3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2022; 13(14):3526-3532. doi:10.7150/jca.67534 This issue Cite
Research Paper
Detection of Kita-Kyushu Lung Cancer Antigen-1, a Cancer/Testis Antigen, in the Stomach Close to a Cancerous Condition
1. Division of Internal Medicine, Kitasato University Medical Centre, Kitamoto, Saitama, Japan.
2. Division of Biomedical Research, Kitasato University Medical Centre, Kitamoto, Saitama, Japan.
3. Division of General and Gastroenterological Surgery, Department of Surgery (Ohashi), Toho University, Tokyo, Japan.
4. Department of General Thoracic Surgery, National Hospital Organization, Saitama Hospital, Wako, Saitama, Japan.
5. Division of Surgery, Kitasato University Medical Centre, Kitamoto, Saitama, Japan.
6. Division of Pathology, Kitasato University Medical Centre, Kitamoto, Saitama, Japan.
7. Department of Microbial Chemistry and Medicinal Research Laboratories, Graduate School of Pharmaceutical Sciences, Kitasato University, Tokyo, Japan.
8. Department of Gastroenterology, School of Medicine, Kitasato University, Sagamihara, Japan.
#Equal contributors to the manuscript.
Received 2021-9-27; Accepted 2022-10-14; Published 2022-10-31
Abstract
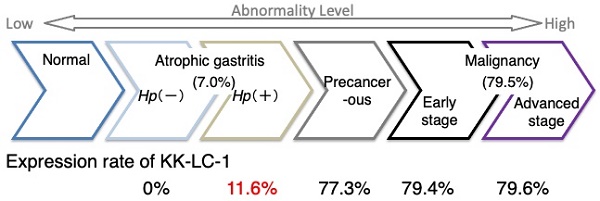
Background: Kita-Kyushu lung cancer antigen-1 (KK-LC-1), encoded by CT83, is a cancer/testis antigen (CTA) and an attractive target for immunotherapy. Our previous study demonstrated frequent CT83 expression in gastric cancers (GCs) and non-tumor sites of the stomach with tumors. Additionally, there was a correlation with Helicobacter pylori (Hp) infection. Since it currently remains unclear whether KK-LC-1 is expressed in the stomach without GC, this study investigated KK-LC-1 expression in non-GC stomach.
Methods: We investigated differences in CT83 gene expression at non-tumor sites of stomachs with or without tumors in 118 GC patients and 115 non-GC patients. Fisher's exact test was used for statistical analyses.
Results: CT83 expression was detected in 77% of non-tumor sites in stomachs with tumors, which was significantly higher than in stomachs without tumors (7%, p < 0.0001). All patients with CT83 expression at non-tumor sites of their stomachs without tumors carried Hp.
Conclusion: CT83 appears to be rarely expressed in the atrophic stomach, and furthermore, a part of patients positive for its expression will develop GC in the future, suggesting that CT83 expression is a useful marker for predicting GC.
Keywords: gastric cancer, Helicobacter pylori, atrophic gastritis, Kita-Kyushu lung cancer antigen-1
Introduction
Gastric cancer (GC) is the third leading cause of cancer-related death worldwide [1]. In Japan, the incidence rate of GC is the second highest of all cancers. Most cases are caused by Helicobacter pylori (Hp) infection [2]. Therefore, detection and eradication of Hp infection may reduce the risk of GC. Furthermore, GC risk can be diagnosed by ABC classification using an anti-Hp antibody and measurement of pepsinogen I/II serum levels [3]. However, this approach has proven to be inaccurate and indicates the occurrence of only 1%-5% of GC in high-risk groups. Thus, more precise methods are needed for risk diagnosis [4].
To date, numerous tumor-associated antigens have been identified in various human cancers [5]. Among them, cancer/testis antigens (CTAs) are particularly attractive targets for immunotherapy. Such antigens have minimal or no expression in normal tissues, except for germline tissues, but they are aberrantly expressed in a range of human cancers [6]. CTAs may be advantageous targets for systemic cancer diagnosis because of their specific and broad expression patterns in various cancer types. However, it has been shown that the individual CTA expression rate is insufficient for diagnostic applications [7].
Among known CTAs, Kita-Kyushu lung cancer antigen-1 (KK-LC-1), encoded by CT83, includes epitope peptides recognized by cytotoxic T lymphocytes (CTLs). CTLs against KK-LC-1 predominantly accumulate among tumor-infiltrating lymphocytes, which leads to a good response to adaptive immunotherapy [8]. Except for the testis, KK-LC-1 is not expressed in normal tissues. It is expressed in 33%, 82%, and 75% of non-small cell lung cancers, GC, and triple-negative breast cancers, respectively [9-12].
In a previous study, we reported that Hp infection induces the expression of specific CTAs in addition to causing malignant transformation of host cells. Additionally, we found a correlation between CT83 expression and Hp infection in clinical GC [13], [14]. These findings suggest a correlation between KK-LC-1 expression and Hp infection, an early cancer-causing event. Therefore, KK-LC-1 may be a novel candidate for cancer prediction and diagnosis.
The expression rate of KK-LC-1 is 79% during early stages of GC [15], which indicates that KK-LC-1 expression occurs at the beginning of a malignancy and is subsequently maintained. Furthermore, we have observed that KK-LC-1 is expressed in premalignant lesions of the stomach [16]. However, to date, it remains to be clarified whether the non-premalignant stomach expresses KK-LC-1. In the present study, we investigated the difference in CT83 expression at non-tumor sites of stomachs with or without tumors. Additionally, we assessed the clinical utility of CT83 for surveillance of GC occurrence.
Materials and methods
Patients
Patients underwent an endoscopic survey of the esophagus, stomach, and duodenum. In cases of an atrophic stomach, two portions, one from the lower and one from the middle corpus of the stomach, were sampled and subjected to a rapid urease test (RUT). After RUT confirmation, we collected gastric specimens. Between March 2016 and August 2017, 412 patients underwent RUTs at Kitasato University Medical Centre. Of these patients, a subset (n = 199) provided informed consent after RUT. Each specimen was checked for RNA quality by expression of β-actin (ATCB), and specimens with a threshold cycle (Ct) greater than 30 were excluded. Finally, 115 samples were used in the present study.
Between August 2012 and June 2017, 196 patients underwent surgical resection for GC at the Department of Surgery, Kitasato University Medical Centre, Kitamoto, Japan. We obtained tumor samples from stomachs with GC, as well as two or four non-tumor samples collected from random locations far from the tumor site and each non-tumor site. On the basis of ACTB expression levels, the patients whose one or more specimens were greater than 30 of ACTB expression were excluded in this study. all samples had sufficient mRNA quality. Finally, we enrolled 118 GC patients in the present study whose clinicopathological characters are shown in Supplementary Table S1. Furthermore, to compare RUT specimens, we selected 22 GC patients with two non-tumor samples from the lower and middle corpuses. The clinicopathological findings were classified in accordance with the Japanese Classification of Gastric Carcinoma (14th edition) [17].
The Human Ethics Review Committee of Kitasato University Medical Centre, Japan (approval nos. 29-16 and 29-18) approved the study protocol. All experiments were performed in accordance with the relevant guidelines and regulations, and all patients signed informed consent prior to resection of the tissue samples used in this study.
Tissue specimens
After collection, each specimen was immediately stored at 4°C overnight in RNAlater (Life Technologies, Carlsbad, CA, USA). Samples were subsequently stored at -80°C until use. For samples from GC patients, we performed hematoxylin-eosin staining on samples from each adjacent site to confirm the predominance of tumor cells at tumor sites and to establish that there was no contamination of tumor cells at non-tumor sites. Furthermore, we categorized the samples from non-tumor sites into fundic gland, borderline (B), or pyloric gland (P) samples.
Expression analysis of CTAs
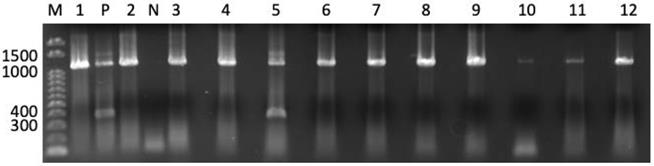
We used the QIACUBE with the RNeasy Tissue Mini Kit (Qiagen, Hilden, Germany) to isolate total RNA from each sample in accordance with the manufacturer's instructions. Total RNA was converted to cDNA using oligo p(dN)6 random primers and Superscript III reverse transcriptase (Life Technologies). ACTB, melanoma antigen gene (MAGE) A1, MAGEA3, MAGEA4, New York esophageal carcinoma-1 (CTAG1A), and synovial sarcoma, X breakpoint-4 (SSX4) expression was measured with TaqMan Gene Expression Assays (IDs: Hs99999903_m1, Hs00607097_m1, H200366532_m1, Hs00365979_m1, Hs00265824_m1, and Hs02341532_m1, respectively). A 7900HT Fast Real-Time PCR System (Life Technologies) was used to perform the analyses. The threshold cycle number of cDNAs converted from RNAs was measured for ACTB. Real-time polymerase chain reaction (PCR) was performed in a 20 µL volume consisting of 5 µL cDNA template, 10 µL FastStart Universal Probe Master Mix (Roche, Mannheim, Germany), and 1 µL TaqMan Gene Expression Assay. Samples were qualified by ≤30 threshold cycles (CT) of ACTB and then assessed for CTA expression. The expression of CTAs except for CT83 was assessed as positive with ≤45 CT. CT83 expression was examined by 40-cycle endpoint reverse transcription (RT)-PCR because an appropriate probe for CT83 mRNA detection was unavailable. PCR amplification was performed in a 20 µL volume consisting of 2 µL cDNA template, rTaq (Takara, Tsu, Japan), dNTPs (Roche, Basel, Switzerland), and 500 nM each of gene-specific primers ATGAACTTCTATTTACTCCTAGCGAGC and TTAGGTGGATTTCCGGTGAGG (Sigma-Aldrich Japan, Tokyo, Japan). The annealing temperature was 67°C. We performed 40 cycles to yield a 342-bp product. PCR products were visualized by ethidium bromide staining and ultraviolet light exposure, following electrophoresis on a 1.5% agarose gel. A representative panel for detection of CT83 expression in tumor-free atrophic stomachs is shown in Figure 1.
Statistical analysis
Fisher's exact test was used for the comparison between CT83 expression and each clinicopathological factor. JMP14.0 (SAS Institute Japan, Tokyo, Japan) was used for the analysis.
Results
CTA expression in GC tumors
We used RT-PCR analysis to determine the fractions of the tumor sites expressing specific CTA genes. Of the 118 GC tumors evaluated, 92 specimens (78.0%) expressed CT83 (Table 1). We found that other CTAs, specifically MAGEA1, MAGEA3, MAGEA4, CTAG1A, and SSX4 had expression frequencies of 29.7%, 32.2%, 19.5%, 13.6%, and 17.8%, respectively.
CT83 expression in non-tumor sites of stomachs with or without tumors
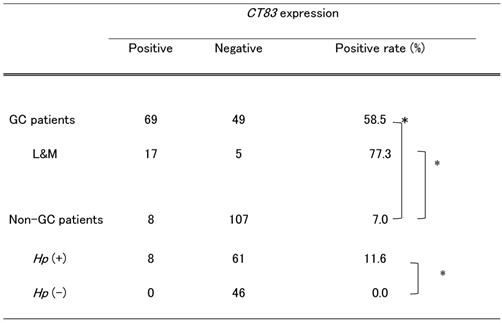
In 69 out of 118 (58.5%) GC patients, CT83 was detected at one or more non-tumor sites (Table 2). There was no significant difference in the positive rate between GC patients with two and four sampled sites (p = 0.8517, Supplementary Table S2).
Cancer/testis antigen (CTA) expression in 118 gastric cancer tumors
| CTA | Positive | Negative | Frequency (%) |
|---|---|---|---|
| CT83 | 92 | 26 | 78.0 |
| MAGEA1 | 35 | 83 | 29.7 |
| MAGEA3 | 38 | 80 | 32.2 |
| MAGEA4 | 23 | 95 | 19.5 |
| CTAG1A | 16 | 102 | 13.6 |
| SSX4 | 21 | 97 | 17.8 |
CT83, Kita-Kyushu lung cancer antigen-1; MAGE, melanoma antigen gene; CTAG1A, New York esophageal squamous cell carcinoma-1; SSX4, synovial sarcoma, X breakpoint-4.
CT83 expression with patient characters of atrophic gastritis
| Clinicopathological parameters | Categories | Total | CT83 expression | |
|---|---|---|---|---|
| (n=115) | Positive | Negative | ||
| Age (average ± SD) | - | 66.1 ± 12.0 | 67.4 ± 8.6 | 66.1 ± 12.2 |
| Gender | Male | 64 (56%) | 5 (8%) | 59 (92%) |
| Female | 51 (44%) | 3 (6%) | 48 (94%) | |
| Hp infection | Positive | 69 (60%) | 8 (12%) | 61 (88%) |
| Negative | 46(40%) | 0 (0%) | 46 (100%) | |
| Atrophic severity | High (O1-O3) | 65 (57%) | 6 (9%) | 59 (91%) |
| Low (C1-C3) | 36 (31%) | 2 (6%) | 34 (94%) | |
| Not recorded | 14 (12%) | 0 (0%) | 14 (100%) | |
| CT83 expression | Positive | 8 (7%) | - | - |
| Negative | 107 (93%) | - | - | |
Values are presented as number (%) or mean ± standard deviation.
CT83; Kita-kyushu lung cancer antigen-1, Hp; Helicobacter pylori.
Atrophic severity was classified as high and low with O1 to O3 and C1 to C3 of Kimura-Takemoto classification, respectively.
Representative panel of CT83 expression in noncancerous gastric specimens. CT83 expression was detected as an amplicon band of 342 bp. CT83 expression was detected in 1 of 12 stomach samples (lane No. 5). Bands between 1000 and 1500 indicate amplicons of genomic DNA of CT83. M, 100 bp ladder; P, positive control (cDNA of MKN45 cell line); N, negative control.
CT83 expression at non-tumor sites of GC and non-GC patients
CT83, Kita-Kyushu lung cancer antigen-1; GC, gastric cancer; L&M, each lower and middle corpus sampled; Hp, Helicobacter pylori. *p < 0.0001.
We investigated GC patients in which samples were obtained from the lower and middle corpuses of the same two non-tumor sites of non-GC patients. In 17 of 22 (77.3%) patients, CT83 was expressed at either of the two or both of the two non-tumor sites at significantly higher rates than in the remaining GC patients (45.7%, p = 0.0276, Supplementary Table S3).
Conversely, among non-GC patients with gastric atrophy, we identified 8 of 115 (7.0%) with CT83 expression (Table 2). Of note, the CT83 expression rate in non-GC patients was significantly lower than that in GC patients (p < 0.0001, Table 3). There were no significant differences in the detection of CT83 expression among age, gender, and atrophic severity. All CT83-positive patients were infected with Hp (Table 3). When exclusively considering Hp-positive non-GC patients, we observed 11.6% with CT83 expression (p < 0.0001, Table 3). Supplementary Table S4 lists non-GC patients with CT83 expression. Except for patient NGC#006, who had a mass in the stomach at the time of endoscopic survey, no specific clinical features except Hp infection were observed in patients with positive CT83 expression; for NGC#006, adenoma was diagnosed on biopsy of a mass in a different site from the biopsy analyzed for CT83 expression. After 2 months of CT83 analysis, patient NGC#006 underwent endoscopic submucosal dissection to remove the adenoma. The final pathological diagnosis was adenocarcinoma in adenoma. Furthermore, the patient relapsed with gastric cancer after 14 months of CT83 analysis, despite successful treatment for Hp eradication. In addition, gastric cancer occurred in patient #003 after 49 months of CT83 expression analysis, even though the patient had received successful treatment for Hp eradication.
Discussion
KK-LC-1 is classified as a CTA because of its absence in normal tissues except for the testis. However, it is expressed in cancers of multiple organs [7, 10]. Furthermore, Fukuyama et al. found a correlation between KK-LC-1 expression and Hp infection. Additionally, we previously showed that CT83 and KK-LC-1 expression was frequently detected at non-tumor sites of the stomach with GC [14, 16]. However, it remained unclear whether KK-LC-1 is expressed in the stomachs of patients with Hp infection and without tumors. In the present study, KK-LC-1, encoded by CT83, was frequently expressed in non-tumor sites of the GC stomach, but infrequently in those of the non-GC stomach (Fig. 2). Furthermore, while CT83 expression was correlated with Hp infection, it was not an exclusive indicator of Hp infection. There is also a similar report by Watari et al. showing greater methylation changes in miR-124a-3 in the order of Hp+/GC, Hp+/AG, and Hp-/AG [18]. The difference between their study and ours is that our subject is detected with higher frequency in GC patients and less frequently in AG patients. Furthermore, we also confirm that carcinogenesis occurs in the object-positive group but not in the object-negative group, although further case accumulation is needed. Hp infection, ABCD stratification, and atrophic severity, which are currently considered predictors of carcinogenesis, have been associated with higher cumulative incidence of gastric cancer in high-risk groups [2, 19, 20]. Other new and potential predictive markers of carcinogenesis, including CT83 expression, also need to be followed up to determine whether their positive cases will show carcinogenesis in the future [21].
Expression rate of CT83 during the transition to gastric abnormality. The expression rate of CT83 is summarized for each stage of gastric abnormality. CT83 was not expressed in the stomach under normal conditions. However, with the development and progression of gastric abnormality, CT83 expression was increased in the following order: atrophic gastritis without and with Hp infection, non-tumor sites of the precancerous stomach with gastric cancer, and early and advanced gastric cancer tumors. This study showed that CT83 expression was clearly discrepant between non-cancerous and cancerous stomachs regardless of Hp infection. The CT83 expression rate in malignancy including early and progressive stages was adopted from Futawatari et al. [15]. Hp, Helicobacter pylori.
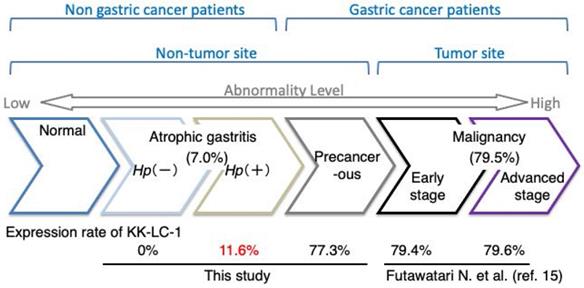
CT83 expression might be a new indicator to measure precancerous levels in the stomach. Higher CT83 expression in non-tumor sites with GC compared with non-GC stomachs may indicate precancerous levels. Of note, epigenetic alterations are precancerous level indicators. Compare et al. reported that global hypomethylation of the gastric mucosa gradually advances from Hp-negative gastritis to Hp-positive gastritis, Hp-positive chronic gastritis, and gastric carcinoma [22]. Conversely, Leodolter et al. showed that global hypomethylation occurs during the early stages of gastritis regardless of Hp infection [23]. Detection of CT83 expression may facilitate determining whether the stomach is close to a malignant level independent of the accumulation of genetic/epigenetic alterations.
CT83 expression was not detected at non-tumor sites of the stomachs in three GC patients. However, its expression was detected in tumor sites of the same patients. Such an issue may be resolved by sampling multiple mucosa sites from the lower corpus. Supplementary Figure S1 and our previous study showed that CT83 expression rates in the lower corpus were 68.1% and 66.1%, respectively [16].
We observed two non-GC patients whose non-tumor sites of the stomach expressed CT83. These patients were later diagnosed with GC. Of note, the area of each specimen of the two patients differed from the area in which the tumor later occurred. This phenomenon suggests that CT83 expression indicate the presence of GC in stomach allopatric sites.
Additionally, CT83 expression was observed in non-tumor sites of GC patients. Of note, GC patients who underwent mucosal resection, including ESD, more frequently presented with metachronous cancers from gastric remnants than during surgical resection [24]. These findings suggest that CT83 expression is a predictive marker of GC occurrence. Non-GC patients in whom CT83 expression was detected are under follow-up to identify possible GC development. However, because all non-GC patients with CT83 had received antibiotics against Hp, genetic/epigenetic alterations caused by Hp will not accumulate, which in return will prevent GC from developing. Nonetheless, GC can occur following Hp eradication [25]. The occurrence of GCs in non-GC patients in whom CT83 expression was detected indicates the potential of KK-LC-1 as a new predictive marker for GC.
Conclusion
CT83 was frequently expressed in non-tumor sites in the GC stomach, but rarely in the non-GC stomach. Furthermore, all non-GC patients with CT83 expression had Hp infection, and part of these patients would develop GC in the future. These results indicate that CT83 is rarely expressed in the atrophic state, but is expressed in those particularly close to the precancerous state, in which carcinogenesis is of high potential. The findings in this study indicate the potential of CT83 as a useful marker for GC prediction.
Abbreviations
GC: gastric cancer; Hp: Helicobacter pylori; CTA: cancer/testis antigen; KK-LC-1: Kita-Kyushu lung cancer antigen-1; CTL: cytotoxic T lymphocyte; RUT: rapid urease test; RT-PCR: reverse transcription-polymerase chain reaction; MAGE: melanoma antigen gene; B: borderline; P: pyloric gland; ESD: endoscopic submucosal dissection.
Supplementary Material
Supplementary figure and tables.
Acknowledgements
The authors thank Ms. Rui Yamamura, Ms. Tomoko Tajima, Ms. Mami Uchida, and Ms. Shizue Shimamura for their technical assistance, and Enago (www.enago.jp) for English language review. We also thank Mitchell Arico and H. Nikki March, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Ethical approval
The study protocol was approved by the Human Ethics Review Committee of Kitasato University Medical Center, Japan (approval nos. 29-16 and 29-18).
Funding
T. Otsuka acknowledges support from a Grant-in-Aid for research from the Kitasato University Medical Center (H27-030). T. Fukuyama acknowledges support from ALL KITASATO PROJECT STUDY, JSPS KAKENHI (19K09226), the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private School of Japan, and the Takeda Science Foundation.
Author contributions
Toshikazu Otsuka: data curation, funding acquisition, formal analysis, writing original draft; Takashi Fukuyama: conceptualization, funding acquisition, investigation, methodology, project administration, resources, validation, writing - review & editing; Nobue Futawatari: conceptualization, resources; Kumiko Tahara: methodology, resources; Masaaki Watanabe: methodology, resources; Yoshinobu Ichiki: conceptualization, supervision; Takafumi Soeno: validation, visualization; Yoshihito Takahashi: validation, visualization; Hitoshi Yamazaki: conceptualization, data curation; Yuma Fujimori: writing - review & editing; Taichi Ohshiro: supervision; Noritada Kobayashi: writing - review & editing; Mitsuhiro Kida: supervision; Wasaburo Koizumi: supervision; Chika Kusano: supervision.
Consent for publication
All patients signed an informed consent form prior to resection of the tissue samples used in this study.
Data availability
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Stewart BW WC, editors World Cancer Report 2014 Lyon, France. International Agency for Research on Cancer. 2014.
2. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M. et al. Helicobacter pylori Infection and the Development of Gastric Cancer. New England Journal of Medicine. 2001;345:784-9
3. Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels — “ABC method”. Proceedings of the Japan Academy, Series B. 2011;87:405-14
4. Mizuno S, Miki I, Ishida T, Yoshida M, Onoyama M, Azuma T. et al. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci. 2010;55:3132-7
5. van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B. et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643-7
6. Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer immunity. 2004;4:1
7. Futawatari N, Fukuyama T, Yamamura R, Kobayashi N. Helicobacter Pylori Infection Induces Gastric Cancer and the Cancer/Testis Antigens Expression. J Infect Dis Ther. 2017;5:e1000345
8. Stevanovic S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B. et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356:200-5
9. Shigematsu Y, Hanagiri T, Shiota H, Kuroda K, Baba T, Mizukami M. et al. Clinical significance of cancer/testis antigens expression in patients with non-small cell lung cancer. Lung Cancer. 2010;68:105-10
10. Fukuyama T, Hanagiri T, Takenoyama M, Ichiki Y, Mizukami M, So T. et al. Identification of a new cancer/germline gene, KK-LC-1, encoding an antigen recognized by autologous CTL induced on human lung adenocarcinoma. Cancer Res. 2006;66:4922-8
11. Shida A, Futawatari N, Fukuyama T, Ichiki Y, Takahashi Y, Nishi Y. et al. Frequent High Expression of Kita-Kyushu Lung Cancer Antigen-1 (KK-LC-1) in Gastric Cancer. Anticancer research. 2015;35:3575-9
12. Paret C, Simon P, Vormbrock K, Bender C, Kolsch A, Breitkreuz A. et al. CXorf61 is a target for T cell based immunotherapy of triple-negative breast cancer. Oncotarget. 2015;6:25356-67
13. Fukuyama T, Yamazaki T, Fujita T, Uematsu T, Ichiki Y, Kaneko H. et al. Helicobacter pylori, a carcinogen, induces the expression of melanoma antigen-encoding gene (Mage)-A3, a cancer/testis antigen. Tumour Biol. 2012;33:1881-7
14. Fukuyama T, Futawatari N, Ichiki Y, Shida A, Yamazaki T, Nishi Y. et al. Correlation Between Expression of the Cancer/Testis Antigen KK-LC-1 and Helicobacter pylori Infection in Gastric Cancer. In vivo. 2017;31:403-7
15. Futawatari N, Fukuyama T, Yamamura R, Shida A, Takahashi Y, Nishi Y. et al. Early gastric cancer frequently has high expression of KK-LC-1, a cancer-testis antigen. World J Gastroenterol. 2017;23:8200-6
16. Fukuyama T, Futawatari N, Yamamura R, Yamazaki T, Ichiki Y, Ema A. et al. Expression of KK-LC-1, a cancer/testis antigen, at non-tumour sites of the stomach carrying a tumour. Scientific reports. 2018;8:6131
17. Japanese classification of gastric carcinoma. 3rd English edition. Gastric Cancer. 2011;14:101-12
18. Watari J, Ito C, Shimoda T, Tomita T, Oshima T, Fukui H. et al. DNA methylation silencing of microRNA gene methylator in the precancerous background mucosa with and without gastric cancer: Analysis of the effects of H. pylori eradication and long-term aspirin use. Scientific reports. 2019;9:12559
19. Yoshida T, Kato J, Inoue I, Yoshimura N, Deguchi H, Mukoubayashi C. et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer. 2014;134:1445-57
20. Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Ushiku T. et al. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointestinal endoscopy. 2016;84:618-24
21. Maeda M, Yamashita S, Shimazu T, Iida N, Takeshima H, Nakajima T. et al. Novel epigenetic markers for gastric cancer risk stratification in individuals after Helicobacter pylori eradication. Gastric Cancer. 2018;21:745-55
22. Compare D, Rocco A, Liguori E, D'Armiento FP, Persico G, Masone S. et al. Global DNA hypomethylation is an early event in Helicobacter pylori-related gastric carcinogenesis. J Clin Pathol. 2011;64:677-82
23. Leodolter A, Alonso S, Gonzalez B, Pa Ebert M, Vieth M, Röcken C. et al. Somatic DNA Hypomethylation in H. pylori-Associated High-Risk Gastritis and Gastric Cancer: Enhanced Somatic Hypomethylation Associates with Advanced Stage Cancer. 2015
24. Kobayashi M, Narisawa R, Sato Y, Takeuchi M, Aoyagi Y. Self-limiting risk of metachronous gastric cancers after endoscopic resection. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2010;22:169-73
25. Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S. et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. The Lancet. 2008;372:392-7
Author contact
![]() Corresponding author: Dr. Takashi Fukuyama, Division of Biomedical Research, Kitasato University Medical Centre, 6-100 Arai, Kitamoto, Saitama 364-8501, Japan. Tel: +81-48-593-1236; Fax: +81-48-593-1262; E-mail: fukuyamkitasato-u.ac.jp.
Corresponding author: Dr. Takashi Fukuyama, Division of Biomedical Research, Kitasato University Medical Centre, 6-100 Arai, Kitamoto, Saitama 364-8501, Japan. Tel: +81-48-593-1236; Fax: +81-48-593-1262; E-mail: fukuyamkitasato-u.ac.jp.

 Global reach, higher impact
Global reach, higher impact