3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(10):3031-3043. doi:10.7150/jca.72793 This issue Cite
Research Paper
Glycolytic Metabolic Remodeling by the Truncate of Glioma-Associated Oncogene Homolog 1 in Triple-Negative Breast Cancer Cells
1. School of Pharmacy, Sungkyunkwan University, 2066, Seobu-ro, Jangan-gu, Suwon, 16419, Republic of Korea.
2. College of Pharmacy, Chonnam National University, Yongbong-ro, Buk-gu, Gwangju, 61186, Republic of Korea.
Abstract
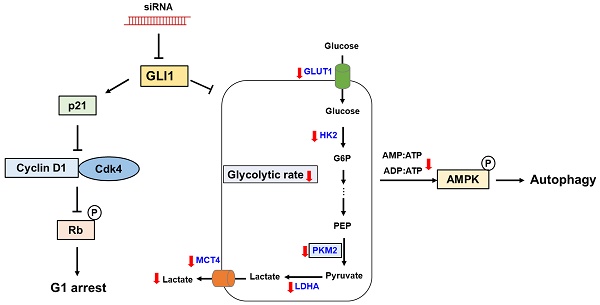
Hedgehog (Hh) signaling pathway plays an essential role in embryonic development, tissue regeneration, and stem cell renewal. In particular, terminal effectors of the Hh signaling pathway are associated with the regulation of glioma-associated oncogene homolog 1 (GLI1) transcription factors. Overexpression of GLI1 is closely associated with poor prognosis in breast cancer. The Hh-GLI1 signaling pathway is activated and participates in the tumorigenesis and progression of breast cancer, especially in the aggressive subtype of triple-negative breast cancer (TNBC). However, the role of GLI1 in regulating TNBC metabolism remains unclear. This study aimed to explore the functional role of GLI1 in glycolytic metabolism in TNBC. Immunohistochemical analysis of GLI1 expression in a tissue microarray revealed significant correlations between GLI1 expression and advanced tumor stage and grade. GLI1 expression levels were drastically increased in MDA-MB-231 cells compared to those in other cell lines. Inhibition of GLI1 expression using GLI1 small interfering RNA (siRNA) in MDA-MB-231 cells resulted in a significant reduction in cell proliferation and induced cell cycle arrest at the G1 phase. Furthermore, GLI1 downregulation significantly reduced the expression of glycolysis-regulated proteins. GLI1 knockdown resulted in reduced glycolytic rates and extracellular lactate levels. Moreover, metabolic stress after GLI1 knockdown activated the energy sensor, adenosine monophosphate-activated protein kinase, which subsequently resulted in autophagy induction. In conclusion, this study indicates that targeting GLI1 reprograms the tumor glucose metabolism to suppress breast cancer cell growth and proliferation.
Keywords: autophagy, glioma-associated oncogene, glycolytic rates, hedgehog (Hh) signaling, TNBC

 Global reach, higher impact
Global reach, higher impact