Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(13):3434-3443. doi:10.7150/jca.77619 This issue Cite
Review
Clinical implications of the interaction between PD-1/PD-L1 and PI3K/AKT/mTOR pathway in progression and treatment of non-small cell lung cancer
Department of Pathology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, China.
Received 2022-7-31; Accepted 2022-9-17; Published 2022-10-3
Abstract
The discovery of immune checkpoints has been well known to provide novel clues for cancer treatments. Immunotherapy against the programmed cell death protein-1 (PD-1) /programmed death-ligand-1 (PD-L1), one of the most popular auxiliary treatments in recent years, has been applied in various tumor treatments, including non-small cell lung cancer (NSCLC). However, inevitable issues such as side effects and drug resistance emerge following the use of immune checkpoint inhibitors. The PI3K/AKT/mTOR pathway may participate in the regulation of PD-L1 expression. Abnormal PI3K/AKT/mTOR pathway activation results in increased PD-L1 protein translation, whereas PD-L1 overexpression can activate the PI3K/AKT/mTOR pathway inversely. Via downstream proteins, including 4E-BP1, STAT3, NF-κB, c-MYC, and AMPK in aberrant energy status, the PI3K/AKT/mTOR pathway can regulate PD-L1 post-transcription and translation. Besides, the regulation of the PI3K pathway by the PD-1/PD-L1 axis involves both tumor cells and the tumor immune microenvironment. Inhibitors targeting the PD-1/PD-L1 have been successfully applied in the treatment of gastrointestinal cancer and breast cancer. Meanwhile, drug resistance from alternative pathway activation also evidently affects clinical progress. To achieve a better therapeutic effect and quality of survival, the combination of multiple treatment modalities presents great research value. Here we reviewed the interaction between PD-1/PD-L1 and PI3K/AKT/mTOR pathway in the progression and treatment of NSCLC and summarized its clinical implications. The intracellular interactions between PD-1/PD-L1 and the PI3K/AKT/mTOR pathway indicate that PD-1/PD-L1 inhibitors have a wide range of potential applications. And we presented the mechanism for combining therapy with monoclonal antibody PD-1/PD-L1 and PI3K/AKT/mTOR inhibitors in this review, to broaden the therapies for NSCLC.
Keywords: PD-1/PD-L1, PI3K/AKT/mTOR pathway, Inhibitors, immunotherapy
Introduction
Lung cancer, primary non-small cell lung cancer (NSCLC), has ranked second in morbidity and first in mortality among cancer diseases worldwide, constructing a serious threat to human life and health [1]. With further study of the mechanism of tumorigenesis and the development of molecular detection, programmed cell death protein-1 (PD-1) /programmed death-ligand-1 (PD-L1) monoclonal antibodies have become the first-line therapy for advanced NSCLC patients [2]. Furthermore, the investigation of immunotherapy has become popular in cancer therapeutic research. Although some patients with PD-L1+ NSCLC have benefited from the application of PD-1/PD-L1 monoclonal antibodies markedly [3, 4], the side effects and drug resistance were still of great concern to patients [4]. Therefore, for patients with PD-L1+ in the advanced stage of NSCLC, combination therapy has become a considerable option, such as the combination of PD-1 monoclonal antibody pembrolizumab with platinum agents' chemotherapy [5]. The clinical trials of the third-generation EGFR-TKI osimertinib combined with PD-L1 monoclonal antibody durvalumab have achieved good experimental results [6]. The PI3K/AKT/mTOR pathway is an essential intracellular signaling pathway that regulates the processes of cancer diseases including cell metabolism, cell proliferation, apoptosis, and gene expression [7]. The PI3K/AKT/mTOR pathway is also reported to participate in the immunosurveillance of the tumor microenvironment [8]. Inhibitors of the PI3K/AKT/mTOR pathway have been under development and in clinical trials [9]. In this review, we will discuss the clinical implications of the correlation between PD-1/PD-L1 and PI3K/AKT/mTOR pathway activation in the progression and treatment of NSCLC, and explore the current situation of relevant targeted drugs and immunotherapy for NSCLC.
PD-L1 expression and PD-1 activation on tumor immunosuppression
NSCLC tumor cells usually express PD-L1 on the membrane and its receptor PD-1 is expressed on the membrane of CD8+ T cells [10]. The coexpression of PD-L1 and PD-1 inactivates CD8+ T cells [11], thereby suppressing anti-tumor immune activity [12]. Researchers find that the activation of PD-1 by PD-L1 inhibits transduction via inactivating the co-receptor CD28 [13]. In addition, CD4+Foxp3+ regulatory T cells (Tregs) belong to the immunosuppressive subpopulation of CD4+ T cells [14], while PD-1 expressed on their surface maintains the immunosuppressive function and enhances immune tolerance. CD4+ T cells are induced to differentiate towards Tregs by the activation of PD-1. And the high expression of Foxp3 mainly through inhibition of mTOR (mammalian target of rapamycin) increases the immunosuppressive effect as well [15]. It has been proved that several mechanisms encoded by CD274 can regulate the expression of PD-L1 [16], intracellular factors, and tumor microenvironment [17]. For example, PTEN influences PD-L1 expression by regulating the mRNA levels, while NF-κB induces PD-L1 gene transcription by directly binding to its promoter and other indirect ways [18]. Plenty of factors and pathways have been found to play a critical role in immunotherapy; more details are still under experiment exploration.
Anti-PD-1/PD-L1 immunotherapy for NSCLC patients with advanced stage
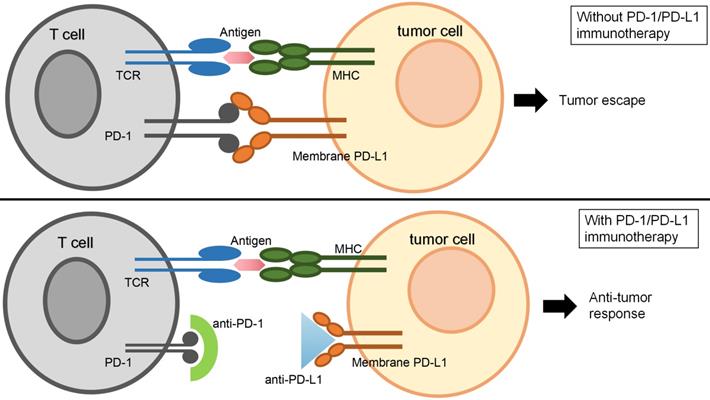
The anti-tumor immune function of T cells can be regained via anti-PD-1/PD-L1 drugs (Figure 1). For NSCLC patients with positive PD-L1 expression, immune checkpoint inhibitor therapy improves the patient's overall survival (OS) rate compared to traditional chemotherapy [19]. Immune checkpoint inhibitors now in clinical use or trials mainly contain the PD-1 monoclonal antibody, such as nivolumab and pembrolizumab [20], and the PD-L1 monoclonal antibody, including atezolizumab and durvalumab [21, 22]. Clinical trial studies have found that the median remission period and median OS significantly increase by using all those four drugs, and compared to conventional chemotherapy, the grade 3/4 adverse events (AEs) are significantly shortened to varying degrees [3, 4].
The schematic of PD-1/PD-L1 immunotherapy. T lymphocyte (T cell) plays an important role in killing cancer cells when surface T cell receptors (TCR) recognize and bind to the major histocompatibility complex (MHC) molecules. Programmed death ligand 1 (PD-L1, CD274) is a kind of immune checkpoint protein, which is highly expressed in part of tumor cells and promotes tumor cell escape from being killed by T-cell. Programmed death 1 (PD-1) expressed on T-lymphocytes can bind to PD-L1 and inhibits T cell proliferation and activity. When anti-PD-L1 drugs antagonized PD-L1 or anti-PD-1 drugs antagonized PD-1, the anti-tumor immune function of T cells recovered.
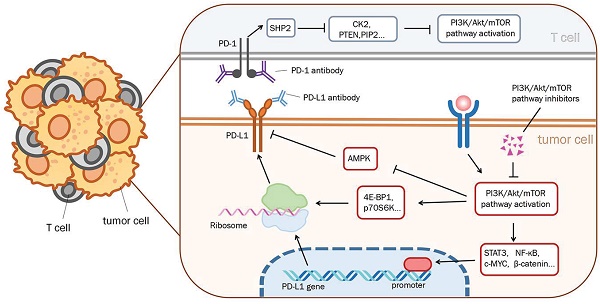
However, the application of a single PD-1 or PD-L1 monoclonal antibody remains flawed. First of all, the indications for only using one type of monoclonal antibody are limited [2, 23]. Nivolumab has been recommended in the NCCS guidelines as a follow-up treatment for metastatic non-squamous NSCLC after first-line chemotherapy or the tumor made progression after chemotherapy [2]. FDA has approved pembrolizumab as first-line therapy for patients with metastatic NSCLC and with PD-L1 expression levels ≥50% (with EGFR mutations, negative or unknown ALK rearrangement test results also available) [24]. Meanwhile, no contraindications (e.g., severe autoimmune disease or organ transplantation) are also required in these patients [25]. In one follow-up treatment for NSCLC [26], different from pembrolizumab, the use of atezolizumab required disease-related information about the patient, rather than the PD-L1 expression level detection. Phase III clinical trial of durvalumab has reported that those NSCLC patients who are unable to apply tumor resection surgically and with PD-L1 ≥25% using durvalumab after radiotherapy obtain significantly better prognosis than those receiving radiotherapy alone [27]. Secondly, tumor cells present resistance to immune checkpoint inhibitors [28] with natural and acquired resistance to PD-1 and PD-L1 inhibitors in various tumors such as melanoma, NSCLC, kidney cancer, and so on [29]. In addition, a certain degree of drug toxicity and side effects exist in any kind of PD-1 or PD-L1 monoclonal antibodies [30]. Therefore, as a popular research topic in recent years [31], the combination therapy of medicine shows great research value and clinical significance for broadening the scope of indications for immune checkpoint inhibitors, alleviating drug resistance, and mitigating the side effects of anti-cancer drugs including targeted drugs [32]. At this stage, the combination of immune checkpoint inhibition therapy with targeted drugs such as EGFR-TKIs [33] or platinum-agent chemotherapy is a popular way of combining therapies for intermediate to advanced NSCLC [34].
Roles of PI3K/AKT/mTOR pathway in tumor development
PI3K/AKT/mTOR pathway upstream gene PIK3CA amplification and PI3K, AKT mutations have been found in NSCLC tissues. The expression of all of these genes increased, while PTEN gene expression is absent, compared to paracancerous tissues [35]. Activation of the PI3K/AKT/mTOR pathway, which is related to multiple upstream and downstream elements, is associated with oncogenesis.
PI3K is an intracellular signal transduction protein with phosphatidylinositol 3- kinase activity, thus Class I PI3Ks are closely associated with cancer, and the PIK3CA gene is involved in encoding the subunits associated with this protein [36]. Overexpression of the PIK3CA gene can directly hyperactivate the PI3K/AKT/mTOR pathway [37]. The activation of PI3K is associated with overexpressed EGFR caused by EGFR gene mutations. The ERBB3 protein from the EGFR receptor tyrosine kinase family also drives PI3K activation. In some cases, EGFR family-related proteins in EGFR-mutant NSCLC can activate PI3K through roles of GAB adaptor proteins, independent of ERBB3 protein [38]. PI3K phosphorylates PIP2 and generates PIP3 then further activates AKT (protein kinase B) [39]. The activation of AKT phosphorylates corresponding enzymes and kinases and regulates a variety of downstream signaling pathways [40], which will indirectly promote the expression of the mammalian target of rapamycin protein (mTOR) [41]. On the contrary, PTEN promotes the conversion of PIP3 to PIP2 [42]. Moreover, the silence of the PTEN gene blocks the conversion of PIP3 to PIP2 [43] and enhances AKT activation [44]. MTOR is an element in two different multiprotein signaling complexes, mTORC1, and mTORC2, both involved in mediating apoptosis and proliferation in different ways [44]. Because of the complexity of the PI3K/AKT/mTOR pathway in regulating cell proliferation and apoptosis-related responses, inhibition of each of the responses in this pathway tends to activate the paracrine pathway, leading to the development of drug resistance [45].
Interaction between PI3K/AKT/mTOR pathway and PD-1/PD-L1
The PI3K/AKT/mTOR pathway can control PD-L1 expression. In lung squamous carcinoma or lung adenocarcinoma tissues with mutations in NRAS, KRAS, EGFR, BARF, PIK3CA, EML4-ALK, activation of PI3K/AKT/mTOR pathway and PD-L1 expression can be detected simultaneously [46]. PI3K/AKT/mTOR-related inhibitors (e.g. mTOR inhibitor rapamycin) decreased PD-L1 expression [47], while stimulation of enhanced AKT/mTOR expression increases PD-L1 expression (validated in mouse experiments) [48]. Multiple responses are linked between PD-L1 and PI3K/AKT/mTOR (Figure 2). For example, in lung squamous carcinoma, deletion of the PTEN gene is found to lead to higher PD-L1 protein translation, while PTEN gene expression deficiency simultaneously promoted AKT activation [49]. As downstream elements of AKT, β-catenin/TCF/LEF transcription complex stimulates CD274 gene transcription by binding to the PD-L1 promoter [50]. Meanwhile, the AKT downstream signaling protein NF-κB also acts on its promoter to induce PD-L1 mRNA expression [18].
PI3K/AKT/mTOR pathway regulating PD-L1 expression. PD-L1 expression is regulated by the PI3K/AKT/mTOR pathway, which controls numerous cell processes including PD-L1 translation and post-transcription. Arrows mean activation, and bars mean inhibition. While dotted lines represent PD-L1 expression and solid lines represent PI3K pathway functions. Phosphatidylinositol 3-kinases (PI3K) is activated by various factors including growth factor receptor tyrosine kinases. PI3K promotes phosphatidylinositol 4, 5-bisphosphate (PIP2) to generate phosphatidylinositol 3, 4, 5-trisphosphate (PIP3), and then activates Protein Kinase B (AKT). AKT mediated PD-L1 regulation is divided into two parts, activating β-catenin and activating the mechanistic target of rapamycin complex 1(mTORC1). β-catenin enhances PD-L1 expression by activating T-cell factor/lymphoid enhancing factor (TCF/LEF) and combining with the PD-L1 promoter. mTORC1 regulates PD-L1 promoter through signal transducer and activator of transcription 3(STAT3), mammalian target of rapamycin (NF-κB), and Myc, activated by beta-transducin repeat-containing protein (β-TrCP) and ribosomal protein S6 kinase beta-1(S6K1) mediated by MAX interactor 1(Mxi1) degradation. Also, mTOC1 induces ribosome biogenesis by promoting p70 protein S6 kinase beta-1(p70S6K1) and inhibiting eukaryotic translation initiation factor 4E-binding protein 1(4E-BP1). Both ways upregulate eukaryotic translation initiation factor 4E (elF4E), which promotes PD-L1 mRNA translation.
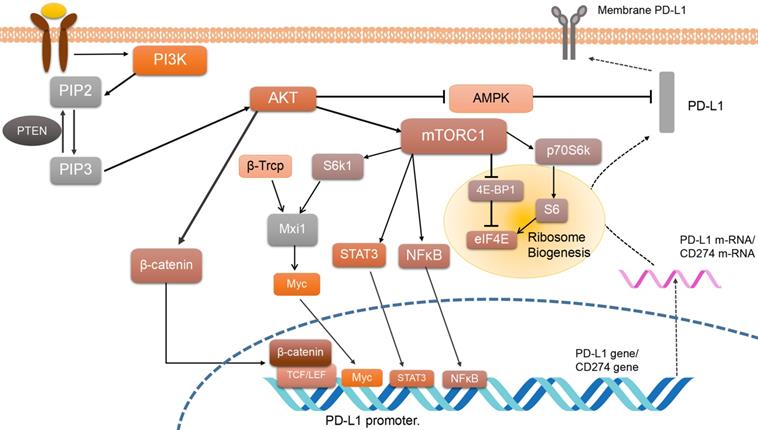
On the other hand, molecules like transcription factors including c-Myc [51] and transcription activating factor STAT3 may play a role in the post-transcription of PD-L1 [52]. STAT3 phosphorylation is relevant to mTOR, but it remains unclear whether mTOR suppressed or promoted STAT3 activity. Some researchers reveal that the phosphorylation of STAT3 by mTOR leads to its maximal activation [53]. As for c-Myc, it has been reported that Mxi1/S6k/β-Trcp can activate c-Myc by promoting Mxi1 degradation and then work on downstream factors such as the CD274 gene [54]. One of the most important mechanisms of mTORC1 is regulating its downstream molecular S6 kinase. High expression of p70 S6 kinase also plays an important role in controlling the expression of PD-L1. The overexpression of mTORC1 can negatively regulate PD-L1 expression, while it suppresses β-TrCP-mediated proteasomal degradation of PD-L1 [55]. Besides, the activation of p70S6K can also promote the translation efficiency of PD-L1 mRNA in the ribosome via activating 4E-BP1. So at least four ways have been mentioned above to regulate PD-L1 expression. One of them is involved in regulating ribosome biogenesis and translation efficiency [56], while another one is referred to as the Mxi1/S6k/β-Trcp pathway [53]. It is supposed that the mTOR pathway may regulate PD-L1 expression through many of the ways mentioned above.
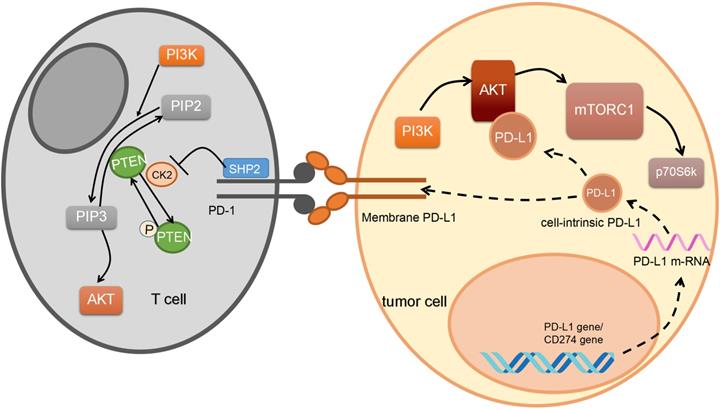
In turn, PD-1/PD-L1 can also regulate the PI3K/AKT/mTOR pathway (Figure 3). PD-L1 activates the PI3K/AKT pathway by stabilizing β-catenin [57], and overexpression of PD-L1 also increases the expression of p-AKT. For example, in gastric cancer, researchers confirm that the PD-1/PD-L1 axis can upregulate AKT phosphorylation [58]. According to another study, in glioma, cell-intrinsic PD-L1 binds to AKT preferentially when compared to other PI3K/AKT signal proteins. Suppressing cell-intrinsic PD-L1 then decreases phosphorylation of mTORC1 and p70S6K in melanoma [59] and ovarian cancer cells [60].
By the way, AMP-activated protein kinase (AMPK) has been reported to be involved in regulating the interaction between PI3K/AKT/mTOR pathway [61] and PD-1/PD-L1 because of aberrant energy status in cancer. Energy deprivation can affect anti-tumor immunity, induce AMPK to phosphorylate PD-L1, and decrease PD-L1 protein abundance [62]. On one hand, activated AMPK phosphorylates PD-L1 on its Ser283 site to block the combination of PD-L1 with CMTM4, a positive regulator of PD-L1, to induce its degradation [62]. On the other hand, researchers demonstrate that the activated AMPK phosphorylated S195 site of PD-L1 results in its abnormal glycosylation and degeneration [63]. Also, a previous study verifies that activated AKT can phosphorylate AMPK directly on its Ser485 site or indirectly on its Thr172 site [64], and down-regulate its expression. In conclusion, activating PI3K/AKT pathway can suppress the AMPK function and then induces a higher PD-L1 expression.
PD-1/PD-L1 activates the PI3K/AKT pathway not just in tumor cells but also in the immune microenvironment. It has been found in breast cancer that PD-1 on an element termed myeloid-driven suppressor cells (MDSC) immune microenvironment bound to PD-L1 on B cell can activate PI3K/AKT/NF-κB signaling pathway in B cell [65]. Then B cell stimulation hindered T cell immune response and promoted tumor cells' immune escape. On the contrary, in T cells, PD-1 collected downstream molecular SHP-2 which suppresses PI3K activation through targeting PTEN phosphorylation mediated by CK2 [66]. The phosphorylated PTEN is in a stable situation, resulting in lower PI3K/AKT expression. Although there is no direct evidence that high expression of PD-L1 is simply mTOR-dependent, it is well known that AKT/mTOR pathway activation can promote the immune escape of cancer cells by promoting high PD-L1 expression.
Value-added regulation of tumor cells by PI3K/AKT/mTOR pathway inhibitors
Several PI3K/AKT/mTOR pathway targeted therapeutic drugs mainly target related genes such as PIK3CA, AKT, TSC1/2, mTOR, PTEN, and so on [67]. Since the development of pathway targeted drugs lags behind the tumorigenesis mechanism research, currently many of them are still in clinical trials (Table 1). At this stage, drugs targeting PI3K are inhibitors of various PI3K isoforms. For example, alpelisib, a drug targeting PIK3CA mutant breast cancer in phase Ⅱ clinical trials [68] displays antitumor activity in pre-initiation studies. Copanlisib and duvelisib are FDA-approved for marketing for specific types of lymphoma or leukemia in the United States. ACP-319 (acertapharma), BYL719 (novartis), and serabelisib are also in clinical trials, but cytotoxic response occurs evidently with each of those drugs used alone [69]. Another discovery indicates that the antipsychotic agent flupentixol can inhibit lung cancer development via inducing apoptosis of oncocytes [70].
AKT inhibitors can be divided into two types: AKT competitive inhibitors and AKT aliasing inhibitors. The former competitively inhibits the ATP binding site on AKT and prevents AKT activation. While the latter inhibits AKT activation and phosphorylation by altering the chemical structure of the AKT PH structural domain, thereby preventing AKT localization on the cell membrane. AKT competitive inhibitors are majorly listed below: Capivasertib (AZD5363) [71], a selective PAN-AKT inhibitor that entered clinical trials for the treatment of breast, gastric, and prostate cancers; Afuresertib (GSK2110183) [72], a monotherapy of relapsed or refractory multiple myeloma treatment; Uprosertib (GSK2141795), which remains in phase Ⅰ and Ⅱ studies [73]; and the AKT inhibitor Ipatasertib (GDC-0068, RG7440), a monotherapy for the treatment of triple-negative breast cancer, still being in phase Ⅰ and Ⅱ studies [74]. Perifosine as an inhibitor of AKT metaplasia to inhibit neuroblastoma tumor cell growth has entered phase Ⅱ studies [75]. Meanwhile, the development of AKT inhibitors still encounters plenty of predicaments. The most notable one is that AKT plays a significant role in maintaining the dynamic balance of cellular physiological functions in normal tissues. AKT inhibitors can cause an unavoidable cytotoxic effort on normal tissues in cancer patients [76]. In addition, like other targeted drugs, the single use of AKT inhibitor tends to induce drug resistance and the substitution related to tumor formation.
There are three generations of mTOR inhibitors [77]. The first generation of mTOR inhibitors includes rapamycin, also known as sirolimus, approved by the FDA as an immunosuppressant and primarily used to prevent immune rejection in organ transplantation [78]. Ridaforolimus also belongs to the first-generation mTOR inhibitors [79]. Everolimus, and temsirolimus have little effect on mTORC2 [80]. Single-agent application of mTOR inhibitor such as everolimus has been applied to treat advanced neuroendocrine tumors, breast cancer, and non-functioning gastrointestinal, and temsirolimus is used to treat lymphangioleiomyomatosis [81]. The second-generation mTOR inhibitors such as Torin1 [82], work on ATP binding sites to block kinase activity of both TORC1 and TORC2 proteins, and PI-103, targets ATP binding sites of mTOR and PI3K. Whereas, drug development is still in the clinical research stage [83]. In contrast, newly developed third-generation mTOR inhibitors named rapalink-1 show potential usage in patients with first- and second-generation drugs resistant tumors [84].
PD-1/PD-L1 regulates PI3K pathway. The PD-1/PD-L1 regulates a number of functions of PI3K pathway. Both the transmembrane part and intracellular part of the PD-1/PD-L1 pathway participate in this process. Reactions can happen in both immune cells and tumor cells. Arrows mean activation, and bars mean inhibition. While dotted lines represent PD-L1 expression. In tumor cells, after PD-L1 mRNA translation, cell-intrinsic PD-L1 activates Protein Kinase B (AKT). After PD-1/PD-L1 binding, Src homology-2-containing protein tyrosine phosphatase 2(SHP2) promotes Phosphatase and tensin homolog (PTEN) phosphorylation through working on casein kinase 2(CK2), further promoting phosphatidylinositol 3, 4, 5-trisphosphate (PIP3) to generate phosphatidylinositol 4, 5-bisphosphate (PIP2).
Inhibitors of the PI3K/AKT/mTOR pathway in clinical development
| Inhibitors | Target(s) | Tumor | Study phase | References |
|---|---|---|---|---|
| Alpelisib | PI3Kα | Advanced Solid Tumor | II | NCT01387321 |
| Duvelisib | PI3K | lymphoma or leukemia | II | NCT04707079 |
| Novartis (BYL719 ) | PI3Kα | SCC of the head and neck | II | NCT01602315 |
| breast cancer | II | NCT02506556 | ||
| Serabelisib (INK1117, TAK117, MLN1117) | PI3Kα | Metastatic Solid Tumors | I | NCT01449370 |
| Flupentixol | ATP binding area of PI3Kα | lung cancer | Preclinical | [70] |
| Capivasertib (AZD5363) | AKT | B-NHL | II | NCT05008055 |
| Afuresertib (GSK2110183) | AKT | Solid tumors, Hematologic malignancies | II | NCT01531894 |
| Uprosertib (GSK2141795) | AKT | Solid Tumors, Lymphoma | I | NCT01266954 |
| Ipatasertib (GDC-0068, RG7440) | AKT | Solid tumor | I | NCT04341259 |
| Perifosine | AKT | Neuroblastoma tumor, RCC, NSCLC | II | NCT00399789, NCT00399789 |
| Everolimus (RAD001) | mTORC1 | advanced NET, breast cancer, MM, non-functioning GI, pulmonary NENs | II (lung, MM) | NCT00401778, NCT00770120 |
| Temsirolimus | mTORC1 | RCC, LAM, lung cancer | II (lung, HL) | NCT00093782, NCT00838955 |
| Torin1 | ATP binding site of mTORC1 and mTORC2 | not mentioned | Preclinical | [86] |
| PI-103 | ATP binding sites of mTOR and PI3K | AML, glioblastoma, melanoma | Preclinical | [87] |
Abbreviations: NCT, ClinicalTrials.gov. No.; SCC, squamous cell carcinoma; B-NHL, B-cell Non-Hodgkin lymphoma; NSCLC, non-small cell lung cancer; GI, gastrointestinal; NENs, pulmonary neuroendocrine neoplasms; MM, malignant mesothelioma; NET, neuroendocrine tumor; LAM, lymphangioleiomyomatosis; AML, acute myeloid leukemia; RCC, renal cell carcinoma; HL, Hodgkin's lymphoma.
Similar to other target agents, resistance can also happen when using PI3K/AKT/mTOR pathway targeted drugs [85]. After using mTOR inhibitors, for example, resistance occurs because of the activation of other tumor-related pathway elements and downstream of mTOR. An experiment shows that after being treated with everolimus or AZD8055, the mice obtained markedly increased activation in EGFR and MEK-ERK signaling pathway in tumor epithelial and stromal cells, respectively [86]. In the PI3K/AKT/mTOR pathway downstream, suppressing the expression or activation of mTOR may lead to the decrease of 4E-BP1 expression. 4E-BP1 suppresses eIF4E expression, and the overexpression of 4E-BP1 will make tumor cells sensitive to rapamycin as eIF4E plays a critical role in controlling translation [87] and tumor progression [88]. In summary, the new generation of target drug development and new combination therapies are under exploration [89].
Anti-tumor immune effects of PI3K/AKT/mTOR pathway inhibitors
The effect of the PI3K/AKT/mTOR pathway on immune cells and the immune microenvironment is complicated and combined with multiple pathways. PI3K/AKT/mTOR inhibitors affect the PD-L1 expression in cancer cells [8]. In NSCLC, the application of rapamycin results in a decrease in PD-L1 expression [47]. In the presence of interferon-γ (IFN-γ), inhibition of PI3K enhances the antitumor effect of IFN-γ, while IFN-γ expression positively is correlated with tumor infiltration of CD3+ T cells. However, IFN-γ also activates AKT/mTOR pathway in cancer diseases, and induces PD-L1 expression, antagonizing its antitumor effect [90]. In consideration of the cytotoxicity and resistance when single-use, PI3K/AKT/mTOR pathway inhibitors are of research interest in combination with other targeted drugs, such as the combination of inhibitors targeting two different components of the pathway. For example, the clinical trials of everolimus combined with EGFR-TKI for the treatment of advanced NSCLC show no significant improvement in therapeutic efficacy compared to EGFR-TKI alone. Nevertheless, the application of everolimus is still suggested for patients with EGFR-TKI-resistant NSCLC [91]. Therefore, further clinical trials of PI3K/AKT/mTOR pathway inhibitors in combination with targeted agents are needed.
PI3K/AKT/mTOR inhibitors also influence the antitumor effects of tumor immune cells infiltrating in cancers [92]. In the tumor environment, PI3Kγ protein expression inhibits NF-κB activity through AKT and mTOR while stimulating C/EBPβ activation in macrophages, resulting in suppression of antitumor immune effects [93]. Selective inhibition of macrophage PI3Kγ stimulates CD8+ T cell activation and enhances cytotoxic effects. Activating PI3K-mTOR signaling in T cells in the tumor environment suppresses autoimmunity by inhibiting activation and differentiation of common T cells and specializing in CD4+Foxp3+ regulatory T cells (Tregs) [94]. PI3K/AKT/mTOR inhibitors restore the anti-tumor immune effect of the body to some extent by blocking pathway activation.
PI3K/AKT/mTOR pathway and anti-tumor immunotherapy
As mentioned above, PI3K/AKT/mTOR pathway activation is closely related to PD-L1 expression and impacts the tumor immune microenvironment [95]. The application of PD-L1 monoclonal antibodies enhances the antitumor immune effects of macrophages by inhibiting the AKT-mTOR pathway [96]. PD-L1 inhibitors have antagonistic effects on AKT and ERK1/2 activation to inhibit tumor proliferation [97]. So that blocking PD-L1 with antibodies in gastrointestinal mesenchymal tumors (GIST) can reduce CD8+ T cell depletion by regulating the PI3K/AKT/mTOR pathway to play an antitumor immune role [98]. Researchers find that in triple-negative breast cancer, atezolizumab can inhibit the mTOR signaling pathway by affecting P53-related genes [99]. At the same time, both PD-1/PD-L1 monoclonal antibodies and PI3K/AKT/mTOR pathway inhibitors may develop resistance through activation of the bypass pathway, and have drug toxicity and side effects when achieving significant cancer suppression [100]. Overall, the combination drug application is a therapeutic modality that will be investigated further (Table 2). For instance, the combination of rapamycin and anti-PD-1 antibody has dampened the progression of NSCLC [47], with the pharmacological effect of rapamycin on inhibiting the activation of the AKT/mTOR pathway from differentiating CD3+ T cells [101]. While both drugs can alleviate the increased production of regulatory T cells (Tregs), PD-L1 enhances the role of Everolimus in the treatment of renal cell carcinoma [102].
Combination therapy of PI3K/AKT/mTOR inhibitors with PD-1/PD-L1 monoclonal antibody
| PI3K/AKT/mTOR Inhibitors | Target | PD-1/PD-L1 monoclonal antibody | Tumor | Study phase | References |
|---|---|---|---|---|---|
| ABI-009 | mTOR | Nivolumab | mTOR Activating Mutated ES, PEComa, DT, Chordoma, NSCLC, UC, Melanoma, RCC, SCC, HCC, cHL, CRC | I and II | NCT03190174 |
| Sirolimus | mTOR | Durvalumab | NSCLC | I | NCT04348292 |
| Ipatasertib | AKT | Atezolizumab | Metastatic or Locally Advanced Malignancies | II | NCT04551521 |
| Copanlisib | PI3K | Nivolumab | Unresectable or MSS Solid Tumor, MSS Colon Cancer | I and II | NCT03711058 |
| Duvelisib (VS-0145, Copiktra) | PI3K | Pembrolizumab (Keytruda) | R/M HNSCC | I and II | NCT04193293 |
| SF1126 | PI3K | Nivolumab | AHCC | I | NCT03059147 |
Abbreviations: ES, Ewing Sarcoma; PEComa, perivascular epithelioid cell tumor; NSCLC, non-small cell lung cancer; DT, Desmoid Tumor; UC, urothelial carcinoma; RCC, renal cell carcinoma; SCC, squamous cell carcinoma; HCC, Hepatocellular Carcinoma; cHL, Classical Hodgkin Lymphoma; CRC, Colorectal Cancer; MSS, Microsatellite Stable; R/M, recurrent or metastatic; HNSCC, head and neck squamous cell carcinoma; AHCC, Advanced Hepatocellular Carcinoma.
Conclusion
This review discussed the tumor immunosuppressive effect of PD-1/PD-L1 inhibitors and the fundamental scenario of immune checkpoint inhibition therapy with the application of monoclonal antibodies. Taking NSCLC as an example, the review explained the components of the PI3K/AKT/mTOR signaling pathway and described their functions in driving carcinogenesis and suppressing antitumor immunity respectively. We also introduced the relevant immunosuppressive agents including the role and situation of single agent use in anti-tumor represented by everolimus, as well as the feasibility of combining multiple targeted agents and multiple adverse medication effects. The viability of a therapeutic strategy combining PI3K/AKT/mTOR pathway inhibitors with PD-1/PD-L1 inhibition will be considered. To date, research into PI3K/AKT/mTOR signaling pathway inhibitors is still currently in progress, and it exhibits great positive significance to investigate the interaction between PD-L1 expression and PI3K/AKT/mTOR signaling pathway activation for addressing anticancer drug resistance, prolonging tumor patient survival, and improving patient prognosis.
Acknowledgements
The work was supported by grants from The National Natural Science Foundation of China (No: 81773218, 81972838, 81703009, 82102805, and 82272722).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021;71:209-249
2. Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR. et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. Journal of the National Comprehensive Cancer Network: JNCCN. 2019;17:1464-1472
3. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455-2465
4. O'Donnell JS, Smyth MJ, Teng MW. Acquired resistance to anti-PD1 therapy: checkmate to checkpoint blockade? Genome Med. 2016;8:111
5. Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM. et al. Systemic Therapy for Stage Ⅳ Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35:3484-3515
6. Yang JC, Shepherd FA, Kim DW, Lee GW, Lee JS, Chang GC. et al. Osimertinib Plus Durvalumab versus Osimertinib Monotherapy in EGFR T790M-Positive NSCLC following Previous EGFR TKI Therapy: CAURAL Brief Report. J Thorac Oncol. 2019;14:933-939
7. Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin Cancer Biol. 2019;59:125-132
8. O'Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91-103
9. Tan AC. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thoracic cancer. 2020;11:511-518
10. Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T. et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112:2149-2155
11. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y. et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nature immunology. 2020;21:1346-1358
12. Wang Z, Wu X. Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer medicine. 2020;9:8086-8121
13. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science (New York, NY). 2017;355:1428-1433
14. Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z. et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunological reviews. 2006;212:8-27
15. Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends in molecular medicine. 2015;21:24-33
16. Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms Controlling PD-L1 Expression in Cancer. Molecular cell. 2019;76:359-370
17. Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. Journal of hematology & oncology. 2019;12:92
18. Antonangeli F, Natalini A, Garassino MC, Sica A, Santoni A, Di Rosa F. Regulation of PD-L1 Expression by NF-κB in Cancer. Front Immunol. 2020;11:584626
19. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D. et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. JAMA oncology. 2018;4:351-357
20. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:123-135
21. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443-2454
22. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N. et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. The New England journal of medicine. 2018;378:2288-2301
23. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR. et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2017;15:504-535
24. Incorvaia L, Fanale D, Badalamenti G, Barraco N, Bono M, Corsini LR. et al. Programmed Death Ligand 1 (PD-L1) as a Predictive Biomarker for Pembrolizumab Therapy in Patients with Advanced Non-Small-Cell Lung Cancer (NSCLC). Advances in therapy. 2019;36:2600-2617
25. Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol. 2020;15:288-293
26. Liu SV, Camidge DR, Gettinger SN, Giaccone G, Heist RS, Hodi FS. et al. Long-term survival follow-up of atezolizumab in combination with platinum-based doublet chemotherapy in patients with advanced non-small-cell lung cancer. European journal of cancer (Oxford, England: 1990). 2018;101:114-122
27. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. The New England journal of medicine. 2017;377:1919-1929
28. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. British journal of cancer. 2018;118:9-16
29. Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nature communications. 2016;7:10501
30. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM. et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2018;36:1714-1768
31. Dafni U, Tsourti Z, Vervita K, Peters S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung cancer (Amsterdam, Netherlands). 2019;134:127-140
32. Zhou Y, Chen C, Zhang X, Fu S, Xue C, Ma Y. et al. Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis. Journal for immunotherapy of cancer. 2018;6:155
33. Remon J, Steuer CE, Ramalingam SS, Felip E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Annals of oncology: official journal of the European Society for Medical Oncology. 2018;29:i20-i27
34. Leonetti A, Wever B, Mazzaschi G, Assaraf YG, Rolfo C, Quaini F. et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist Updat. 2019;46:100644
35. Divecha N, Irvine RF. Phospholipid signaling. Cell. 1995;80:269-278
36. Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486-5496
37. Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15:273-291
38. Song X, Fan PD, Bantikassegn A, Guha U, Threadgill DW, Varmus H. et al. ERBB3-independent activation of the PI3K pathway in EGFR-mutant lung adenocarcinomas. Cancer Res. 2015;75:1035-1045
39. Carnero A, Paramio JM. The PTEN/PI3K/AKT Pathway in vivo, Cancer Mouse Models. Frontiers in oncology. 2014;4:252
40. Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169:381-405
41. Brown JS, Banerji U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacology & therapeutics. 2017;172:101-115
42. Song M, Chen D, Lu B, Wang C, Zhang J, Huang L. et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PloS one. 2013;8:e65821
43. Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z. et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer cell. 2014;25:590-604
44. Fumarola C, Bonelli MA, Petronini PG, Alfieri RR. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol. 2014;90:197-207
45. LoRusso PM. Inhibition of the PI3K/AKT/mTOR Pathway in Solid Tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34:3803-3815
46. Luo M, Xia Y, Wang F, Zhang H, Su D, Su C. et al. PD0325901, an ERK inhibitor, enhances the efficacy of PD-1 inhibitor in non-small cell lung carcinoma. Acta pharmaceutica Sinica B. 2021;11:3120-3133
47. Sun SY. Searching for the real function of mTOR signaling in the regulation of PD-L1 expression. Translational oncology. 2020;13:100847
48. Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR. et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016;76:227-238
49. Rennier K, Shin WJ, Krug E, Virdi G, Pachynski RK. Chemerin Reactivates PTEN and Suppresses PD-L1 in Tumor Cells via Modulation of a Novel CMKLR1-mediated Signaling Cascade. Clin Cancer Res. 2020;26:5019-5035
50. Du L, Lee JH, Jiang H, Wang C, Wang S, Zheng Z. et al. β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. The Journal of experimental medicine. 2020;217:e20191115
51. Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN. et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science (New York, NY). 2016;352:227-231
52. Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM. et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. Journal for immunotherapy of cancer. 2019;7:305
53. Cai Y, Xue F, Qin H, Chen X, Liu N, Fleming C. et al. Differential Roles of the mTOR-STAT3 Signaling in Dermal γδ T Cell Effector Function in Skin Inflammation. Cell Rep. 2019;27:3034-3048.e5
54. Huang Y, Hu K, Zhang S, Dong X, Yin Z, Meng R. et al. S6K1 phosphorylation-dependent degradation of Mxi1 by β-Trcp ubiquitin ligase promotes Myc activation and radioresistance in lung cancer. Theranostics. 2018;8:1286-1300
55. Deng L, Qian G, Zhang S, Zheng H, Fan S, Lesinski GB. et al. Inhibition of mTOR complex 1/p70 S6 kinase signaling elevates PD-L1 levels in human cancer cells through enhancing protein stabilization accompanied with enhanced beta-TrCP degradation. Oncogene. 2019;38:6270-6282
56. Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nature medicine. 2007;13:84-88
57. Yu W, Hua Y, Qiu H, Hao J, Zou K, Li Z. et al. PD-L1 promotes tumor growth and progression by activating WIP and beta-catenin signaling pathways and predicts poor prognosis in lung cancer. Cell Death Dis. 2020;11:506
58. Wu L, Cai S, Deng Y, Zhang Z, Zhou X, Su Y. et al. PD-1/PD-L1 enhanced cisplatin resistance in gastric cancer through PI3K/AKT mediated P-gp expression. International immunopharmacology. 2021;94:107443
59. Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E. et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell. 2015;162:1242-1256
60. Gao H, Zhang J, Ren X. PD-L1 regulates tumorigenesis and autophagy of ovarian cancer by activating mTORC signaling. Bioscience reports. 2019;39:BSR20191041
61. Han F, Li CF, Cai Z, Zhang X, Jin G, Zhang WN. et al. The critical role of AMPK in driving Akt activation under stress, tumorigenesis and drug resistance. Nature communications. 2018;9:4728
62. Dai X, Bu X, Gao Y, Guo J, Hu J, Jiang C. et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Molecular cell. 2021;81:2317-2331.e6
63. Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO. et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Molecular cell. 2018;71:606-620.e7
64. Magnone M, Emionite L, Guida L, Vigliarolo T, Sturla L, Spinelli S. et al. Insulin-independent stimulation of skeletal muscle glucose uptake by low-dose abscisic acid via AMPK activation. Scientific reports. 2020;10:1454
65. Liu M, Wei F, Wang J, Yu W, Shen M, Liu T. et al. Myeloid-derived suppressor cells regulate the immunosuppressive functions of PD-1(-)PD-L1(+) Bregs through PD-L1/PI3K/AKT/NF-κB axis in breast cancer. Cell Death Dis. 2021;12:465
66. Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. The New England journal of medicine. 2016;375:1767-1778
67. Polivka J Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacology & therapeutics. 2014;142:164-175
68. Ippen FM, Grosch JK, Subramanian M, Kuter BM, Liederer BM, Plise EG. et al. Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. 2019;21:1401-1411
69. Janku F. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: From laboratory to patients. Cancer Treat Rev. 2017;59:93-101
70. Dong C, Chen Y, Li H, Yang Y, Zhang H, Ke K. et al. The antipsychotic agent flupentixol is a new PI3K inhibitor and potential anticancer drug for lung cancer. International journal of biological sciences. 2019;15:1523-1532
71. Banerji U, Dean EJ, Pérez-Fidalgo JA, Batist G, Bedard PL, You B. et al. A Phase I Open-Label Study to Identify a Dosing Regimen of the Pan-AKT Inhibitor AZD5363 for Evaluation in Solid Tumors and in PIK3CA-Mutated Breast and Gynecologic Cancers. Clin Cancer Res. 2018;24:2050-2059
72. Arceci RJ, Allen CE, Dunkel I, Jacobsen ED, Whitlock J, Vassallo R. et al. Evaluation Of Afuresertib, An Oral Pan-AKT Inhibitor, In Patients With Langerhans Cell Histiocytosis. Blood. 2013;122:2907
73. Aghajanian C, Bell-McGuinn KM, Burris HA 3rd, Siu LL, Stayner LA, Wheler JJ. et al. A phase I, open-label, two-stage study to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral AKT inhibitor GSK2141795 in patients with solid tumors. Investigational new drugs. 2018;36:1016-1025
74. de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A. et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin Cancer Res. 2019;25:928-936
75. Hyman DM, Smyth LM, Donoghue MTA, Westin SN, Bedard PL, Dean EJ. et al. AKT Inhibition in Solid Tumors With AKT1 Mutations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35:2251-2259
76. Johnson SM, Gulhati P, Rampy BA, Han Y, Rychahou PG, Doan HQ. et al. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. Journal of the American College of Surgeons. 2010;210:767-776 776-768
77. Xu T, Sun D, Chen Y, Ouyang L. Targeting mTOR for fighting diseases: A revisited review of mTOR inhibitors. European journal of medicinal chemistry. 2020;199:112391
78. Ventura-Aguiar P, Campistol JM, Diekmann F. Safety of mTOR inhibitors in adult solid organ transplantation. Expert opinion on drug safety. 2016;15:303-319
79. Spreafico A, Mackay HJ. Current phase II clinical data for ridaforolimus in cancer. Expert Opin Investig Drugs. 2013;22:1485-1493
80. Kakiuchi Y, Yurube T, Kakutani K, Takada T, Ito M, Takeoka Y. et al. Pharmacological inhibition of mTORC1 but not mTORC2 protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism through Akt and autophagy induction. Osteoarthritis and cartilage. 2019;27:965-976
81. Piha-Paul SA, Hong DS, Kurzrock R. Response of lymphangioleiomyomatosis to a mammalian target of rapamycin inhibitor (temsirolimus) -based treatment. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:e333-335
82. Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y. et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. The Journal of biological chemistry. 2009;284:8023-8032
83. Keniry M, Parsons R. mTOR inhibition, the second generation: ATP-competitive mTOR inhibitor initiates unexpected receptor tyrosine kinase-driven feedback loop. Cancer Discov. 2011;1:203-204
84. Kuroshima K, Yoshino H, Okamura S, Tsuruda M, Osako Y, Sakaguchi T. et al. Potential new therapy of Rapalink-1, a new generation mammalian target of rapamycin inhibitor, against sunitinib-resistant renal cell carcinoma. Cancer science. 2020;111:1607-1618
85. Dong C, Wu J, Chen Y, Nie J, Chen C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Frontiers in pharmacology. 2021;12:628690
86. Fujishita T, Kojima Y, Kajino-Sakamoto R, Taketo MM, Aoki M. Tumor microenvironment confers mTOR inhibitor resistance in invasive intestinal adenocarcinoma. Oncogene. 2017;36:6480-6489
87. Wang J, Ye Q, Cao Y, Guo Y, Huang X, Mi W. et al. Snail determines the therapeutic response to mTOR kinase inhibitors by transcriptional repression of 4E-BP1. Nature communications. 2017;8:2207
88. Pelletier J, Graff J, Ruggero D, Sonenberg N. Targeting the eIF4F translation initiation complex: a critical nexus for cancer development. Cancer Res. 2015;75:250-263
89. De Vera AA, Reznik SE. Chapter 14 - Combining PI3K/Akt/mTOR Inhibition With Chemotherapy. In: Chen Z-S, Yang D-H, editors. Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy: Academic Press. 2019:229-242
90. Gao Y, Yang J, Cai Y, Fu S, Zhang N, Fu X. et al. IFN-gamma-mediated inhibition of lung cancer correlates with PD-L1 expression and is regulated by PI3K-AKT signaling. Int J Cancer. 2018;143:931-943
91. Fang W, Huang Y, Gu W, Gan J, Wang W, Zhang S. et al. PI3K-AKT-mTOR pathway alterations in advanced NSCLC patients after progression on EGFR-TKI and clinical response to EGFR-TKI plus everolimus combination therapy. Transl Lung Cancer Res. 2020;9:1258-1267
92. Sai J, Owens P, Novitskiy SV, Hawkins OE, Vilgelm AE, Yang J. et al. PI3K Inhibition Reduces Mammary Tumor Growth and Facilitates Antitumor Immunity and Anti-PD1 Responses. Clin Cancer Res. 2017;23:3371-3384
93. Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S. et al. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539:437-442
94. Essig K, Hu D, Guimaraes JC, Alterauge D, Edelmann S, Raj T. et al. Roquin Suppresses the PI3K-mTOR Signaling Pathway to Inhibit T Helper Cell Differentiation and Conversion of Treg to Tfr Cells. Immunity. 2017;47:1067-1082.e12
95. Huang H, Zhou J, Chen H, Li J, Zhang C, Jiang X. et al. The immunomodulatory effects of endocrine therapy in breast cancer. Journal of Experimental & Clinical Cancer Research. 2021;40:19
96. Hartley GP, Chow L, Ammons DT, Wheat WH, Dow SW. Programmed Cell Death Ligand 1 (PD-L1) Signaling Regulates Macrophage Proliferation and Activation. Cancer Immunol Res. 2018;6:1260-1273
97. Wang X, Yang X, Zhang C, Wang Y, Cheng T, Duan L. et al. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proceedings of the National Academy of Sciences. 2020;117:6640-6650
98. Zhao R, Song Y, Wang Y, Huang Y, Li Z, Cui Y. et al. PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif. 2019;52:e12571
99. Saleh R, Taha RZ, Sasidharan Nair V, Alajez NM, Elkord E. PD-L1 Blockade by Atezolizumab Downregulates Signaling Pathways Associated with Tumor Growth, Metastasis, and Hypoxia in Human Triple Negative Breast Cancer. Cancers (Basel). 2019;11:1050
100. Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer metastasis reviews. 2016;35:515-524
101. Breslin EM, White PC, Shore AM, Clement M, Brennan P. LY294002 and rapamycin co-operate to inhibit T-cell proliferation. British journal of pharmacology. 2005;144:791-800
102. Hirayama Y, Gi M, Yamano S, Tachibana H, Okuno T, Tamada S. et al. Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer science. 2016;107:1736-1744
Author contact
![]() Corresponding author: Songqing Fan, Department of Pathology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, China. E-mail address: songqingfanedu.cn.
Corresponding author: Songqing Fan, Department of Pathology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, China. E-mail address: songqingfanedu.cn.

 Global reach, higher impact
Global reach, higher impact