3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(13):3396-3403. doi:10.7150/jca.74107 This issue Cite
Research Paper
Real-world experience of safety and effectiveness of regorafenib for treatment of metastatic colorectal cancer, advanced gastrointestinal stromal tumors, and hepatocellular carcinoma: a post-marketing surveillance study in Korea
1. Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Republic of Korea.
2. Department of Surgery, Busan Paik Hospital, Inje University College of Medicine, Busan, Republic of Korea.
3. Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang-si, Gyeonggi-do, Republic of Korea.
4. Bayer Korea Ltd, Seoul, Republic of Korea.
5. Division of Hemato-Oncology, Department of Internal Medicine, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea.
6. Division of Hematology/Oncology, Department of Internal Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
7. Division of Medical Oncology, Department of Internal Medicine, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
8. Division of Medical Oncology, Department of Internal Medicine, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
9. Department of Surgery, Inje University Sanggye Paik Hospital, Seoul, Korea.
10. Department of Medical Oncology, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea.
11. Division of Hematology-oncology, Department of Internal Medicine, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea.
Abstract
Purpose: This regulatory post-marketing surveillance (PMS) study was performed to evaluate the safety and effectiveness of regorafenib on Korean patients with colorectal cancer (CRC), gastrointestinal stromal tumors (GIST), and hepatocellular carcinoma (HCC) in a real-world clinical setting.
Methods: This PMS was conducted as a multi-center, prospective, observational study at 34 centers in Korea from August 2013 to August 2019. The primary objective was to evaluate the safety of regorafenib in real-world practice, with the secondary objective to investigate its effectiveness, including its overall response rate (ORR), progression-free survival (PFS), and overall survival (OS).
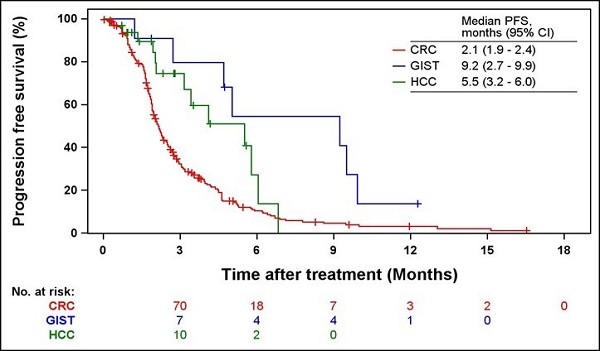
Results: In total, 301 patients were included in the analysis (254 patients with CRC, 14 patients with GIST, and 33 patients with HCC). The incidence rates of adverse events (AEs) were 85.0%, 78.6%, and 81.8% in patients with CRC, GIST, and HCC, respectively. The most frequent AE related to regorafenib in the three cancer types was palmar-plantar erythrodysesthesia syndrome (PPES). The ORRs of patients with CRC, GIST, and HCC were 4.7%, 0%, and 41.4%, respectively. The median PFS and OS were 2.1 and 6.1 months for CRC, respectively; 9.2 and 16.4 months for GIST, respectively; and 5.5 months and not estimated (NE) for HCC, respectively. Patients who experienced a dose modification or discontinuation of regorafenib showed significantly shorter median PFS and OS (2.2 vs. 2.6 months, respectively, P = 0.0335 for PFS; 5.3 vs. 8.5 months, respectively, P = 0.0010 for OS).
Conclusion: This PMS study, which is the largest surveillance study of CRC in Korea, found no newly identified safety concerns for patients who received regorafenib in the real-world setting. Additionally, the results of this study were consisted with those previously reported in phase III trials.
Keywords: Regorafenib, Colorectal cancer, Gastrointestinal stromal tumors, Hepatocellular carcinoma, Real-world data, Post-marketing surveillance.

 Global reach, higher impact
Global reach, higher impact