Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(9):2922-2932. doi:10.7150/jca.74617 This issue Cite
Research Paper
Clinical outcomes of Atezolizumab Therapy for Previously-Treated Advanced-Stage Non-Small Cell Lung Cancer: A Real-World Study in Taiwan
1. Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University, Taipei, Taiwan.
2. Department of Internal Medicine, National Taiwan University Cancer Center, National Taiwan University, Taipei, Taiwan.
3. Department of Chest Medicine, Taipei Veterans General Hospital, Taipei, Taiwan.
4. School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
5. Institute of Clinical Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
6. Division of Pulmonary and Critical Care Medicine, Department of Medicine, Chang Gung Memorial Hospital-Kaohsiung Medical Center, Chang Gung University College of Medicine, Kaohsiung, Taiwan.
7. Department of Internal Medicine, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
8. Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
9. Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
10. Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, China Medical University and China Medical University Hospital, Taichung, Taiwan.
11. Division of Lung Cancer and Interventional Bronchoscopy, Department of Thoracic Medicine, College of Medicine, Chang Gung Memorial Hospital, Chang Gung University, Taipei, Taiwan.
Received 2022-5-1; Accepted 2022-7-5; Published 2022-7-18
Abstract
Immune checkpoint inhibitors (ICIs) are the standard treatment for non-small-cell lung cancer (NSCLC). We assessed the clinical prognostic factors in NSCLC patients receiving atezolizumab as a second- or later-line (2L+) treatment. Data were retrospectively collected for NSCLC patients treated with atezolizumab from July 2017 to June 2019 at six medical centers in Taiwan. Clinical characteristics, treatment course and responses of patients were recorded. A total of 128 NSCLC patients received 2L+ atezolizumab, and the outcomes included a response rate of 10.2%, median progression-free survival (mPFS) of 3.5 months, and median overall survival (mOS) of 10.7 months. Eleven patients who had received osimertinib treatment before atezolizumab had a shorter mPFS (2.3 versus 3.5 months; p = 0.002) and mOS (4.8 versus 11.2 months; p < 0.001) than those without prior osimertinib treatment. Even for the subgroup of patients with EGFR-mutant non-squamous NSCLC, prior osimertinib was still associated with shorter PFS (2.3 versus 4.1 months; p = 0.006) and OS (4.8 versus 11.7 months; p < 0.001). Multivariate analysis revealed that prior osimertinib treatment correlated with not only shorter PFS (hazard ratio [HR]: 2.94; 95% confidence interval [CI], 1.34-6.47; p = 0.007) but also shorter OS (HR, 3.55; 95% CI, 1.57-8.03; p = 0.002). Patients with prior ICIs treatment (HR, 3.18; p = 0.002) or poor performance status (HR, 2.70; p = 0.001) had shorter OS. In conclusion, osimertinib treatment before atezolizumab therapy was associated with a shorter PFS and a poor prognosis in NSCLC patients in real-world settings. Further studies with larger sample sizes are needed to validate these observations.
Keywords: Atezolizumab, Epidermal growth factor receptor mutation, Non-small-cell lung cancer, Tyrosine kinase inhibitor, Osimertinib, Immune checkpoint inhibitor
Introduction
Lung cancer is the leading cause of cancer-related deaths [1]. Traditionally, platinum-doublet chemotherapy has been the standard first-line treatment for advanced non-small-cell lung cancer (NSCLC), but its prognosis is poor [2-4]. In the recent decade, precision-targeted therapies for patients with oncogenic alterations, such as epidermal growth factor receptor (EGFR) and B-type Raf kinase V600E (BRafV600E) mutations or anaplastic lymphoma kinase (ALK) and ROS proto-oncogene 1 (ROS1) fusions, have provided favorable effectiveness and survival benefits [5-7]. Unfortunately, almost all patients ultimately acquire resistance to targeted therapies. Hence, novel treatment strategies are urgently needed.
Antibodies targeting the programmed death-ligand 1 (PD-L1)/programmed death-1 (PD-1) pathway represent an important advance in the management of metastatic NSCLC. In recent years, anti-PD-L1/anti-PD-1 immune checkpoint inhibitors (ICIs) have become the standard of care for previously treated NSCLC without a targetable oncogene, and have rapidly become first-line treatment for patients with advanced NSCLC [2, 3, 8-11].
Atezolizumab, an anti-PD-L1 antibody, has been shown to provide clinically relevant improvement of overall survival (OS) versus docetaxel in patients with previously treated metastatic NSCLC, regardless of PD-L1 expression [12]. In addition, patients with EGFR mutation-positive disease also have similar overall survival benefit with atezolizumab and docetaxel [12]. Atezolizumab has also shown a promising efficacy and an acceptable safety profile when combined with platinum-doublet chemotherapy in patients who have not previously received chemotherapy for NSCLC [13, 14]. Even as a first-line treatment, atezolizumab monotherapy provides a significantly longer OS than platinum-based chemotherapy in patients with NSCLC with high PD-L1 expression, regardless of the histologic type [8]. In the phase 3 IMpower150 study, the addition of atezolizumab to bevacizumab plus carboplatin and paclitaxel as the first-line treatment for nonsquamous metastatic NSCLC resulted in a significant improvement in progression-free survival (PFS) and OS [15]. A survival benefit was observed across key subgroups, including those with varying levels of PD-L1 expression, with no new safety risks identified with the combination treatment.
Atezolizumab has been approved for patients with metastatic NSCLC in Taiwan since 2017. Despite its efficacy in clinical trials, little is known about real-world clinical outcomes of atezolizumab in patients with lung cancer, especially in the Asian population. Additionally, it remains unclear whether the choice of frontline treatment affects the effectiveness of subsequent atezolizumab treatment in patients with metastatic NSCLC. Especially, osimertinib has been reimbursed by the National Health Insurance (NHI) of Taiwan since April 2020. The purpose of this study was to investigate the impact of frontline treatments in patients who received subsequent atezolizumab-based regimens in real-world settings in Taiwan.
Material and Methods
Patients
This multicenter, observational, retrospective study was conducted at six medical centers in Taiwan. The participating institutions included three hospitals in northern Taiwan (National Taiwan University Hospital, Taipei Veterans General Hospital, and Chang Gung Memorial Hospital Linkou Branch), one in central Taiwan (China Medical University Hospital), and two in southern Taiwan (Chang Gung Memorial Hospital Kaohsiung Branch and Kaohsiung Medical University Chung-Ho Memorial Hospital). The study was approved by the Institutional Review Board of each participating medical center, which waived the requirement for informed consent.
The inclusion criteria included patients who had tumors which were histologically or cytologically confirmed diagnosis of advanced or metastatic lung cancers, and received at least one dose of atezolizumab before June 30, 2019. Exclusion criteria included patients participating the interventional clinical trials of atezolizumab, or patients whose follow-up duration after the first administration of atezolizumab was less than 4 weeks were excluded. Lung cancer histology was classified according to the World Health Organization classification of lung tumors [16]. The lung cancer stage was determined according to the 8th Edition of the International Association for the Study of Lung Cancer tumor-node-metastasis staging system [17]. The patients had previously received at least one line of systemic therapy for unresectable, locally advanced, or metastatic NSCLC. Patients were included in the analysis irrespective of the PD-L1 status.
The clinical and demographic characteristics of the patients, treatment medications, and responses were recorded. The treatment medications included chemotherapy, TKI therapy, ICIs, or radiotherapy. Smoking history before lung cancer diagnosis, smoking duration, and the number of packs of cigarettes were recorded. Patients who had smoked <100 cigarettes in their lifetime were defined as nonsmokers, and all others were categorized as smokers [18].
Response evaluation of patients with NSCLC
The treatment responses were evaluated and recorded according to the Response Evaluation Criteria in Solid Tumors guidelines (version 1.1), which included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) [19]. PFS was defined as the period from the initiation of atezolizumab treatment to disease progression or death. OS was defined as the period from the date of initial atezolizumab treatment to the date of death.
Statistical analysis
The SPSS software (version 26.0 for Mac; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Categorical variables were analyzed using the chi-squared test. If the sampling variability was ≤ 5, Fisher's exact test was applied. Statistical significance was set at a two-sided P-value of < 0.05. The nonparametric Mann-Whitney U-test was used to compare the median ages between two groups.
The propensity score (PS) for the probability of atezolizumab monotherapy or combination therapy was created through a logistic regression model, which included potential confounders such as, sex, age, Eastern Cooperative Oncology Group performance status (ECOG PS), smoking, histology (adenocarcinoma vs. non-adenocarcinoma), brain metastasis, PD-L1 expression, line of atezolizumab, prior EGFR-TKI, prior platinum therapy, prior ICIs, prior pemetrexed, and prior osimertinib. A 1:1 matched cohort group of atezolizumab monotherapy and combination therapy was created. The survival outcome analysis of both original cohort and PSM cohort were performed.
The Kaplan-Meier method was used to plot survival curves, and the log-rank test was used for comparison between groups. The predictive factors of PFS and the potential prognostic factors of OS were evaluated by multivariate Cox regression model. The selection of possible predictors and prognostic factors were on the basis of previous studies investigating the prognostic factors of survival in lung cancer, especially for 2nd-line ICIs [20, 21], including: sex, smoking, performance status, tumor histology, brain metastasis, PD-L1 tumor proportion scores (TPS), EGFR mutation status, line of atezolizumab, atezolizumab monotherapy or combination therapy, prior treatments (EGFR-TKI, platinum therapy, ICIs, pemetrexed, and osimertinib).
Results
Patient distribution and baseline clinical characteristics
There were 160 patients who received atezolizumab, including 144 patients with NSCLC and 16 with small-cell lung cancer. Of the 144 patients with NSCLC, 16 received atezolizumab as the first-line treatment. 128 patients receiving atezolizumab as the second- or later-line (2L+) treatment were enrolled in this study. The median follow-up period of the 128 patients were 41.2 (95% confidence interval [CI]: 37.6-45.8) months.
The 128 patients had a median age of 60.8 (range: 32.8-83.2) years (Table 1). Sixty-four (50.0%) patients were females, and 75 (58.6%) were nonsmokers. The tumor histology included 97 (75.8%) adenocarcinoma. Twenty-four patients (18.8%) had tumor with high PD-L1 TPS (≥50%). The EGFR mutation status were 42 (32.8%) EGFR mutations, 66 (51.6%) wild type of EGFR, and 20 (15.6%) no recording data.
Clinical characteristics of NSCLC patients who had received atezolizumab as the second- or later-line (2L+) treatment
| Factor | Patients (%) |
|---|---|
| Total patients, n (%) | 128 (100.0%) |
| Age (median, years) (range) | 60.8 (32.8-83.2) |
| Sex | |
| Female | 64 (50.0%) |
| Male | 64 (50.0%) |
| Smoking status | |
| Nonsmokers | 75 (58.6%) |
| Smokers | 53 (41.4%) |
| ECOG PS | |
| 0-1 | 103 (80.5%) |
| ≥2 | 25 (19.5%) |
| Histology | |
| Non-adenocarcinoma | 31 (24.2%) |
| Adenocarcinoma | 97 (75.8%) |
| Brain Metastasis | |
| No | 78 (60.9%) |
| Yes | 50 (39.1%) |
| PD-L1 TPS | |
| <50% | 58 (45.3%) |
| ≥50% | 24 (18.8%) |
| No data | 46 (35.9%) |
| EGFR | |
| Wild type | 66 (51.6%) |
| Mutant | 42 (32.8%) |
| No data | 20 (15.6%) |
| Line of atezolizumab | |
| Second | 38 (29.7%) |
| ≥Third | 90 (70.3%) |
| Atezolizumab therapy | |
| Monotherapy | 57 (44.5%) |
| Combination | 71 (55.5%) |
| Prior medications | |
| EGFR-TKI | 62 (48.4%) |
| ICIs | 13 (10.2%) |
| Platinum | 110 (85.9%) |
| Pemetrexed | 85 (66.4%) |
EGFR, epidermal growth factor receptor gene; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; ECOG PS, Eastern Cooperative Oncology Group performance status; TPS, tumor proportion score; ICIs, immune checkpoint inhibitors.
Thirty-eight (29.7%) patients were treated with atezolizumab as the second-line treatment, and 90 (70.3%) patients received atezolizumab as the third- or subsequent-line treatment. The median number of prior lines of systemic treatment was 4 (range: 2-11). A total of 57 (44.5%) patients received atezolizumab monotherapy, and 71 (55.5%) patients received atezolizumab combination therapy, including 44 (62.0%; 44 of 71) dual therapy, 20 (28.2%) triple therapy and 7 (9.9%) quadruple therapy (Supplementary Table S1). Before atezolizumab-containing treatment, there were 13 patients treated with ICIs (Supplementary Table S2), 62 patients with EGFR-TKIs, 110 patients with platinum-based chemotherapy, and 85 patients with pemetrexed.
Treatment responses and survival analysis in patients with atezolizumab as 2L+ atezolizumab-containing treatment
Among the 128 patients with NSCLC who received 2L+ atezolizumab, the maximum response to atezolizumab treatment was CR in 1 patient (0.8%), PR in 12 patients (9.4%), SD in 37 patients (28.9%), and PD in 78 patients (60.9%). The response rate was 10.2%. The median PFS was 3.5 (95% CI: 2.9-4.1) months, and median OS was 10.7 (95% CI: 9.4-12.0) months.
PFS was used to evaluate the potential predictive factor and the impact of front-line treatments before atezolizumab-containing treatment (Table 2). There was no significant difference in mPFS between patients with different PD-L1 TPS (<50% versus ≥50% versus No data: 3.2 months versus 3.1 months versus 3.8 months; p = 0.724) (Supplementary Figure S1A). There was also no significant difference in mPFS between the patients with EGFR mutations (3.2 months), wild type of EGFR (3.4 months) and without EGFR mutation data (3.9 months; p = 0.344) (Supplementary Figure S1B). Thirteen patients who had received ICIs before atezolizumab-containing treatment had a shorter mPFS (2.4 months versus 3.5 months; p = 0.009) than those without prior ICIs (Supplementary Figure S2A). There was no significant difference in mPFS between patients with and without prior EGFR-TKI (3.5 months versus 3.3 months; p = 0.341).
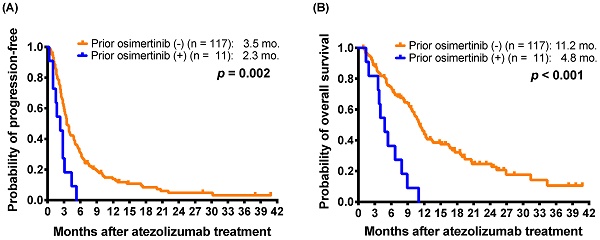
Furthermore, 11 patients had received osimertinib treatment before atezolizumab-containing treatment. The 11 patients received osimertinib after acquiring resistance to EGFR-TKI therapy, including gefitinib, erlotinib, and afatinib (seven, two, and two patients, respectively). All of the patients were nonsmokers and received atezolizumab in the third- or subsequent-line settings (range: 4-10, median: 6). The 11 patients who had received osimertinib treatment before atezolizumab had a shorter mPFS than those who had not received prior osimertinib (2.3 months versus 3.5 months; p = 0.002) (Figure 1A).
For mOS, the patients who received EGFR TKIs before atezolizumab had shorter mOS than those who had not received prior EGFR TKI (10.1 months versus 12.0 months; p = 0.048) (Table 3). Furthermore, the patients who received osimertinib before atezolizumab also had shorter mOS than those who had not received prior osimertinib (4.8 months versus 11.2 months; p < 0.001) (Figure 1B). The patients who had received ICIs before an atezolizumab-containing regimen had a shorter mOS (6.3 versus 11.0 months; p = 0.030) than those without prior ICI treatment (Supplementary Figure S2B). In addition, patients with better performance status (ECOG PS 0-1) (11.7 months versus 5.7 months; p < 0.001), patients with adenocarcinoma histology (11.1 months versus 6.6 months; p = 0.021) had longer mOS.
Multivariate analysis of predictive factors for PFS in patients who received atezolizumab as a second- or subsequent-line treatment
| Factor | Number of patients | PFS (months) | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|---|
| P | HR (95% CI) | P | |||
| Sex | |||||
| Female | 64 | 3.5 | 1 | ||
| Male | 64 | 3.4 | 0.688 | 1.46 (0.80-2.64) | 0.215 |
| Smoking history | |||||
| Nonsmokers | 75 | 3.5 | 1 | ||
| Smokers | 53 | 3.5 | 0.744 | 1.07 (0.59-1.93) | 0.832 |
| ECOG PS | |||||
| 0-1 | 103 | 3.5 | 1 | ||
| ≥2 | 25 | 3.5 | 0.249 | 1.13 (0.66-1.93) | 0.651 |
| Histology | |||||
| Non-adenocarcinoma | 31 | 3.5 | 1 | ||
| Adenocarcinoma | 97 | 3.5 | 0.246 | 0.58 (0.31-1.08) | 0.087 |
| Brain metastasis | |||||
| No | 78 | 3.9 | 1 | ||
| Yes | 50 | 2.9 | 0.131 | 1.41 (0.91-2.30) | 0.125 |
| PD-L1 TPS | |||||
| <50% | 58 | 3.2 | 1 | ||
| ≥50% | 24 | 3.1 | 1.29 (0.72-2.31) | 0.398 | |
| No data | 46 | 3.8 | 0.724 | 1.09 (0.69-1.70) | 0.724 |
| EGFR | |||||
| Wild type | 66 | 3.4 | 1 | ||
| Mutant | 42 | 3.2 | 0.82 (0.42-1.58) | 0.544 | |
| No data | 20 | 3.9 | 0.344 | 1.22 (0.66-2.24) | 0.528 |
| Line of atezolizumab | |||||
| second | 38 | 3.5 | 1 | ||
| ≥third | 90 | 3.2 | 0.820 | 1.24 (0.76-2.03) | 0.395 |
| Atezolizumab therapy | |||||
| Monotherapy | 57 | 3.1 | 1 | ||
| Combination | 71 | 3.9 | 0.890 | 0.86 (0.55-1.35) | 0.503 |
| Prior EGFR-TKI | |||||
| No | 66 | 3.3 | 1 | ||
| Yes | 62 | 3.5 | 0.463 | 1.19 (0.66-2.15) | 0.570 |
| Prior platinum therapy | |||||
| No | 18 | 3.3 | 1 | ||
| Yes | 110 | 3.5 | 0.341 | 0.56 (0.29-1.12) | 0.100 |
| Prior immunotherapy | |||||
| No | 115 | 3.5 | 1 | ||
| Yes | 13 | 2.4 | 0.009 | 3.03 (1.54-5.95) | 0.001 |
| Prior pemetrexed | |||||
| No | 43 | 3.9 | 1 | ||
| Yes | 85 | 3.1 | 0.886 | 1.22 (0.69-2.17) | 0.496 |
| Prior osimertinib | |||||
| No | 117 | 3.5 | 1 | ||
| Yes | 11 | 2.3 | 0.002 | 2.94 (1.34-6.47) | 0.007 |
PFS, progression-free survival; HR, hazards ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed death-ligand 1; TPS, tumor proportion score; EGFR, epidermal growth factor receptor gene; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor.
To more clarify the impact of osimertinib on the effectiveness of subsequent atezolizumab treatment, we extracted a subgroup including 41 patients harboring nonsquamous NSCLC with EGFR mutations (Supplementary Table S3). After exclusion of one osimertinib-treated patient without EGFR mutation data, the subgroup enrolled 10 patients who had received osimertinib after acquired resistance to first- or second-generation EGFR-TKIs and before atezolizumab-containing treatment. The difference in the response rates between the patients with and without osimertinib treatment before atezolizumab therapy did not reach statistical significance (0.0% [0/10] vs. 9.7% [3/31], p = 0.564, by Fisher's exact test). The patients who received osimertinib before atezolizumab had a shorter mPFS (2.3 months versus 4.1 months; p = 0.006) and a mOS (4.8 months versus 11.7 months; p < 0.001) than those who had not received prior osimertinib (Figure 2A and 2B).
Predictive and prognostic factors in patients with atezolizumab as 2L+ atezolizumab-containing treatment
Multivariate analysis was performed using the Cox regression model to determine potential predictive factors of PFS (Table 2). Patients who had received osimertinib before atezolizumab-containing treatment had significantly shorter PFS than those who did not receive prior Osimertinib (HR, 2.94; 95% CI, 1.34-6.47; p = 0.007). In addition, prior ICIs (HR, 3.03; 95% CI, 1.54-5.95; p = 0.001) was significantly associated with shorter PFS.
For OS, multivariate analysis was carried out to identify potential prognostic factors for survival and showed that prior osimertinib treatment (HR: 3.55; 95% CI, 1.57-8.03; p = 0.002), prior ICI treatment (HR: 3.18; 95% CI, 1.53-6.61; p = 0.002) and a poor performance status (HR: 2.70; 95% CI, 1.46-4.97; p = 0.001) were associated with shorter OS. Meanwhile, adenocarcinoma histology was a favorable prognostic factor (HR: 0.31; 95% CI, 0.16-0.60; p < 0.001) (Table 3).
Comparing treatment efficacy and survival of matched monotherapy and combination therapy of atezolizumab
To compare the clinical efficacy between atezolizumab monotherapy and combination therapy, we conducted a propensity-scored 1:1 matched cohort, and there were total of 34 patient pairs (PSM cohort) who received monotherapy/combination therapy of atezolizumab from the original cohort. The demographic and clinical characteristics were balanced between the matched groups (Table 4).
In the PSM cohort, the response rate were 2.9% (1 of 34) in monotherapy group and 11.8% (4 of 34) in the combination therapy group (p = 0.356, by Fisher's exact test). In the original cohort, there were no significant differences in mPFS (3.1 months versus 3.9 months; p = 0.890) and mOS (11.2 months versus 10.6 months; p = 0.677) between monotherapy group and combination therapy group (Figure 3A and 3B). Pertaining to the PSM cohort, the results of outcomes analysis were highly consistent with the original cohort. There were also no significant differences in mPFS (2.8 months versus 3.9 months; p = 0.054) and mOS (11.6 months versus 10.3 months; p = 0.878) between patients who received monotherapy and combination therapy of atezolizumab (Figure 3C and 3D).
Multivariate analysis of prognostic factors for OS in patients who received atezolizumab as a second- or subsequent-line treatment
| Factor | Number of patients | OS (months) | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|---|
| P | HR (95% CI) | P | |||
| Sex | |||||
| Female | 64 | 10.3 | 1 | ||
| Male | 64 | 11.1 | 0.977 | 0.73 (0.39-1.37) | 0.329 |
| Smoking history | |||||
| Nonsmokers | 75 | 10.1 | 1 | ||
| Smokers | 53 | 11.6 | 0.707 | 0.94 (0.47-1.88) | 0.871 |
| ECOG PS | |||||
| 0-1 | 103 | 11.7 | 1 | ||
| ≥2 | 25 | 5.7 | <0.001 | 2.70 (1.46-4.97) | 0.001 |
| Histology | |||||
| Non-adenocarcinoma | 31 | 6.6 | 1 | ||
| Adenocarcinoma | 97 | 11.1 | 0.021 | 0.31 (0.16-0.60) | <0.001 |
| Brain metastasis | |||||
| No | 78 | 10.7 | 1 | ||
| Yes | 50 | 10.3 | 0.563 | 0.82 (0.49-1.36) | 0.442 |
| PD-L1 TPS | |||||
| <50% | 58 | 10.2 | 1 | ||
| ≥50% | 24 | 7.6 | 1.84 (0.96-3.54) | 0.069 | |
| No data | 46 | 11.7 | 0.341 | 1.10 (0.65-1.88) | 0.728 |
| EGFR | |||||
| Wild type | 66 | 11.0 | 1 | ||
| Mutant | 42 | 10.2 | 0.65 (0.29-1.47) | 0.299 | |
| No data | 20 | 11.4 | 0.241 | 1.53 (0.78-3.01) | 0.221 |
| Line of atezolizumab | |||||
| second | 38 | 11.2 | 1 | ||
| ≥third | 90 | 10.2 | 0.630 | 1.58 (0.88-2.84) | 0.129 |
| Atezolizumab therapy | |||||
| Monotherapy | 57 | 11.2 | 1 | ||
| Combination | 71 | 10.6 | 0.677 | 0.85 (0.50-1.44) | 0.541 |
| Prior EGFR-TKI | |||||
| No | 66 | 12.0 | 1 | ||
| Yes | 62 | 10.1 | 0.048 | 1.40 (0.73-2.70) | 0.317 |
| Prior platinum therapy | |||||
| No | 18 | 8.3 | 1 | ||
| Yes | 110 | 10.9 | 0.115 | 0.68 (0.30-1.54) | 0.351 |
| Prior immunotherapy | |||||
| No | 115 | 11.0 | 1 | ||
| Yes | 13 | 6.3 | 0.030 | 3.18 (1.53-6.61) | 0.002 |
| Prior pemetrexed | |||||
| No | 43 | 11.2 | 1 | ||
| Yes | 85 | 10.3 | 0.473 | 1.08 (0.57-2.06) | 0.819 |
| Prior osimertinib | |||||
| No | 117 | 11.2 | 1 | ||
| Yes | 11 | 4.8 | <0.001 | 3.55 (1.57-8.03) | 0.002 |
OS, overall survival; HR, hazards ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed death-ligand 1; TPS, tumor proportion score; EGFR, epidermal growth factor receptor gene; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor.
Kaplan-Meier estimates of (A) progression-free survival and (B) overall survival of patients with and without osimertinib before atezolizumab treatment (log-rank test) in the patients with atezolizumab as 2L+ atezolizumab-containing treatment.
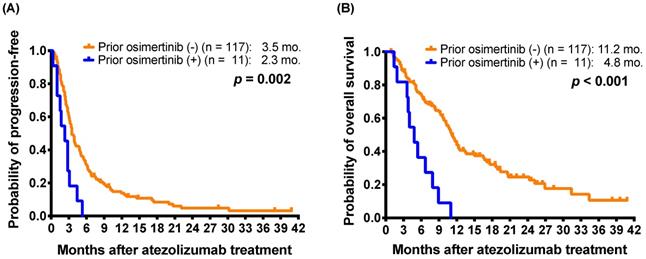
For 41 patients harboring non-squamous NSCLC with EGFR mutations, Kaplan-Meier estimates of (A) progression-free survival and (B) overall survival of patients with and without osimertinib exposure before atezolizumab treatment (log-rank test).
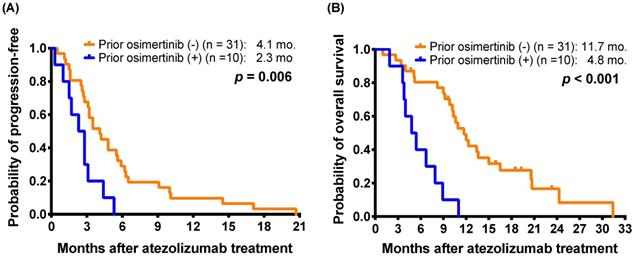
Kaplan-Meier estimates of (A) progression-free survival and (B) overall survival of patients who received monotherapy or combination therapy of atezolizumab (log-rank test) in the original cohort. For propensity-score matching cohort, (C) progression-free survival and (D) overall survival of patients who received monotherapy or combination therapy.
Clinical characteristics of patients who had received monotherapy or combination therapy of atezolizumab
| Factor | Original cohort of atezolizumab | P | Propensity-score matching cohort of atezolizumab | P | ||
|---|---|---|---|---|---|---|
| Monotherapy | Combination | Monotherapy | Combination | |||
| Total patients, n (%) | 57 (44.5%) | 71 (55.5%) | 34 (50.0%) | 34 (50.0%) | ||
| Age (median, years) (range) | 62.0 (32.8-80.2) | 58.5 (35.1-83.2) | 0.294§ | 62.6 (42.5-80.2) | 61.6 (36.1-83.2) | 0.536§ |
| Sex | 0.594 | 0.808 | ||||
| Female | 30 (52.6%) | 34 (47.9%) | 16 (47.1%) | 15 (44.1%) | ||
| Male | 27 (47.4%) | 37 (52.1%) | 18 (52.9%) | 19 (55.9%) | ||
| Smoking status | 0.112 | 1.000 | ||||
| Nonsmokers | 29 (50.9%) | 46 (64.8%) | 18 (52.9%) | 18 (52.9%) | ||
| Smokers | 28 (49.1%) | 25 (35.2%) | 16 (47.1%) | 16 (47.1%) | ||
| ECOG PS | 0.611 | 0.752* | ||||
| 0-1 | 47 (82.5%) | 56 (78.9%) | 29 (85.3%) | 27 (79.4%) | ||
| ≥2 | 10 (17.5%) | 15 (21.1%) | 5 (14.7%) | 7 (20.6%) | ||
| Histology | 0.031 | 0.770 | ||||
| Non-adenocarcinoma | 19 (33.3%) | 12 (16.9%) | 8 (23.5%) | 7 (20.6%) | ||
| Adenocarcinoma | 38 (66.7%) | 59 (83.1%) | 26 (76.5%) | 27 (79.4%) | ||
| Brain metastasis | 0.055 | 1.000 | ||||
| No | 40 (70.2%) | 38 (53.5%) | 23 (67.6%) | 23 (67.6%) | ||
| Yes | 17 (29.8%) | 33 (46.5%) | 11 (32.4%) | 11 (32.4%) | ||
| PD-L1 TPS | 0.159 | 0.275 | ||||
| <50% | 21 (36.8%) | 37 (52.1%) | 17 (50.0%) | 11 (32.4%) | ||
| ≥50% | 14 (24.6%) | 10 (14.1%) | 5 (14.7%) | 9 (26.5%) | ||
| No data | 22 (38.6%) | 24 (33.8%) | 12 (35.3%) | 14 (41.2%) | ||
| EGFR mutations | 0.007 | 0.592 | ||||
| Wild type | 33 (57.9%) | 33 (46.5%) | 18 (52.9%) | 21 (61.8%) | ||
| Mutant | 11 (19.3%) | 31 (43.7%) | 9 (26.5%) | 9 (26.5%) | ||
| No data | 13 (22.8%) | 7 (9.9%) | 7 (20.6%) | 4 (11.8%) | ||
| Line of atezolizumab | 0.675 | 0.604 | ||||
| Second | 18 (31.6%) | 20 (28.2%) | 10 (29.4%) | 12 (35.3%) | ||
| ≥Third | 39 (68.4%) | 51 (71.8%) | 24 (70.6%) | 22 (64.7%) | ||
| Prior medications | ||||||
| EGFR-TKIs | 16 (28.1%) | 46 (64.8%) | <0.001 | 16 (47.1%) | 16 (47.1%) | 1.000 |
| ICIs | 4 (7.0%) | 9 (12.7%) | 0.383* | 4 (11.8%) | 1 (2.9%) | 0.356* |
| Platinum | 49 (86.0%) | 61 (85.9%) | 0.994 | 30 (88.2%) | 29 (85.3%) | 1.000* |
| Pemetrexed | 35 (61.4%) | 50 (70.4%) | 0.283 | 24 (70.6%) | 24 (70.6%) | 1.000 |
| osimertinib | 3 (5.3%) | 8 (6.3%) | 0.344* | 3 (8.8%) | 2 (5.9%) | 1.000* |
*Fisher's exact test; §Mann-Whitney U-test;
ECOG PS, Eastern Cooperative Oncology Group performance status; TPS, tumor proportion score; TKI, tyrosine kinase inhibitor; ICIs, immune checkpoint inhibitors; EGFR, epidermal growth factor receptor gene; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor.
In addition, multivariate analysis for PFS and OS of the PSM cohort also supported the results of the original cohort (Supplementary Table S4). Patients who had received osimertinib before atezolizumab-containing treatment had significantly shorter PFS (HR, 2.73; 95% CI, 1.05-7.07; p = 0.039) and OS (HR: 3.30; 95% CI, 1.16-9.41; p = 0.026) than those who did not receive prior osimertinib. Prior ICI treatment (HR: 3.17; 95% CI, 1.19-8.45; p = 0.021) and a poor performance status (HR: 3.26; 95% CI, 1.55-6.86; p = 0.002) were also associated with shorter OS.
Discussion
This multicenter observational study explored the clinical prognostic factors in patients with NSCLC who received atezolizumab-containing regimens as a 2L+ treatment in real-world settings in Taiwan. Patients who had received osimertinib treatment before atezolizumab experienced a shorter mPFS and mOS than those who had not received prior osimertinib. Moreover, poor performance status and prior ICIs exposure before atezolizumab treatment were associated with a poor prognosis. Given the reported impacts of prior osimertinib treatment in patients who received subsequent ICIs, this is an important issue for future studies.
Osimertinib has been approved by the FDA as a frontline treatment in patients with EGFR-mutant, metastatic NSCLC. In Taiwan, osimertinib has not been reimbursed until April 2020. However, it is unknown whether osimertinib impacts the effectiveness of subsequent treatments, especially ICIs. The current study showed that patients with prior osimertinib treatment had a shorter mPFS than those without osimertinib treatment. This finding was consistent with the post-hoc analysis results of a phase 2 trial, which showed that patients with acquired resistance to osimertinib had a shorter PFS with atezolizumab combination therapy than those without osimertinib exposure [22]. Furthermore, the current study showed that prior osimertinib treatment was a poor prognosis factor, likely because patients exposed to osimertinib represented a subgroup that had exhausted multiple lines of TKI therapy and/or potentially acquired resistance EGFR mutations, such as T790M. This observation may be hinted by the results of the phase 3 KEYNOTE-789 trial (NCT03515837), which evaluates the efficacy and safety of pemetrexed plus platinum chemotherapy (carboplatin or cisplatin), with or without pembrolizumab, in the treatment of adults with EGFR-TKI-resistant, EGFR-mutated, metastatic nonsquamous NSCLC tumors, including first-line osimertinib failure.
Recently, clinical trials of frontline immunotherapy demonstrated disappointing results for patients with NSCLC with EGFR or ALK alterations [23-25]. Several double-blind, randomized controlled clinical trials have shown poor second-line treatment outcomes for EGFR-mutant NSCLC treated with a single ICI agent [12, 26, 27]. In addition to treatment effectiveness, safety issues should be considered for concurrent or sequential immunotherapy and targeted therapy. Patients who received a combination of immunotherapy and EGFR-TKIs demonstrated more toxicities, including pyrexia, pneumonitis, and abnormal liver function [28, 29]. Furthermore, osimertinib plus durvalumab treatment was terminated early in the TATTON trial owing to increased reporting of interstitial lung disease [30]. Lisberg et al. reported treatment of seven patients with PD-L1-positive, EGFR-mutant, advanced NSCLC with pembrolizumab before EGFR-TKI therapy [25]. Apart from treatment futility, one patient developed fatal pneumonitis on erlotinib, and another patient died. Although these adverse effects may have resulted from an EGFR-TKI, their increased occurrence with pembrolizumab is concerning [31]. Sequential ICI and ALK-TKI (crizotinib) treatment was associated with a significantly increased risk of hepatotoxicity in patients with ALK, ROS1, or MNNG HOS transforming gene (MET) exon 14 alterations [32]. Thus, it is important to arrange the combination or sequence of targeted therapy and ICIs during the entire treatment course of NSCLC.
A randomized phase 3 study, the OAK trial (NCT02008227), revealed that atezolizumab improved OS over that with docetaxel in patients with locally advanced or metastatic NSCLC who had previously received one to two lines of chemotherapy, including at least one platinum-based treatment (HR: 0.75; 95% CI, 0.64-0.89; p = 0.0006) [33], and the updated response rate (RR), PFS, and OS were 14.6%, 2.8 months, and 13.8 months, respectively [33]. In addition, the global phase III/IV TAIL study showed an RR of 11.1%, mPFS of 2.7 months, and mOS of 11.1 months [34]. The current study in real-world settings showed a RR of 10.2%, mPFS of 3.5 months, and mOS of 10.7 months, which was similar to the results of the clinical trial [33]. However, the proportions of patients with EGFR mutation-positive status were 10% in the OAK trial and 4% in the TAIL trial [12, 34], which were significantly lower than the current study (32.8%). The OAK trial showed that EGFR mutation status did not impact on the efficacy of atezolizumab as 2nd-line treatment, and it is similar to the current studies. Furthermore, the above two clinical trials did not explore the impact of osimertinib exposure on the treatment effectiveness of atezolizumab. The current study showed that osimertinib exposure before atezolizumab was associated with shorter mPFS and mOS.
PD-L1 is currently widely validated and accepted as a biomarker of response to ICIs [35]. Although atezolizumab as first-line monotherapy showed clinical survival benefit in patients with NSCLC with high PD-L1 expression [8], the OAK trial revealed that patients with previously treated metastatic NSCLC have survival benefit from atezolizumab treatment regardless of PD-L1 expression, histology, or EGFR mutation status [12]. The current study also showed the same results that neither PD-L1 expression nor EGFR mutation status were associated with PFS or OS. The mechanism of the different response in NSCLC patients with high- or low-expression PD-L1 when treating with atezolizumab as first-line or second-line is unclear.
Whether immunotherapy has a potential role in patients with driver mutations remains debated. Most of the ICI clinical trials excluded patients with EGFR mutations or ALK fusions because the response rate to ICI monotherapy was low for NSCLC with EGFR mutations [12, 23]. Cohorts E and F in the phase 1/2 KEYNOTE-021 study (NCT02039674) also showed that pembrolizumab plus gefitinib or erlotinib was not a feasible treatment option because of grade 3/4 liver toxicity or no improvement in the response rate, respectively [36]. However, the phase 3 IMpower150 trial (NCT02366143) demonstrated that the four-drug combination of atezolizumab, bevacizumab, carboplatin, and paclitaxel, when used as the frontline treatment for patients with metastatic nonsquamous NSCLC, improved OS (HR: 0.78; 95% CI, 0.64-0.96; p = 0.02) and PFS (HR: 0.62; 95% CI, 0.52-0.74; p < 0.001) vs. bevacizumab plus carboplatin/paclitaxel [15]. Notably, a survival benefit was also observed in patients with EGFR mutations who had received prior treatment with EGFR-TKIs [37]. In 2020, Lam et al. reported the results of a phase 2 trial in which atezolizumab/bevacizumab/pemetrexed/carboplatin provided a response rate of over 60% and an mPFS of 9.4 months in patients with TKI-resistant, EGFR-mutant, metastatic lung cancer [22]. More clinical trials are necessary to clarify whether atezolizumab combination therapy may be effective in patients with acquired resistance to EGFR-TKIs.
Although the U.S. Food and Drug Administration (FDA) has approved ICIs as a standard of care for previously treated patients with NSCLC, it remains unclear whether the frontline medications affect the effectiveness of subsequent immunotherapy in these patients. Limited data are available regarding the prognosis of patients who receive immunotherapy in the frontline setting prior to atezolizumab treatment. Hernando-Calvo et al. reported that prior ICIs were not associated with the best treatment responses to subsequent ICIs, and only metastatic burden was a significant predictor of PFS by multivariate analysis [38]. Although our data also showed that there was no significant difference in the response rates to atezolizumab between patients with and without prior ICI treatment, the former group had shorter PFS and OS. The difference may be due to the fact that the previous study enrolled patients with various tumor types, including melanoma (45%), NSCLC (21%), and head and neck squamous cell carcinoma [38]. Further, different ICIs may have different treatment effects.
Variable laboratory biomarkers have been associated with treatment efficacy or prognosis of immunotherapy [39-43]. Mezquita et al. reported that pretreatment lung immune prognostic index (LIPI), combining derived neutrophils/(leukocytes minus neutrophils) ratio (dNLR) greater than 3 and LDH greater than upper limit of normal (ULN), was correlated with worse outcomes for ICI [42]. Zho et al. showed that circulating immune cell ratio and tumor markers could be as the potential predict factors of atezolizumab for lung cancer patients [40]. In addition, higher neutrophil to lymphocyte ratio (NLR), higher ALP, increasing white blood cell counts, and abnormally low albumin and low chloride levels associated with poor prognosis [44-47]. Although identification of biomarkers for patients who had favorable response to ICIs is urgent and important, most of these trials were retrospectively designed or had small case numbers. Further prospective randomized controlled trials and unified companion diagnostic devices are necessary to validate these candidate biomarkers. Although we did not enrolled the serum biomarkers as prognosis factors due to heterogenicity background of the enrolled patients, the results of the current study may serve as the basis for conducting future prospective studies using these factors to identify the patients who had directly benefit from atezolizumab treatment.
This study had some limitations. First, we retrospectively extracted patient-level data, and the nature of the retrospective study design may not completely exclude inherent biases. The atezolizumab-containing treatments were heterogeneous, and the results of this study may have limited generalizability to all patients. Second, ICIs and osimertinib has not been reimbursed by NIH of Taiwan until early 2020, and ICIs reimbursement criteria excluded patients with EGFR mutations. So, the number of osimertinib-treated patients before atezolizumab was less during the enrollment period of the study. Nonetheless, the enrollment of a large population from the six medical centers across the country and the nationwide data provided valuable information for future clinical decision making. Future randomized controlled designs will help strengthen the data of the hypothesis-generating research. Third, we did not collect the delicate subsequent therapies after disease progression to atezolizumab treatment, and it may have impact on OS.
In conclusion, prior osimertinib exposure was associated with poor clinical outcomes of subsequent atezolizumab-containing treatment of NSCLC. Additional studies with larger sample sizes are required to validate these findings.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
The authors thank all the patients and their families for their support.
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of participating medical center (NTUH IRB protocol code 202001071RSA).
Funding
This was supported by F. Hoffmann-La Roche, Ltd. The funder was not involved in the study design, data collection, analysis, and interpretation, or the decision to submit the manuscript for publication.
Author contributions
S.-G. W. and J.-Y. S. designed the study. S.-G. W., C.-L. C., C.-C. W., J.-Y. H., T.-C. H., C.-H. K. and J.-Y. S. collected patients and clinical data. S.-G. W. and J.-Y. S. performed literature search and drafting of the manuscript. J.-Y. S. and C.-H. K. supervised the study. All authors approved the final draft of the submitted manuscript.
Competing Interests
The authors declare that this study received funding from F. Hoffmann-La Roche, Ltd. The funder was not involved in the study design, patient collection, data analysis, interpretation of data, or the decision to submit it for publication. All authors met the authorship criteria as recommended by the International Committee of Medical Journal Editors (ICMJE) and retained full control of the manuscript content.
SG Wu has received speaking honoraria from Roche, AstraZeneca, and Pfizer. CL Chiang has received honoraria from AstraZeneca, Boehringer Ingelheim, and Roche. CH Kuo, CC Wang, and JY Hung declare no conflict of interest. TC Hsia has received research grants from Eli Lilly. JY Shih has received personal fees for advisory boards from AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Ono Pharmaceutical, Chugai Pharmaceutical, and Bristol-Myers Squibb; speaking honoraria from AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Ono Pharmaceutical, Chugai Pharmaceutical, and Bristol-Myers Squibb; and travel expense from Roche, Pfizer, Merck Sharp & Dohme, Chugai Pharmaceutical, and Bristol-Myers Squibb.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33
2. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A. et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw. 2021;19:254-66
3. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C. et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192-iv237
4. Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW. et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:171-210
5. Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013;31:1105-11
6. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II. et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998-2006
7. Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol. 2018;15:694-708
8. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH. et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med. 2020;383:1328-39
9. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ. et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819-30
10. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F. et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-92
11. Park K, Vansteenkiste J, Lee KH, Pentheroudakis G, Zhou C, Prabhash K. et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. 2020;31:191-201
12. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-65
13. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M. et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Nonsquamous NSCLC: Results From the Randomized Phase 3 IMpower132 Trial. J Thorac Oncol. 2021;16:653-64
14. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ. et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924-37
15. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N. et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288-301
16. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB. et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-60
17. Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF. et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2015;10:1675-84
18. Centers for Disease C, Prevention. Cigarette smoking among adults-United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:1157-61
19. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-47
20. Alonso-Garcia M, Sanchez-Gastaldo A, Munoz-Fuentes MA, Molina-Pinelo S, Boyero L, Benedetti JC. et al. Real-World Analysis of Nivolumab and Atezolizumab Efficacy in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer. Pharmaceuticals (Basel). 2022 15
21. Fujimoto D, Miura S, Yoshimura K, Wakuda K, Oya Y, Haratani K. et al. A Real-World Study on the Effectiveness and Safety of Pembrolizumab Plus Chemotherapy for Nonsquamous NSCLC. JTO Clin Res Rep. 2022;3:100265
22. Lam TC, Tsang K, Choi H, Lee VHF, Lam KO, Chiang CL. et al. 380MO A phase II trial of atezolizumab, bevacizumab, pemetrexed and carboplatin combination for metastatic EGFR-mutated NSCLC after TKI failure. Annals of Oncology. 2020;31:S1389
23. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z. et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res. 2016;22:4585-93
24. Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H. et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19:521-36
25. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T. et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naive Patients With Advanced NSCLC. J Thorac Oncol. 2018;13:1138-45
26. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-39
27. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-50
28. Ma BBY, Rudin CM, Cervantes A, Dowlati A, Costa D, Schmid P. et al. 441O Preliminary safety and clinical activity of erlotinib plus atezolizumab from a Phase Ib study in advanced NSCLC. Annals of Oncology. 2016;27:ix141
29. Gibbons DL, Chow LQ, Kim DW, Kim SW, Yeh T, Song X. et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. Journal of Thoracic Oncology. 2016;11:S79
30. Oxnard GR, Yang JCH, Yu H, Kim SW, Saka H, Horn L. et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in <em>EGFR</em>-mutant lung cancer. Annals of Oncology. 2020;31:507-16
31. McCoach CE. A Cautionary Analysis of Immunotherapy Prior to Targeted Therapy. J Thorac Oncol. 2019;14:8-10
32. Lin JJ, Chin E, Yeap BY, Ferris LA, Kamesan V, Lennes IT. et al. Increased Hepatotoxicity Associated with Sequential Immune Checkpoint Inhibitor and Crizotinib Therapy in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol. 2019;14:135-40
33. Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S. et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2018;13:1156-70
34. Ardizzoni A, Azevedo S, Rubio-Viqueira B, Rodriguez-Abreu D, Alatorre-Alexander J, Smit HJM. et al. Primary results from TAIL: a global single-arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer. J Immunother Cancer. 2021 9
35. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345-62
36. Yang JC-H, Gadgeel SM, Sequist LV, Wu C-L, Papadimitrakopoulou VA, Su W-C. et al. Pembrolizumab in Combination With Erlotinib or Gefitinib as First-Line Therapy for Advanced NSCLC With Sensitizing <em>EGFR</em> Mutation. Journal of Thoracic Oncology. 2019;14:553-9
37. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F. et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387-401
38. Hernando-Calvo A, Valdivia A, Santa Gadea OS, Braña I, Berché R, Matos I. et al. 573P Efficacy of immunotherapy (IT) after prior immune checkpoint inhibitors (ICIs) exposure. Annals of Oncology. 2020;31:S489-S90
39. Katayama Y, Yamada T, Chihara Y, Tanaka S, Tanimura K, Okura N. et al. Significance of inflammatory indexes in atezolizumab monotherapy outcomes in previously treated non-small-cell lung cancer patients. Sci Rep. 2020;10:17495
40. Zhuo M, Chen H, Zhang T, Yang X, Zhong J, Wang Y. et al. The potential predictive value of circulating immune cell ratio and tumor marker in atezolizumab treated advanced non-small cell lung cancer patients. Cancer Biomark. 2018;22:467-76
41. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC. et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27:732-8
42. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D. et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4:351-7
43. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL. et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1-7
44. Ulas A, Turkoz FP, Silay K, Tokluoglu S, Avci N, Oksuzoglu B. et al. A laboratory prognostic index model for patients with advanced non-small cell lung cancer. PLoS One. 2014;9:e114471
45. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69
46. Zhang K, Lai Y, Axelrod R, Campling B, Hyslop T, Civan J. et al. Modeling the overall survival of patients with advanced-stage non-small cell lung cancer using data of routine laboratory tests. Int J Cancer. 2015;136:382-91
47. Huang P, Lan M, Peng AF, Yu QF, Chen WZ, Liu ZL. et al. Serum calcium, alkaline phosphotase and hemoglobin as risk factors for bone metastases in bladder cancer. PLoS One. 2017;12:e0183835
Author contact
![]() Corresponding authors: Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University, Taipei, Taiwan. E-mail: jyshihedu.tw (J.Y. Shih); Division of Lung Cancer and Interventional Bronchoscopy, Department of Thoracic Medicine, College of Medicine, Chang Gung Memorial Hospital, Chang Gung University, Taipei, Taiwan. E-mail: chihhsikuocom (C.H. Kuo).
Corresponding authors: Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University, Taipei, Taiwan. E-mail: jyshihedu.tw (J.Y. Shih); Division of Lung Cancer and Interventional Bronchoscopy, Department of Thoracic Medicine, College of Medicine, Chang Gung Memorial Hospital, Chang Gung University, Taipei, Taiwan. E-mail: chihhsikuocom (C.H. Kuo).

 Global reach, higher impact
Global reach, higher impact