3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(12):3368-3377. doi:10.7150/jca.71592 This issue Cite
Research Paper
Hexokinase 2 Is a Pivot for Lovastatin-induced Glycolysis-to-Autophagy Reprogramming in Triple-Negative Breast Cancer Cells
1. Key Laboratory of Translational Cancer Stem Cell Research, Department of Pathophysiology, Hunan Normal University School of Medicine, Changsha, Hunan 410013, China.
2. Department of Biochemistry and Molecular Biology, Jishou University, Jishou, Hunan, China.
3. Department of Nursing, Hunan Normal University School of Medicine, Changsha, Hunan 410013, China.
* These authors contributed equally to this work.
Abstract
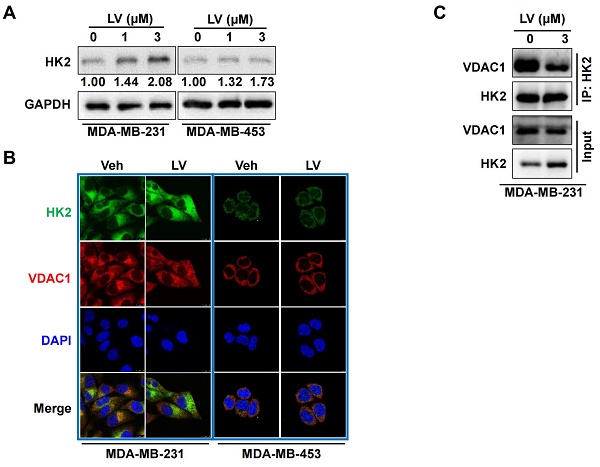
Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer with limited therapeutic options available. We have recently demonstrated that lovastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, suppresses TNBC cell proliferation and stemness properties in vitro and in vivo. However, the mechanisms through which lovastatin inhibits TNBC cells are not fully understood. Here, we used 1H NMR-based metabolomic profiling to investigate lovastatin-induced metabolic changes in TNBC cell line MDA-MB-231. Among the 46 metabolites identified, lactate demonstrated the highest variable importance in projection (VIP) score. Glycolysis stress test revealed that lovastatin significantly decreased the extracellular acidification rate (ECAR) in MDA-MB-231 cells. Furthermore, lovastatin treatment down-regulated the levels of glycolysis-related proteins including GLUT1, PFK1, and PKM2 in MDA-MB-231 but not non-TNBC MDA-MB-453 cells. In addition, lovastatin induced autophagy as evidenced by increased LC3 puncta formation and LC3-II/I ratio, increased AMPK phosphorylation, and decreased Akt phosphorylation. We also revealed the interaction between the glycolytic enzyme hexokinase 2 (HK2) and the mitochondrial membrane protein voltage-dependent anion channel 1 (VDAC1), an important regulator of autophagy. Further bioinformatics analysis revealed that VDAC1 was expressed at a higher level in breast cancer than normal tissues and higher level of VDAC1 predicted poorer survival outcomes in breast cancer patients. The present study suggests that lovastatin might exert anti-tumor activity by reprogramming glycolysis toward autophagy in TNBC cells through HK2-VDAC1 interaction.
Keywords: Lovastatin, Triple-negative breast cancer, Metabolomics, Glycolysis, Autophagy

 Global reach, higher impact
Global reach, higher impact