Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(10):3044-3050. doi:10.7150/jca.76052 This issue Cite
Hypothesis
Hypothesis: Mutations and Immunosurveillance in Obesity-Associated Colorectal Cancer
Department of Medical Education, Geisinger Commonwealth School of Medicine, 525 Pine Street, Scranton, PA 18509, USA.
Abstract
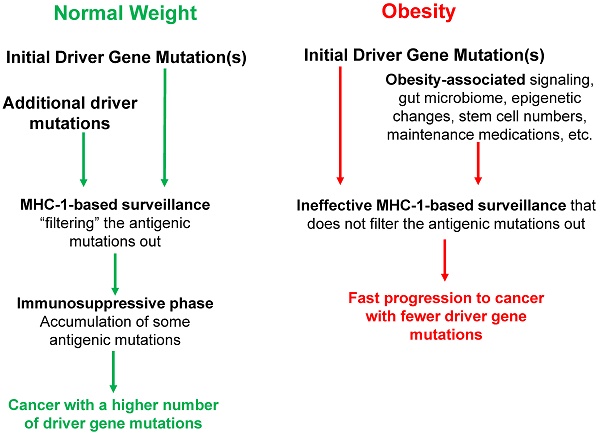
Tumorigenesis typically requires the accumulation of several driver gene mutations; therefore, there is a mutation threshold for the completion of the neoplastic process. Obesity increases the risk of cancer, and we have proposed that one mechanism whereby obesity raises the risk of microsatellite stable (MSS) colon cancer is by decreasing the mutation threshold. Therefore, obese MSS colon cancer patients should exhibit fewer driver gene mutations compared to normal body-mass index (BMI) patients. Our hypothesis is supported by results from analyses of The Cancer Genome Atlas (TCGA) data, which revealed that cancer genomes of obese MSS colon patients exhibit both fewer somatic mutations and fewer driver gene mutations. These findings could be explained by the high levels of obesity-associated cytokines and factors, the signaling pathways of which substitute for the additional driver gene mutations detected in normal-weight MSS colon cancer patients. Therefore, obesity-induced aberrant cell signaling might cooperate with initiating driver gene mutations to promote neoplastic development. Consistent with this possibility, we observed a lower number of KRAS mutations in high-BMI MSS colon cancer patients. This paper extends our hypothesis to address the interactions between obesity, immune surveillance in neoplastic development, and colorectal cancer (CRC) risk. A better understanding of these interactions will inform future preventive and therapeutic approaches against MSS CRC. We propose that the individual variations in the major histocompatibility class 1 (MHC-1) genotype interact with obesity to shape the tumor mutational landscape. Thus, the efficiency of the immune surveillance mechanisms to select against specific mutations may depend on both the MHC-1 genotype variant and the BMI of an individual. A high BMI is expected to reduce the number of driver gene mutations required to evade the MHC-1 surveillance mechanism and support an accelerated cancer progression.
Keywords: Colon cancer, obesity, mutations, BMI, cell signaling

 Global reach, higher impact
Global reach, higher impact