3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(9):2945-2953. doi:10.7150/jca.75622 This issue Cite
Review
A 2022 Update on Extensive Stage Small-Cell Lung Cancer (SCLC)
1. EpicentRx Inc., Department of Clinical Research, 11099 North Torrey Pines Road, Suite 160, La Jolla, CA 92037, USA
2. SciClone Pharmaceuticals Co., Ltd. Department of Clinical Research, 22 Floor, Shanghai Central Plaza, No. 381 Middle Huaihai Road, Huangpu, Shanghai 200020, China
3. Clinical Trials Innovations, Mountain View, CA, 94043, USA
Abstract
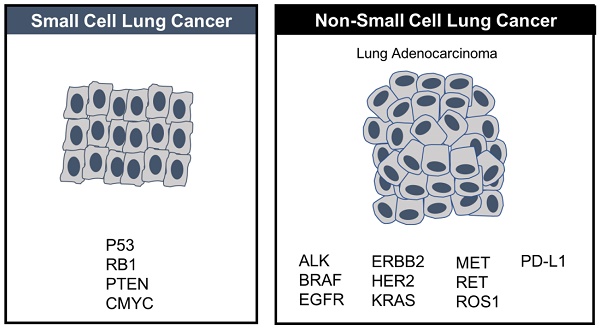
For close to 40 years small-cell lung cancer (SCLC) was adrift, as listless, and as idle as a painted ship on a painted ocean, with nary a breeze to blow in the direction of clinical progress or change. The preferred decades-old first line regimen was etoposide-platinum, to which ≥50% of patients respond, followed by decades-old, tired topotecan in second line for platinum sensitive patients, full stop, because there were no approved therapeutic options (nor generally any compelling experimental ones) in third line or beyond. In 2012 SCLC was designated by the U.S. Congress as a “recalcitrant” tumor type and for good reason: because most patients relapse, after the generally favorable response in first line, respond poorly, if at all to subsequent therapies, and rarely survive beyond 1 year.
A significant sea change occurred in 2018 with the approval of nivolumab followed by pembrolizumab and atezolizumab in 2019, durvalumab in 2020, accelerated approval for lurbinectedin in 2020 and trilaciclib in 2021 for myelosuppression. In 2021, the US indications for nivolumab and pembrolizumab were withdrawn. Suddenly, a tumor type, whose name was virtually synonymous with stalled progress and movement, and which was much less well studied and funded than its more prevalent cousin, non-small cell lung cancer (NSCLC), finds itself in the eye of the storm, that is, at the epicenter of an intense flurry and ferment of activity, not all of it positive. This review surveys approved drugs and select up-and-coming ones in development for extensive stage SCLC.
Keywords: SCLC, platinum chemotherapy, immunotherapy, checkpoint inhibitor therapy

 Global reach, higher impact
Global reach, higher impact