alterations in bladder cancer and their clinical applications in molecular tumor staging. Nat Clin Pract Urol. 2006;3:642-652
3. Kim WT, Cho NH, Ham WS. et al. Comparison of the efficacy of urine cytology, Nuclear Matrix Protein 22 (NMP22), and Fluorescence in situ Hybridization (FISH) for the diagnosis of bladder cancer. Korean J Urol. 2009;50:6-11
4. Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol Oncol. 2008;26:646-51
5. Horstmann M, Patschan O, Hennenlotter J. et al. Combinations of urine-based tumour markers in bladder cancer surveillance. Scand J Urol Nephrol. 2009;43:461-6
6. Todenhöfer T, Hennenlotter J, Esser M. et al. Combined application of cytology and molecular urine markers to improve the detection of urothelial carcinoma. Cancer Cytopathol. 2013;121:252-60
7. He H, Han C, Hao L, Zang G. ImmunoCyt test compared to cytology in the diagnosis of bladder cancer: a meta-analysis. Oncol Lett. 2016;12:83-8
8. Van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005;47:736-748
9. Mitra AP. Molecular substratification of bladder cancer: moving towards individualized patient management. Ther Adv Urol. 2016;8(3):215-33
10. Hall A, Nelson K, Poole LB. et al. Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid Redox Signal. 2011;15:795-815
11. Wood ZA, Schroder E, Robin Harris J. et al. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32-40
12. Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal. 2005;7:768-77
13. Yo YD, Chung YM, Park JK. et al. Synergistic effect of peroxiredoxin II antisense on cisplatin-induced cell death. Exp Mol Med. 2002;34:273-277
14. Soini Y, Haapasaari KM, Vaarala MH. et al. 8-hydroxydeguanosine and nitrotyrosine are prognostic factors in urinary bladder carcinoma. International Journal of Clinical and Experimental Pathology. 2011;4(3):267
15. Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutation research/fundamental and molecular mechanisms of mutagenesis. 2005;591(1-2):93-102
16. Kang SW, Rhee SG, Chang TS. et al. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med. 2005;11:571-8
17. Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38(12):1543-1552
18. Cao J, Schulte J, Knight A. et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28(10):1505-1517
19. Hoshino I, Matsubara H, Akutsu Y, et al Tumor suppressor PRDX1 is a prognostic factor in esophageal squamous cell carcinoma patients. Oncol reports. 2007; 18(4):867-872.
20. Rhee SG, Chae HZ, Kim K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543-1552
21. Kinnula VL, Lehtonen S, Sormunen R. et al. Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J Pathol. 2002;196:316-323
22. Noh DY, Ahn SJ, Lee RA. et al. Overexpression of peroxiredoxin in human breast cancer. Anticancer Res. 2001;21:2085-2090
23. Kropotov A, Gogvadze V, Shupliakov O. et al. Peroxiredoxin V is essential for protection against apoptosis in human lung carcinoma cells. Exp Cell Res. 2006;312:2806-2815
24. Ren P, Ye H, Dai L. et al. Peroxiredoxin 1 is a tumor-associated antigen in esophageal squamous cell carcinoma. Oncol reports. 2013;30(5):2297-2303
25. Li HX, Sun XY, Yang SM. et al. Peroxiredoxin 1 promoted tumor metastasis and angiogenesis in colorectal cancer. Pathol Res Pract. 2018;214(5):655-660
26. Chen YT, Chen CL, Chen HW. et al. Discovery of novel bladder cancer biomarkers by comparative urine proteomics using iTRAQ technology. J Proteome Res. 2010;9(11):5803-5815
27. Gao L, Meng J, Yue C. et al. Integrative analysis the characterization of peroxiredoxins in pan-cancer. Cancer Cell International. 2021;21(1):1-7
28. Sun YL, Cai JQ, Liu F. et al. Aberrant expression of peroxiredoxin 1 and its clinical implications in liver cancer. World Journal of Gastroenterol. 2015;21(38):10840-52
Author contact
![]() Corresponding author: Dr Usha Agrawal, ICMR-National Institute of Pathology, New Delhi, Safdarjung Hospital 110029, India. E-mail: ushakaggarwal.nipin; Phone no: +91-11-26716473-480.
Corresponding author: Dr Usha Agrawal, ICMR-National Institute of Pathology, New Delhi, Safdarjung Hospital 110029, India. E-mail: ushakaggarwal.nipin; Phone no: +91-11-26716473-480.

 Global reach, higher impact
Global reach, higher impact