Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(9):2768-2774. doi:10.7150/jca.73347 This issue Cite
Research Paper
Pretreatment BAN Score Based on Body-mass-index, Albumin and Neutrophil-lymphocyte Ratio Could Predict Long-term Survival for Patients with Operable Esophageal Squamous Cell Carcinoma
Department of Oncology, the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, P. R. China.
*These authors contributed equally to this work.
Received 2022-3-28; Accepted 2022-5-21; Published 2022-6-13
Abstract
Background: The present study was designed to examine the prognostic value of a systemic inflammation marker-BAN score, which was established based on body-mass-index (BMI), albumin (ALB) and neutrophil-lymphocyte ratio (NLR) in resectable esophageal squamous cell carcinoma (ESCC) patients.
Methods: A total of 420 newly diagnosed ESCC patients in our hospital between January 2008 and December 2013 were included. Their baseline characteristics were retrospectively reviewed and collected. BAN score was calculated as (BMI × ALB/ NLR). The optimal cutoff value for BAN score was defined as 28.0 in terms of survival. Patients were then allocated to high BAN (≥ 28.0) and low BAN (< 28.0) score groups.
Results: Pretreatment BAN score was significantly associated with tumor length, white blood cell count, BMI, ALB and NLR levels. However, no significant difference was observed in patients' age, gender, tumor location, degree of differentiation, depth of invasion, lymph node involvement, tumor-node-metastasis (TNM) stage or other variables between groups. Moreover, those with high pretreatment BAN scores (≥ 28.0) tended to have favorable disease free survival (DFS) [hazard ratio (HR), 0.650; 95% confidence interval (CI), 0.481-0.877; P = 0.005] and overall survival (OS) (HR, 0.608; 95% CI, 0.445-0.829; P = 0.002) by univariate analysis. Furthermore, multivariate Cox regression analysis suggested that high BAN score (≥ 28.0) could serve as an independent predictor for both DFS (HR, 0.726; 95% CI, 0.532-0.993; P = 0.045) and OS (HR, 0.670; 95% CI, 0.485-0.927; P = 0.016).
Conclusions: Pretreatment BAN score could independently predict long-term survival for resectable ESCC patients.
Keywords: Body-mass-index, Albumin, Neutrophil-lymphocyte-ratio, Esophagectomy, Prognostic marker, Esophageal squamous cell carcinoma
Introduction
Esophageal cancer (EC) is one of the most commonly seen malignancies and the fourth leading cause of cancer-related death in China [1]. Unlike Western countries, more than 90% of EC cases are classified as esophageal squamous cell carcinoma (ESCC) in Chinese population [1-3]. And approximately two thirds of the patients are diagnosed with advanced or metastatic diseases at first presentation. Although great advances have been achieved in early screening, diagnosis and treatment for the last two decades, ESCC remains one of the most deadliest malignant diseases, with a reported 5-year survival rate of about 30% [1].
Results from previous studies have confirmed that systemic inflammation response plays crucial roles in the process of tumor development, progression and metastasis [4-5]. Additionally, a broad range of cancer related symptoms including anorexia, fatigue and cancer cachexia have also been recognized to be attributable to systemic inflammation response [6]. Furthermore, the degree of systemic inflammation response has been reported to be significantly associated with impaired survival in cancer patients [4, 7].
Recently, several systemic inflammation-based prognostic variables such as modified Glasgow prognostic score (GPS), hypoalbuminaemia, neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) [8-10] have been established to help risk classification and optimal treatment for ESCC. On the one hand, hypoalbuminemia has been suggested not only as an indicator of poor nutritional condition but also significantly correlated with chronic inflammation in cancer patients [11], and it could also predict worse survival for ESCC cases [12-13]. On the other hand, as a novel and promising blood marker of systemic inflammation response, an elevated NLR has been shown to be associated with a higher risk of postoperative recurrence and poor prognosis in various malignancies, including ESCC [14-15]. Most recently, Arigami T, et al. found that preoperative NLR was a valuable indicator for predicting disease progression and prognosis in ESCC subjects who received esophagectomy with lymphadenectomy [16]. Furthermore, Sun and his colleagues suggested that BMI could be used as a prominent marker for evaluation of nutritional status and a low BMI was an independent indicator for unfavorable OS in Chinese patients with ESCC [17].
Therefore, we proposed a novel and promising inflammation index-BAN score based on a combination of patient's BMI, ALB and NLR to reflect the degree of systemic inflammation response in resectable ESCC. However, its clinical significance has not yet been determined. The purpose of this study was to examine if pretreatment BAN score could predict long-term survival in patients with operable ESCC.
Patients and methods
Patients
A cohort of 420 newly and histopathologically diagnosed ESCC patients who underwent esophagectomy with lymphadenectomy at the Department of Thoracic Surgery, the First Affiliated Hospital of Anhui Medical University between January 2008 and December 2015 were selected in the present study. Patients who were diagnosed with malignancies other than ESCC, who received neoadjuvant chemotherapy and/or radiotherapy and those who were diagnosed with autoimmune disorder or infection were excluded. This study was approved by the independent ethics committees at our hospital and was performed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). Informed consent was obtained from all included subjects and patient anonymity was preserved. Disease free survival (DFS) was calculated from the date of surgery to local recurrence/distant metastasis, OS was defined as the time interval from the date of diagnosis to death or to the most recent follow-up.
Treatment
All patients underwent transthoracic esophagectomy with at least a two-field regional lymphadenectomy, including standard, extended, or total dissection of the cervical, thoracic and abdominal lymph nodes, with a median number of dissected lymph nodes of 19 (range, 12-89). Adjuvant chemotherapy or radiotherapy was delivered based on the tumor stage, doctor's selection and patient's treatment desire. Actually, a total of 73 patients received adjuvant chemotherapy, with the aim to decrease the rate of local recurrence and/or distant metastasis. Fluorouracil-based two-drug combination chemotherapy was delivered to four-fifths of the cases, whereas the remaining subjects underwent fluorouracil monotherapy.
Clinical and laboratory parameters
Patients' baseline information, such as age, gender, weight, height, etc. were retrospectively reviewed and collected from the medical records. Patients were staged based on the AJCC/UICC TNM staging system (the 8th edition). The length of primary tumor and the degree of differentiation were also classified accordingly.
Pretreatment serum albumin level, neutrophil and lymphocyte counts were examined in samples obtained within one week before the initiation of treatment. Serum albumin level was examined by an automatic biochemical analyzer (Roche 501, Japan). Neutrophils and lymphocytes were detected using an XE-2100 automated hematology analyzer (Sysmex Co., Kobe, Japan).
BMI, ALB and NLR (BAN) score
BAN score was defined as follows:
BAN = (BMI × ALB) / NLR
Where: BMI = weight (kg) / [height (m)]2 ; ALB = serum albumin (g/dL); NLR = neutrophil count / lymphocyte count.
In this study, the optimal cutoff value for BAN score was determined with the method established by Jan Budczieset al. at http://molpath.charite.de/cutoff/ [18]. Thus, BAN score was classified as low (< 28.0) and high (≥ 28.0) groups in the subsequent analysis.
Statistical analysis
The difference of baseline characteristics was examined by Chi-square test. Kaplan-Meier method with log-rank test was utilized to estimate survival curves. Prognostic factors were determined by univariate and multivariate analysis with Cox proportional hazards regression models and hazard ratios (HRs) for variables respecting to DFS and OS were also calculated. HRs with 95% confidence intervals (CIs) and two-sided P value were reported. All statistical analysis was carried out with SPSS 21.0 (SPSS Inc., Chicago, IL, USA). And a two-sided P value of less than 0.05 was considered to be with statistical significance.
Results
Patient baseline characteristics
A total of 420 patients were selected in the final analysis. Patients' baseline characteristics were demonstrated in Table 1. The median age was 60.0 years (ranged, 20.0-87.0 years), and 75.7% of the cases were males. Tumors with well differentiation, moderate differentiation and poor/none-differentiation were observed in 107 (25.5%), 213 (50.7%) and 100 (23.8%) patients, respectively. The primary tumor invasion depth of T1, T2, T3, and T4 were found in 40 (9.5%), 66 (15.7%), 280 (66.7%), and 34 (8.1%) of the subjects, respectively. Lymph node was involved in 205 (48.8%) of the patients. And the numbers of subjects diagnosed with stage I, II, and III disease were 24 (9.2%), 127 (48.8%) and 107 (41.9%), respectively (Table 1).
Correlation between pretreatment BAN score and clinicopathologic parameters
Patients were classified into two groups: those with low (< 28.0, more inflammation) and high (≥ 28.0, less inflammation) BAN scores. The baseline parameters were listed in Table 1. Results showed that pretreatment BAN score was significantly correlated with tumor length, pretreatment WBC count, BMI, ALB and NLR. Whereas no significant difference was seen in age, gender, tumor location, degree of differentiation, depth of invasion, lymph node status, tumor-node-metastasis (TNM) stage, alcohol consumption, smoking or adjuvant treatment between the two groups (Table 1).
Prognostic value of pretreatment BAN score in resectable ESCC
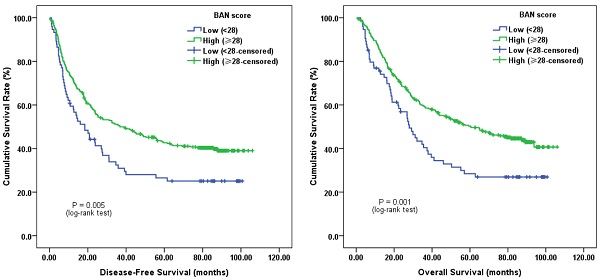
Survival analysis demonstrated that subjects with low pretreatment BAN score (< 28.0, more inflammation) tended to have a worse median DFS of 18.4 months (95% CI, 11.2-25.6 months) and OS of 28.0 months (95% CI, 23.2-32.7 months), respectively. Whereas the median DFS and OS for those with high pretreatment BAN score (≥ 28.0, less inflammation) were 36.8 months (95% CI, 21.4-52.2 months) and 60.2 months (95% CI, 40.9-80.0 months), respectively. Log-rank test showed that both DFS and OS were significantly different between the two groups (P = 0.005 and P = 0.001, respectively) (Figure 1).
Patient baseline characteristics, clinicopathological features, and pretreatment BAN score (N=420)
| Clinicopathologic characteristics | Patients | BAN score (No., %) | P value | |
|---|---|---|---|---|
| No. (%) | Low (< 28.0) | High (≥ 28.0) | ||
| Age (years) | 0.435 | |||
| < 60 | 244 (58.1) | 46 (62.2) | 198 (57.2) | |
| ≥ 60 | 176 (41.9) | 28 (37.8) | 148 (42.8) | |
| Gender | 0.772 | |||
| Male | 318 (75.7) | 57 (77.0) | 261 (75.4) | |
| Female | 102 (24.3) | 17 (23.0) | 85 (24.6) | |
| Tumor location | 0.476 | |||
| Upper | 37 (8.8) | 6 (8.1) | 31 (9.0) | |
| Middle | 260 (61.9) | 42 (56.8) | 218 (63.0) | |
| Lower | 123 (29.3) | 26 (35.1) | 97 (28.0) | |
| Tumor length (cm) | < 0.001* | |||
| < 5 | 226 (53.8) | 20 (27.0) | 206 (59.5) | |
| ≥ 5 | 194 (46.2) | 54 (73.0) | 140 (40.5) | |
| Differentiation | 0.461 | |||
| Well | 107 (25.5) | 22 (29.7) | 85 (24.6) | |
| Moderate | 213 (50.7) | 38 (51.4) | 175 (50.6) | |
| Poor/Undifferentiated | 100 (23.8) | 14 (18.9) | 86 (24.8) | |
| T stage | 0.087 | |||
| T1 | 40 (9.5) | 3(4.1) | 37 (10.7) | |
| T2 | 66 (15.7) | 8 (10.7) | 58 (16.8) | |
| T3 | 280 (66.7) | 54 (73.0) | 226 (65.3) | |
| T4 | 34 (8.1) | 9 (12.2) | 25 (7.2) | |
| N stage | 0.518 | |||
| N0 | 215 (51.2) | 34 (45.9) | 181 (52.3) | |
| N1 | 111 (26.4) | 20 (27.0) | 91 (26.3) | |
| N2 | 73 (17.4) | 17 (23.0) | 56 (16.2) | |
| N3 | 21 (5.0) | 3 (4.1) | 18 (5.2) | |
| TNM stage | 0.116 | |||
| I | 38 (9.0) | 3 (4.1) | 35 (10.1) | |
| II | 192 (45.7) | 31 (41.9) | 161 (46.5) | |
| III | 190 (45.3) | 40 (54.0) | 150 (43.4) | |
| Alcohol consumption | 0.788 | |||
| Never | 278 (66.2) | 45 (60.8) | 233 (67.3) | |
| Ever | 142 (33.8) | 29 (39.2) | 113 (32.7) | |
| Smoking | 0.855 | |||
| Never | 155 (36.9) | 28 (37.8) | 127 (36.7) | |
| Ever | 265 (63.1) | 46 (62.2) | 219 (63.3) | |
| Adjuvant treatment | 0.963 | |||
| No | 347 (82.6) | 61 (82.4) | 286 (82.7) | |
| Yes | 73 (17.4) | 13 (17.6) | 60 (17.3) | |
| Pretreatment WBC level (10^9/L) | 7.5 ± 2.4 | 9.2 ± 3.2 | 7.1 ± 2.0 | < 0.001* |
| Pretreatment BMI (kg/m2) | 22.1 ± 3.2 | 20.5 ± 3.4 | 22.5 ± 3.0 | < 0.001* |
| Pretreatment albumin level (g/L) | 43.8 ± 8.1 | 40.2 ± 5.0 | 44.5 ± 8.4 | < 0.001* |
| Pretreatment NLR level | 2.5 ± 2.1 | 5.0 ± 4.1 | 1.9 ± 0.6 | < 0.001* |
BAN, BMI-Albumin-NLR; ESCC, esophageal squamous cell carcinoma; TNM, tumor-node-metastasis; WBC, white blood cell; BMI, body mass index; NLR, neutrophil lymphocyte ratio.
*P < 0.05.
Kaplan-Meier survival curves of A, disease-free survival (DFS) and B, overall survival (OS) stratified by pretreatment body-mass-index, albumin and neutrophil-lymphocyte ratio (BAN) score in 420 esophageal squamous cell carcinoma (ESCC) patients (with log-rank test).
Clinicopathological factors, BAN score, and DFS: univariate and multivariate analysis (N=420)
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years) | ||||||
| < 60 vs. ≥ 60 | 1.024 | 0.801-1.309 | 0.852 | NI | ||
| Gender | ||||||
| Male vs. Female | 0.748 | 0.555-1.007 | 0.056 | NI | ||
| Tumor location | ||||||
| Middle vs. Non-middle | 0.987 | 0.797-1.218 | 0.890 | NI | ||
| Tumor length (cm) | ||||||
| < 5 vs. ≥ 5 | 1.504 | 1.179-1.920 | 0.001* | 1.095 | 0.843-1.421 | 0.498 |
| Differentiation | ||||||
| Moderate/Well vs. Poor/Undifferentiated | 1.111 | 0.839-1.472 | 0.462 | NI | ||
| Depth of invasion | ||||||
| T1-T2 vs. T3-T4 | 2.115 | 1.536-2.911 | < 0.001* | 1.508 | 1.072-2.123 | 0.018* |
| Lymph node involvement | ||||||
| Negative vs. Positive | 2.634 | 2.045-3.392 | < 0.001* | 2.192 | 1.670-2.878 | < 0.001* |
| TNM stage | ||||||
| I-II vs. III | 2.248 | 1.755-2.880 | < 0.001* | NI | ||
| Alcohol consumption | ||||||
| Never vs. Ever | 1.408 | 1.094-1.812 | 0.008* | 1.139 | 0.851-1.524 | 0.381 |
| Smoking | ||||||
| Never vs. Ever | 1.328 | 1.024-1.724 | 0.033* | 1.117 | 0.828-1.506 | 0.468 |
| Adjuvant treatment | ||||||
| No vs. Yes | 1.561 | 1.156-2.109 | 0.004* | 1.191 | 0.875-1.622 | 0.266 |
| BAN score | ||||||
| Low (< 28.0) vs. High (≥ 28.0) | 0.650 | 0.481-0.877 | 0.005* | 0.726 | 0.532-0.993 | 0.045* |
DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; NI, not included.
*P < 0.05.
In univariate analysis of DFS, the results revealed that low pretreatment BAN score was significantly associated with impaired DFS (HR, 0.650; 95% CI, 0.481-0.877; P = 0.005; Figure 1A). Tumor length (<5 vs. ≥5 cm), depth of invasion (T1-2 vs. T3-4), lymph node involvement (Negative vs. Positive), TNM stage (I-II vs. III), alcohol consumption (Never vs. Ever), smoking (Never vs. Ever) and adjuvant treatment (No vs. Yes) were classified as other significant prognostic indicators (P < 0.05). In addition, pretreatment BAN score (HR, 0.726; 95% CI, 0.532-0.993; P = 0.045) could independently predict DFS identified by multivariate analysis (Table 2).
Univariate analysis of OS suggested that cases with low pretreatment BAN score tended to have worse OS (HR, 0.608; 95% CI, 0.445-0.829; P = 0.002; Figure 1B). Besides, tumor length, depth of invasion, lymph node involvement, TNM stage, alcohol consumption and adjuvant treatment could also significantly predict OS. Moreover, multivariate cox regression analysis found that pretreatment BAN score could also serve as a significant predictor for OS. BAN score of ≥ 28.0 had a HR of 0.670 (95% CI, 0.485-0.927; P = 0.016) for OS (Table 3).
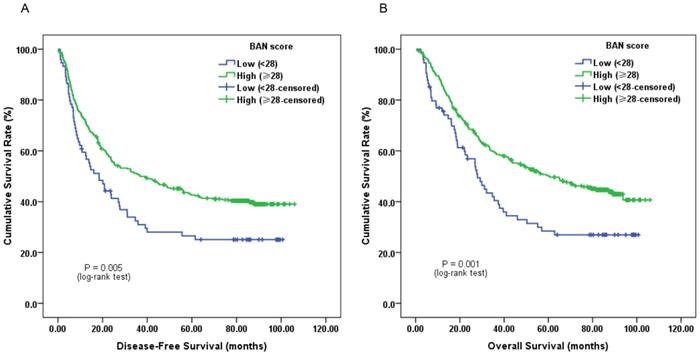
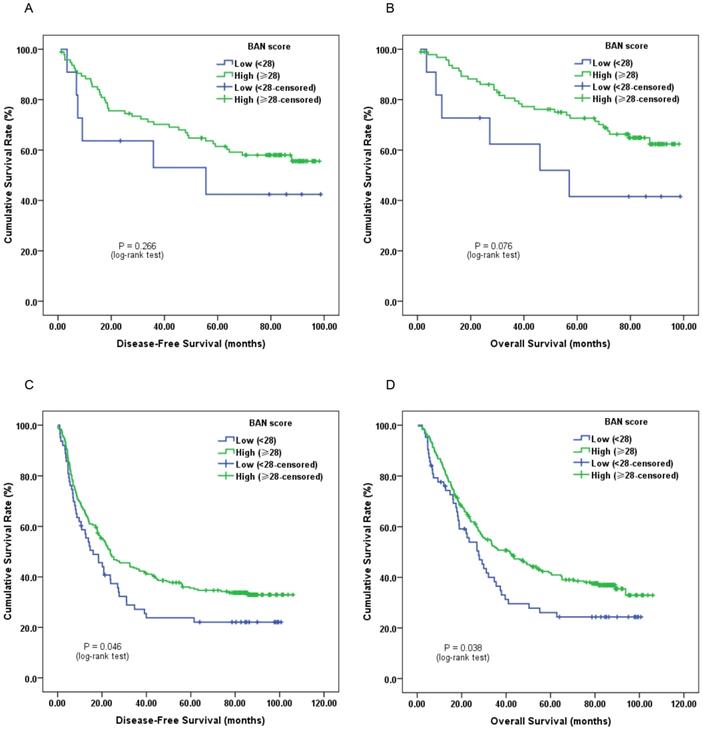
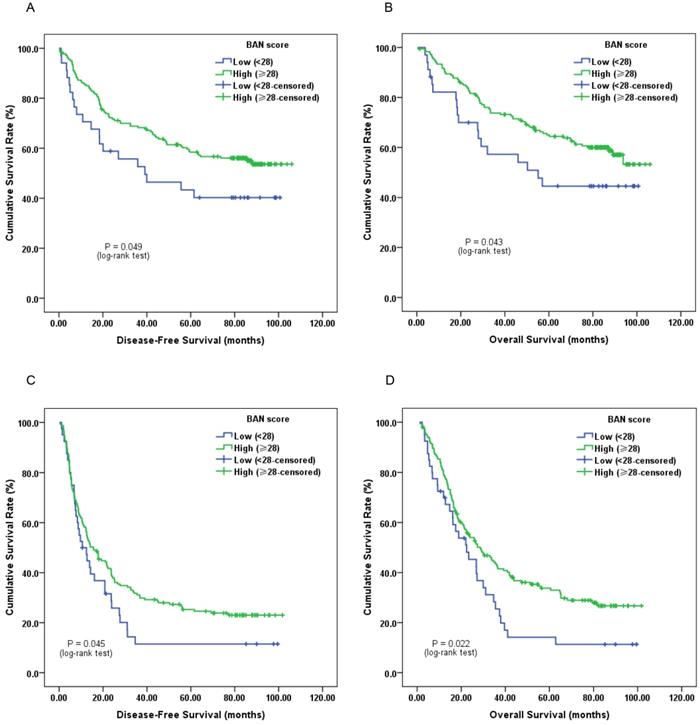
Furthermore, subgroup analysis classified by depth of invasion and lymph node status indicated that low pretreatment BAN score was significantly associated with worse DFS (Figure 2C, 3A and 3C; P < 0.05) and OS (Figure 2D, 3B and 3D; P < 0.05) in certain cases, but not DFS in T1-2 stage ESCC subjects (Figure 2A and 2B; P > 0.05).
Discussion
Systemic inflammation response has been identified to be involved in the development, progression and distant metastasis, and associated with unfavorable survival in a broad range of malignant diseases, including ESCC [4-7]. To the best of our knowledge, this study revealed for the first time that patients with low pretreatment BAN score had more advanced and progressive diseases, and were significantly correlated with worse prognosis. The present study shed light on the importance of evaluating systemic inflammation response in ESCC patients as a prognostic marker.
Clinicopathological factors, BAN score, and OS: univariate and multivariate analysis (N=420)
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years) | ||||||
| < 60 vs. ≥ 60 | 1.134 | 0.878-1.465 | 0.336 | NI | ||
| Gender | ||||||
| Male vs. Female | 0.770 | 0.566-1.047 | 0.096 | NI | ||
| Tumor location | ||||||
| Middle vs. Non-middle | 0.957 | 0.765-1.196 | 0.698 | NI | ||
| Tumor length (cm) | ||||||
| < 5 vs. ≥ 5 | 1.429 | 1.107-1.844 | 0.006* | 0.980 | 0.745-1.290 | 0.888 |
| Differentiation | ||||||
| Moderate/Well vs. Poor/Undifferentiated | 1.103 | 0.823-1.478 | 0.512 | NI | ||
| Depth of invasion | ||||||
| T1-T2 vs. T3-T4 | 2.393 | 1.690-3.389 | < 0.001* | 1.804 | 1.248-2.607 | 0.002* |
| Lymph node involvement | ||||||
| Negative vs. Positive | 2.602 | 1.996-3.391 | < 0.001* | 2.135 | 1.611-2.829 | < 0.001* |
| TNM stage | ||||||
| I-II vs. III | 2.320 | 1.789-3.008 | < 0.001* | NI | ||
| Alcohol consumption | ||||||
| Never vs. Ever | 1.521 | 1.170-1.978 | 0.002* | 1.338 | 1.023-1.748 | 0.033* |
| Smoking | ||||||
| Never vs. Ever | 1.294 | 0.987-1.698 | 0.062 | NI | ||
| Adjuvant treatment | ||||||
| No vs. Yes | 1.577 | 1.153-2.156 | 0.004* | 1.189 | 0.864-1.635 | 0.288 |
| BAN score | ||||||
| Low (< 28.0) vs. High (≥ 28.0) | 0.608 | 0.445-0.829 | 0.002* | 0.670 | 0.485-0.927 | 0.016* |
OS, overall survival.
*P < 0.05.
Kaplan-Meier survival curves of A, DFS and B, OS stratified by pretreatment BAN score in T1-2 stage ESCC patients (N = 106); C, DFS and D, OS stratified by pretreatment BAN score in T3-4 stage ESCC patients (N = 314) (with log-rank test).
Kaplan-Meier survival curves of A, DFS and B, OS stratified by pretreatment BAN score in ESCC patients without lymph node involvement (N = 215); C, DFS and D, OS stratified by pretreatment BAN score in ESCC patients with lymph node involvement (N = 205) (with log-rank test).
Clinically, serum CRP, white blood cell, neutrophil and lymphocyte counts are the most commonly measured parameters to evaluate the degree of systemic inflammation response in cancer patients [7]. Besides, hypoalbuminemia is also recognized to play vital roles in systemic inflammatory response [11]. Moreover, combinations of such variables have been proposed to establish several inflammation-based prognostic formulas.
On the one hand, the modified GPS is determined based on serum CRP and albumin concentrations, and is widely used as a valuable and promising prognostic marker in various malignancies, including ESCC [19-22]. However, Tian et al. failed to identify modified GPS as an independent prognostic indicator in 260 ESCC patients who received transthoracic esophagectomy [22]. Neither did Arigami et al. found its independent prognostic significance in 238 ESCC subjects who underwent esophagectomy with lymphadenectomy [16]. On the other hand, the NLR, a marker of systemic inflammation has also been found to predict unfavorable survival in cases with different cancers including ESCC [14-15]. Results from Chen MF' study showed that an elevated pretreatment NLR was significantly associated with advanced-stage disease and impaired OS in 926 ESCC patients who underwent concomitant chemoradiotherapy, but not in the 242 subjects treated with surgical intervention [15]. Recently, Arigami T and his colleagues suggested that NLR could serve as a valuable indicator for predicting disease progression and prognosis in patients with ESCC who received esophagectomy with lymphadenectomy [16].
Furthermore, to the best of our knowledge, weight loss was also associated with ongoing systemic inflammation response [23-24]. Therefore, we established BAN score, which was calculated based on BMI, albumin and NLR, to assess the ongoing systemic inflammation response in these patients. Subjects with BAN score of < 28.0 indicating high systemic inflammation response were found to have more advanced tumor length. However, no significant difference was observed in BAN score based on age, gender, tumor location, degree of differentiation, depth of invasion, lymph node status or tumor-node-metastasis, suggesting that both groups could generate similar degree of systemic inflammation response. In addition, the multivariate analysis revealed that depth of invasion, lymph node metastasis, alcohol consumption and pretreatment BAN score were independently associated with impaired long-term survival. Of these, depth of invasion, lymph node metastasis and alcohol consumption were non-modifiable variables. Thus, high systemic inflammation response as manifested by ALI < 28.0 provided a novel and prominent therapeutic area for improving the outcome of such cases.
Although the main limitations of this study were the retrospective and single institution design, the findings suggested that pretreatment BAN score could significantly predict long-term survival, help risk stratification more accurately and design optimal therapeutic strategies for resectable ESCC.
Acknowledgements
This study was supported by grants from 2021 Anhui Natural Science Foundation (NO. 2108085QH345) and 2018 In-House Youth Development Program of the First Affiliated Hospital of Anhui Medical University (NO. 2877).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;65:115-32
2. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381-7
3. Lin Y, Totsuka Y, He Y. et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233-42
4. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-7
5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer related Inflammation. Nature. 2008;454:436-44
6. Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
7. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149-63
8. Kimura J, Kunisaki C, Makino H. et al. Evaluation of the Glasgow Prognostic Score in patients receiving chemoradiotherapy for stage III and IV esophageal cancer. Dis Esophagus. 2016;29:1071-80
9. Zhang P, Xi M, Li QQ. et al. The modified Glasgow Prognostic Score is an independent prognostic factor in patients with inoperable thoracic esophageal squamous cell carcinoma undergoing chemoradiotherapy. J Cancer. 2014;5:689-95
10. Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23:31-9
11. McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39:210-3
12. Wang CY, Hsieh MJ, Chiu YC. et al. Higher serum c-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol. 2009;92:270-5
13. Lindenmann J, Fink-Neuboeck N, Koesslbacher M. et al. The influence of elevated levels of C- reactive protein and hypoalbuminemia on survival in patients with advanced inoperable esophageal cancer undergoing palliative treatment. J Surg Oncol. 2014;110:645-50
14. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophillymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218-30
15. Chen MF, Chen PT, Kuan FC, Chen WC. The Predictive Value of Pretreatment Neutrophil-To-Lymphocyte Ratio in Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2018 Oct 25. doi: 10.1245/s10434-018-6944-1
16. Arigami T, Okumura H, Matsumoto M. et al. Analysis of the fibrinogen and neutrophil-lymphocyte ratio in esophageal squamous cell carcinoma. Medicine (Baltimore). 2015;94:e1702
17. Sun P, Zhang F, Chen C. et al. Comparison of the prognostic values of various nutritional parameters in patients with esophageal squamous cell carcinoma from Southern China. J Thorac Dis. 2013;5:484-91
18. Budczies J, Klauschen F, Sinn BV. et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE. 2012;7:e51862
19. Proctor MJ, Morrison DS, Talwar D. et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726-34
20. Nakagawa K, Tanaka K, Nojiri K. et al. The modified Glasgow prognostic score as a predictor of survival after hepatectomy for colorectal liver metastasis. Ann Surg Oncol. 2014;21:1711-8
21. Fan H, Shao ZY, Xiao YY. et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol. 2016;142:1285-97
22. Tian R, Zhang F, Sun P. et al. The Preoperative Sensitive-Modified Glasgow Prognostic Score is Superior to the Modified Glasgow Prognostic Score in Predicting Long-term Survival for Esophageal Squamous Cell Carcinoma. Oncotarget. 2016;7:67485-94
23. Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87:264-7
24. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158
Author contact
![]() Corresponding author: Wan-ren Peng, Department of Oncology, the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, P. R. China. Tel.: +86-551-65908800; Fax: +86-551-65908800; E-mail: pengwanrenedu.cn.
Corresponding author: Wan-ren Peng, Department of Oncology, the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, P. R. China. Tel.: +86-551-65908800; Fax: +86-551-65908800; E-mail: pengwanrenedu.cn.

 Global reach, higher impact
Global reach, higher impact