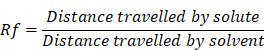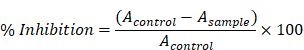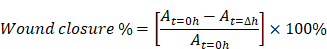Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(3):490-504. doi:10.7150/jca.77110 This issue Cite
Research Paper
Drug Standardization through Pharmacognostic Approaches and Estimation of Anticancer Potential of Chamomile (Matricaria chamomilla L.) using Prostate-Cancer cell lines: An In-vitro Study
1. Department of Ilmul Advia (Pharmacology), RRIUM, University of Kashmir, Srinagar, J&K, India, 190006
2. Department of Moalajat (Medicine), RRIUM, University of Kashmir, Srinagar, J&K, India, 190006
3. Transcriptomics Laboratory (K-Lab), Division of Plant Biotechnology, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar, Srinagar, J&K, India, 190025
4. Mountain Research Centre for Field Crops, Khudwani, Anantnag, 192101, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Srinagar, J&K, India, 192102
5. Division of Forest Products & Utilization, Faculty of Forestry, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Benhama, Ganderbal, J&K, India 191202
6. Allelopathy Laboratory, Department of Botany, Aligarh Muslim University, Aligarh, India 202002
7. Biochemistry and Pathology Lab, RRIUM, Srinagar, University of Kashmir, J&K, India, 190006
8. Phytochemistry lab, RRIUM, Srinagar, University of Kashmir, J&K, India, 190006
9. SMPU Unit RRIUM, Srinagar, University of Kashmir, J&K, India, 190006
10. Division of Agricultural Statistics, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar, Srinagar, J&K, India, 190025
11. Department of Biology, College of Science, Al-Imam Muhammad Ibn Saud Islamic University, Riyadh, Saudi Arabia
12. Department of Pharmacy Practice, College of Pharmacy, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh 11671, Saudi Arabia
13. Basic Health Sciences Department, College of Medicine, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh 11671, Saudi Arabia
14. SAMRC Precision Oncology Research Unit (PORU), DSI/NRF SARChI Chair in Precision Oncology and Cancer Prevention (POCP), Pan African Cancer Research Institute (PACRI), University of Pretoria, Hatfield 0028, South Africa
15. Department of Biotechnology, University of Kashmir, Srinagar, J&K, India, 190006
16. Department of Medical Lab Technology, Indian Institute of Health and Technology (IIHT), Deoband, Saharanpur, UP, India, 247554
17. Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia, 11451
Received 2022-7-14; Accepted 2022-12-25; Published 2023-2-5
Abstract
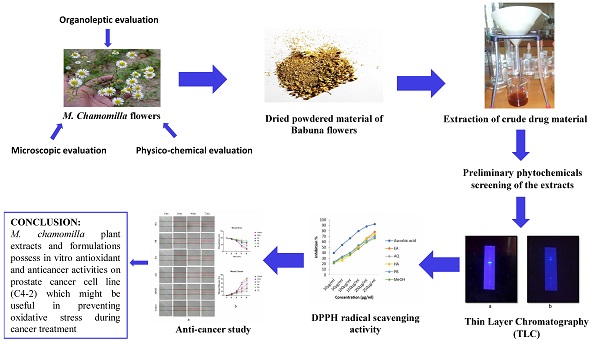
Cancer is the major challenge across world and the adenocarcinoma of prostate malignancy is the second most prevalent male cancer. Various medicinal plants are used for the treatment and management of various cancers. Matricaria chamomilla L., is one of the extensively used Unani medicament for the treatment of various type of diseases. In the current study we evaluated most of the parameters prescribed for drug standardization using pharmacognostic approaches. The 2,2 Diphenyl-1-picryl hydrazyl (DPPH) method was utilized for the analysis of antioxidant activity in the flower extracts of M. chamomilla. Moreover, we analyzed the antioxidant and cytotoxic activity of M. chamomilla (Gul-e Babuna) through in-vitro method. DPPH (2,2-diphenyl-1-picryl-hydrazl-hydrate) method was utilized for the analysis of antioxidant activity in the flower extracts of M. chamomilla. CFU and wound healing assay were performed to determine the anti-cancer activity. The results demonstrated that various extracts of M. chamomilla fulfilled most of the parameters of drug standardization and contained good antioxidant and anticancer activities. The ethyl acetate showed higher anticancer activity followed by aqueous, hydroalcoholic, petroleum benzene and methanol by CFU method. Also, the wound healing assay demonstrated that ethyl acetate extract has more significant effect followed by methanol and petroleum benzene extract on prostate cancer cell line (C4-2). The current study concluded that the extract of M. chamomilla flowers could act as good source of natural anti-cancer compounds.
Keywords: Gul-e-Babuna, Anti-cancer, Antioxidant, Saraṭān, Prostate cancer, CFU, DPPH, TLC, phytomedicine
Introduction
The plant world has provided a never-ending supply of medicinal plants having diverse collection of biological traits and pharmacological uses. Plants have been utilized in many ways for thousands of years, including herbal teas, syrups, infusions, liniments, powder, and so on [1]. According to the WHO, over 21,000 plant species can be utilized for medicinal approaches. Phytomedicine is a well-established and documented practice, and its applications are growing especially for balancing out the effects of contemporary lifestyle and diets. Herbs contain some of the most potent phytochemicals, that have been identified to have curative and preventive abilities synthesize secondary metabolites with various chemical structures including tannins, terpenoids, alkaloids, and flavonoids, which are involved in several therapeutic pharmacological effects due to presence of free radicals. Through various studies, it is found that polyphenols, flavonoids, alkaloids and terpenes present in the herbs act as antioxidant and anticancer agents [2]. They provide nourishment for normal cell growth, repair, impede carcinogens, stimulate the immune system, and act as antioxidants, anti-ageing, and microbicidal agent [3, 4]. German chamomile (Matricaria chamomilla L.) is a well-known specie of Asteraceae family sharing its origin from Mediterranean Basin and Eastern Europe and is grown all over the world. The scientific name "Matricaria" is derived from the Latin word matrix (uterus), referring to its tendency to relax uterine muscles related to mensus and postpartum abnormalities.
Medicinal plants are characterized in priority as an exhaustive source of bioactive compounds that are used in drug development [5, 6]. Prostate cancer affects around one out of every nine men at some point in their lives, and in males prostate cancer is the major cause of mortality. In United States during year 2019, 33,330 deaths were estimated due to prostate cancer and 191,930 new cases were recorded [7]. Males over the age of 50 and African American men are prone to prostate cancer. Out of 10 approximately 6 males above 65 are affected with this cancer, while males below forty are exceptionally uncommon. The average age at which a person is diagnosed is around 66 [7, 8]. There have been no published documents yet for confirming bioactive compounds from nutraceuticals and medicinal plants that have capability of lowering the nuclear localization of AR in CRPC cells directly and efficiently.
M. chamomilla is a vital drug in Unani medicine, and our study was carried out due to use it in the cancer treatment. Its effect on cancer may be due to its anti-inflammatory, demulcent, liquefier of the matters, and relaxant, diaphoretic and relaxant effect internally as well as externally by the mechanism of diversion and evacuation of matters causing the disease. Polyphenols' cytotoxicity on a variety of cancer cells has been established, and their antioxidant characteristics have been determined [9, 10]. Purified flavonoids have demonstrated to have anticancer properties against hepatoma (Hep-G2), cervical carcinoma (Hela), and breast cancer in humans (MCF-7). Flavonoids suppress NF-B expression, which is important for cancer progression, angiogenesis, and proliferation [11]. The most essential constituents of this medicine are, Flavonoids, Sesquiterpenes, Coumarins, Poly acetylenes, phenyl carboxylic acids, Mucins, Amino acids, Choline, Phytosterols, Mineral substances. In chamomile extract eleven bioactive compounds that include herniarin and umbelliferone (coumarin) and apigenin were identified. In M. chamomilla coumarins are delinated by presence of herniarin, umbelliferone and others [12, 13]. So, the bioactive compounds present in Babuna (M. chamomilla) strengthen the hypothesis that this drug may be effective in the case of cancer due to its antioxidant property. The goal of this research was to assess the antioxidant and anti-malignancy activities of M. chamomilla. These goals may open new approaches to volarize sources of this plant species to fight against deadly diseases especially cancer.
Materials and Methodology
Plant Sample collection, identification and authentication
Test drug namely Gul-e-Babuna (M. chamomilla) flowers, were collected from the nearby market. The samples of plants were verified by the University of Kashmir's Center for Biodiversity and Taxonomy and were submitted to the museum of Centre for Biodiversity and Taxonomy under specimen voucher no.
Parameters for drug standardization Macroscopic and organoleptic evaluation
Organoleptic evaluation of the procured samples was done to differentiate them from the related species having similar appearance. Parameters like shape, texture, colour, odour, taste were observed [14]
Microscopic evaluation
Microscopic evaluation aided by the stains allows a more detailed examination of the histological characters of the powdered drug for the correct identification. The flowers of M. chamomilla were crushed into powder form and boiled in solution of chloral-hydrate for 15-20 minutes. Small amount of powdered flower extract was observed under microscopic for various microscopic characters. Both stained and unstained slides were prepared. The stain used was phloroglucinol solution (1-2 drops of 0.1% W/V) and a drop of concentrated HCl to stain lignified cells pink [14].
Physico-chemical evaluation
Certain Physico-chemical investigations including determination of ash values and extractive values were carried out for the formulations prepared [14, 15].
Ash value
The proportion of inorganic substances present in a sample is determined by the ash value. The method was used to ascertain total ash, water-soluble ash and sulphated ash.
Extractive values
The powder formulation of plant medication was air-dried and 5g of this powder was mashed using 100ml alcohol and water sequentially in a closed flask for 24 hours to determine extractive value. During the first 6 hours, the solutions were shaken repeatedly and left undisturbed for 18 hours. After that, filtration was done quickly to avoid solvent loss, followed by drying of, 25ml of filtrate at 50°C d through evaporation using a sunken dish having flat bottom to a consistent weight. At the end of this process, calculations for the alcohol percentage and water-soluble extract with reference to an air-dried drug were performed [15].
Loss on drying
5 gm drug sample (in powdered form) was set on a sunken dish for evaporation at 105°C without being dried first, and the substance was weighed after 6 hours of drying. The drying procedure was continued until the difference between two consecutive weighing was less than 0.25 percent or two consecutive measurements were identical. Constant weight was achieved when the difference between two successive weights was less than 0.01g after drying for 30 minutes in a desiccator [16].
Foaming index
One gram of Babuna flower powder was ground into a coarse powder and placed in a flask with 100 mL of boiling water. The concentrated liquid was made followed by filtration and was poured into 10 stopper vials (height 16 cm, diameter 16 mm) in increments of 1 ml, 2 ml, 3 ml, and so on, with level of liquid in all vials being corrected to 10 ml with water. After covering the test tube with a stopper, it was shaken for 15 seconds lengthwise. After resting the tubes upto 15 minutes, measurement of the foam height was recorded [15].
Swelling index
One gram of finely ground and carefully weighed Babuna flowers were put in a stoppered measuring cylinder of 25 ml to which water was added at the rate of 25 ml, then mixture was vigorously jiggled at an interval of 10 minutes upto1 hour. The cylinder was left at room temperature for 3 hours before measurements were taken. Using 1g of plant material as a reference, the mean of the various readings was calculated [15].
Fluorescence analysis
Many plants glow when cut surfaces or powders are subjected to Ultraviolet light, which may aid for their recognization. Fluorescence of the plant powder (40 mesh) was investigated in natural day light and under Ultraviolet light (254 and 366 nm), as well as after therapy with various chemicals such as picric acid, sodium hydroxide, acetic acid, ferric chloride, nitric acid, iodine, and hydrochloric acid [17].
pH values
For the preparation of solutions, 1g and 10g of the accurately weighed drug was deliquesce in 100 ml of distilled water separately. Then the filtered extract was collected and pH values of solutions (1% and 10%) of M. chamomilla flowers were checked using a standardized pH meter and placed in tabulated form.
Extraction of crude drug material
The dried and coarsely powdered material of Babuna (350gm) flowers were subjected to consecutive extraction in soxhlet extractor using different solvents in ascending order of their polarity e.g., petroleum benzene, ethyl acetate, methanol, hydro alcohol and aqueous. Extraction was performed using continuous hot percolation soxhlation [18].
Preliminary phytochemicals screening of the extracts
The Pet. Benzene (PB), Ethyl acetate (EA), Methanol (MeOH), Hydro-alcohol (HA) and Aqueous (AQ) extracts of Matricaria chamomilla flowers were subjected to phytochemical screening. Phytochemical studies that were accomplished for identifying different constituents contained in the flowers.
Test for alkaloids
Each extract (PB, EA, MeOH, HA and AQ) of the M. chamomilla flowers were assessed for the occurrence of alkaloids using Mayer's test, Tannic acid test, Wagner's test, Dragendroff's test and Hager's Test. Several grams of each extract were mixed in their respective solvents for preparing stock solution.
Tests for glycosides
Each extract of the drug was tested for the availability of glycosides using Borntrager's test, Keller Killiani test and Legal's test.
A few grams of each extract were properly mixed in their respective solvents for preparing stock solution.
Test for tannins
Each extract of the drug was tested for the occurrence of tannin using the Ferric chloride test and Lead acetate test. A few grams of each extract were properly mixed in their respective solvents for preparing stock solution.
Test for carbohydrates
Each drug extract was tested for the availability of carbohydrates using Molisch's Test (for the presence of general sugars), Benedict's test (for reducing sugars), Fehling's test (for reducing sugars) and Barfoed's test (for reducing sugars).
Test for flavonoids
Each drug extract was tested for the availability of flavonoids using the alkaline reagent test and Zinc test.
Test for proteins
Each drug extract was tested for the availability of proteins using the Ninhydrin test and Millon's test.
Test for saponins
Each drug extract was tested for the availability of saponins using the Lead acetate test, Froth test and Foam test.
Test for terpenoids
Each drug extract was tested for the availability of terpenoids using the Salkowski test.
Test for phytosterols
Each drug extract was tested for the availability of phytosterols using the Salkowski test.
Thin Layer Chromatography (TLC)
Ethyl acetate and methanolic extracts from M. chamomilla were subjected to TLC profiling. The samples were soaked in appropriate solvents before being applied to TLC plates via capillary tubes. An appropriate solvent system was developed to act as a mobile phase for these extracts solutions. For ethyl acetate extract of M. chamomilla flower, the solvent system is made up of toluene, ethyl acetate and formic acid (3.5:3.5:1) respectively. The ethyl acetate decoction of M. chamomilla flower was implemented to a TLC plate using an appropriate capillary tube and established TLC plate, which then was independently placed in a TLC chamber for development using solvent system noted above as mobile phase. The TLC plate that was created was air-dried and then viewed in a UV chamber. Spots on the TLC plate were identified and the retention factor was calculated on the plate. The retention factor (Rf) was calculated by the following method.
Similarly, Methanolic extract from M. chamomilla was treated with a distinct solvent system. The extract was applied on a TLC plate, which was then placed separately in a solvent system made up of toluene, ethyl acetate, formic acid (3:4:1) respectively. Spots on both TLC plates were identified and the retention factor was calculated on the plate by the method already mentioned [19].
DPPH radical scavenging activity
According to the method described by Silva and Soysa the DPPH radical scavenging activity of the samples was decided, in which 950l of DPPH solution (100M in absolute methanol) was blended with 50l of varying extract concentrations (10, 50, 100, 150, 200, and 250g/ml). Further, the mixtures were shaken, followed by placing it in dark for 30 minutes and at 517 nm absorbance was measured [20, 21].
The radical scavenging activity was determined using below equation:
where, Acontrol represents the absorbance of control at t=0 min and Asample represents the absorbance of the sample at t=30 mins. For refrence ascorbic acid was used as standard and for each test solution IC50 values were calculated i.e., the concentration essential for inhibiting the formation of 50% DPPH radical.
Anti-cancer study
Cell lines
The C4-2 cell line of prostate cancer was procured from the University of Pittsburg, USA under MTA with the University of Chicago. C4-2 cell line was used in the current study as C4-2 cell line is highly cancerous and shows migration at a faster rate.
Methodology
The following procedures were utilized to test the materials (Medicinal plant, Cell line) used in the study:
Preparing crude plant extracts for cell culture
The dried and powdered flowers were subjected to the Soxhlet method of extraction with petroleum benzene, ethyl acetate, methanol, hydroalcoholic and aqueous solvents at their respective boiling temperatures for 48hrs. The extracts so obtained were dried in a rotatory vacuum evaporator (Perfit, India, Cat no. R300), further weighed and properly mixed in 10ml DMSO (Dimethyl sulphoxide), followed by filter sterilization using 0.2µm nylon filters (HiMedia). The extracts were formatted at a concentration of 12.5mg/ml and preserved in -20°C for rest of the analysis [22].
Cell line establishment
The RPMI media supplemented with 10% FBS, 1% L-glutamine and 100µg/ml penicillin-streptomycin was used for maintaining cell lines in 5% CO2 incubator at 37°C.
Proliferation and migration studies
Proliferation and migration studies were conducted through Colony Formation Unit assay (CFU) and cell migration using wound healing assay [22].
Wound closure was calculated by using formula
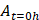 is the area of the wound measured immediately after scratching (t= 0 hour)
is the area of the wound measured immediately after scratching (t= 0 hour)
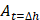 is the area of the wound measured h hours after the scratch is performed
is the area of the wound measured h hours after the scratch is performed
Statistical analysis
Graph Pad 7.0 Prism (Graph Pad, Inc. software) and MS (Microsoft) Excel 2007 were used for statistical analysis and diagrammatic construction. Data was represented as mean +/- SD and statistically significance was determined using ANOVA or Student's t-test as appropriate, three replications were used in the experiment. P values of < 0.05 were referred as significant. CFU and wound healing assays were measured using Image J.
Results
Microscopic evaluation
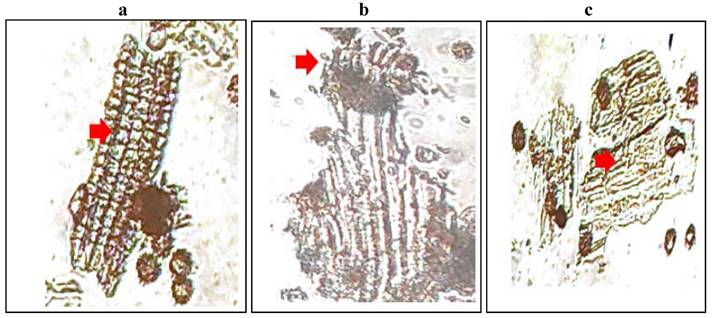
The histological character of M. chamomilla flower powder is shown in Figure 1.
Organoleptic evaluation
The organoleptic characters of dried flowers of M. chamomilla were evaluated and are tabulated in Table 1. From data, it was found that the dried flowers of Babuna were yellow-brownish in colour, moderately sour and specific fragrance and rough texture.
(a) Sclereids from the central region of a bract or palea. (b) Papillose stigma and part of the style in surface view with associated cluster crystals of calcium oxalate. (c) Outer epidermis near the base of the corolla in surface view.
Macroscopic characters of dried flowers of M. chamomilla L
| S. No | Parameters | Matricariachamomilla L. (Dried flowers) |
|---|---|---|
| 1 | Colour | Yellowish brown |
| 2 | Taste | Slightly bitter |
| 3 | Odour | Specific |
| 4 | Texture | Rough |
Physicochemical parameters
Determination of ash values
The overall ash value of plant matter is reported in Table 2. Babuna had a total ash value of 61 percent, acid insoluble ash of 1.8 percent, and sulphated ash value of 6.4 percent, according to data obtained.
Determination of solvent extraction values
Polar elements such as phenols, alkaloids etc. in the plant sample is indicated by the ethanol-soluble extractive values. As shown in Table 2, the ethanol-soluble extractive values of Babuna flowers were 2.8 percent (hot extractive value) and 4.8 percent (cold extractive value). While as the water-soluble extractive values were found to be 11.8% and 15.8% as hot extractive value and cold extractive value respectively.
Losses due to drying
The data pertaining to losses due to drying is tabulated in Table 2. From data, it was found that there was a 6.8% loss on drying of Babuna flowers.
Determination of pH values
The pH values of Babuna flowers were found to be 5.6 and 5.52 for 1% and 10% solution respectively as shown in Table 2.
Determination of swelling and foaming index
The swelling and foaming index of dried flowers of Babuna were found to be 2 (swelling index) and <100 (foaming index).
Analysis for checking powdered drug fluorescence
Fluorescence characteristics of powder formulated drugs are present in Table 3 with various chemical reagents under visible and ultraviolet light.
Phytochemical screening
The analysis of phytochemicals of various solvents extracts of Babuna are tabulated in Table 4. The alkaloids were found in almost all extracts. Anthraquinone glycosides were present in only ethyl acetate, methanolic and hydro-alcoholic extracts while cardiac glycosides were present in only petroleum benzene, ethyl acetate and aqueous extracts. Tannins, carbohydrates, protein and saponins were absent in petroleum benzene extracts. Moreover, the aqueous extract also lacked the presence of tannins. Saponins were only present in hydroalcoholic and aqueous extracts. Moreover, terpenoids and sterols were present in all extracts.
Physicochemical parameters of flowers of M. chamomilla L.
| Ash values | Particulars | Wt. of drug (gm) | Wt. of ash (gm) | % Yield of ash | |
| Total ash value | 5 | 3.05 | 61 | ||
| Acid insoluble ash value | 5 | 0.09 | 1.8 | ||
| Sulphated ash value | 5 | 0.32 | 6.4 | ||
| Solvent extractive values | Solvent | Type of extractive value | Wt. of drug (gm) | Wt. of dried extract (gm) | % Yield of extract (w/w) |
| Ethanol | Hot extractive value | 5 | 0.28 | 2.8 | |
| Cold extractive value | 5 | 0.048 | 4.8 | ||
| Aqueous | Hot extractive value | 5 | 0.118 | 11.8 | |
| Cold extractive value | 5 | 0.158 | 15.8 | ||
| Loss on drying | Part used | Wt. of drug (gm) | Loss on drying (gm) | % Loss on drying | |
| Dried Flowers | 5 | 0.068 | 6.8 | ||
| pH value | Sample | pH | |||
| 1% solution | 5.6 | ||||
| 10% solution | 5.52 | ||||
| Swelling and foaming index | Sample | Swelling index | Foaming index | ||
| Matricaria chamomilla (Dried Flowers) | 2 | <100 | |||
Fluorescence analysis of powdered drug with various chemical reagents under visible light, short and long wavelength.
| Drug Treatment | Day light | UV (254nm) | UV (366nm) |
|---|---|---|---|
| Powder drug+ Distilled water | Light brown | Yellowish | Whitish |
| Powder drug + Conc. HCl | Yellowish green | Green | Dark brown |
| Powder drug +Dil. HCl | Transparent | Light yellow | Milky white |
| Powder drug + Conc. H2SO4 | Dark brown | Brown | Milky white |
| Powder drug + Conc. HNO3 | Light yellow | Yellowish green | Violet |
| Powder drug +chloroform | Transparent | Transparent | Violet |
| Powder drug + 10% NaOH | Yellow | Light green | Milky |
| Powder drug + picric acid | Yellow | Green | Black |
| Powder drug + Methanol | Transparent | Light green | Whitish |
| Powder drug + Ethyl acetate | Transparent | Light green | Transparent |
| Powder drug + glacial acetic acid | Transparent | Transparent | Light milky |
| Powder drug + Pet. Ether | Transparent | Light yellow | Brown |
| Powder drug + 10% Fecl3 | Brown | Green | Black |
| Powder drug + Ammonia solution | Light green | Green | Milky white |
Phytochemical screening of Petroleum benzene, Ethyl acetate, Methanol, Hydro alcoholic, Aqueous extracts of flowers of Matricaria chamomilla L.
| Phytochemicals | Tests | Petroleum benzene | Ethyl acetate | Methanol | Hydro-alcoholic | Aqueous |
|---|---|---|---|---|---|---|
| Alkaloids | Mayer's test | +ve | +ve | +ve | +ve | +ve |
| Hager's test | +ve | +ve | +ve | +ve | -ve | |
| Wagner's test | +ve | +ve | +ve | +ve | -ve | |
| Dragendroff's test | +ve | +ve | +ve | +ve | +ve | |
| Anthraquinone glycosides | Borntrager's test | -ve | +ve | +ve | +ve | -ve |
| Cardiac glycosides | Keller Killiani test. | +ve | +ve | -ve | -ve | +ve |
| Legal's test | -ve | +ve | -ve | -ve | -ve | |
| Tannins | Ferric chloride test | -ve | -ve | +ve | +ve | -ve |
| Lead acetate test | -ve | +ve | +ve | +ve | -ve | |
| Carbohydrates | Molisch's test | -ve | -ve | -ve | +ve | +ve |
| Benedict's test | -ve | +ve | +ve | +ve | -ve | |
| Fehling's test | -ve | -ve | -ve | -ve | -ve | |
| Barford's test | -ve | -ve | -ve | -ve | -ve | |
| Flavonoids | Alkaline reagent test | +ve | +ve | +ve | +ve | +ve |
| Zinc test | -ve | +ve | +ve | +ve | -ve | |
| Proteins | Ninhydrin test | -ve | +ve | -ve | +ve | -ve |
| Millon's test | -ve | +ve | +ve | -ve | +ve | |
| Saponins | Lead acetate test | -ve | -ve | -ve | -ve | -ve |
| Froth test | -ve | -ve | -ve | +ve | +ve | |
| Foam test | -ve | -ve | -ve | -ve | -ve | |
| Terpenoids | Salkowski test | +ve | +ve | +ve | +ve | +ve |
| Sterols | Salkowski test | +ve | +ve | +ve | +ve | +ve |
Thin layer chromatography
TLC profiling of Gul-e-Babuna ethyl acetate extract in the Touluene: ethyl acetate: formic acid (3.5:3.5:1) solvent system indicated four bands with Rf values of 0.11, 0.51, 0.55, and 0.67. Thin layer chromatography of methanolic extract in Touluene: ethyl acetate: formic acid (3:4:1) solvent solution indicated existence of three bands with Rf values of 0.227, 0.606, and 0.636 (Table 5). Figure 2 shows TLC profiling pictures for various extracts (a, b).
TLC profiling images of (a) Ethyl acetate extract. (b) Methanolic extract
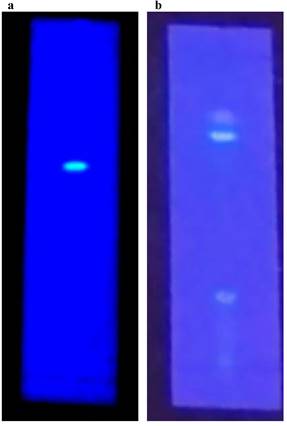
Thin Layer Chromatography (TLC) of EA and MeOH extracts of M. chamomilla flowers.
| Extracts | Solvent system | Number of spots | Rf values | Remarks |
|---|---|---|---|---|
| Ethyl acetate | Touluene: ethyl acetate: formic acid (3.5:3.5:1) | 4 | 0.11, 0.51, 0.55, 0.67 | UV active |
| Methanol | Touluene: ethyl acetate: formic acid (3:4:1) | 3 | 0.227, 0.606, 0.636 | UV active |
DPPH radical scavenging activity
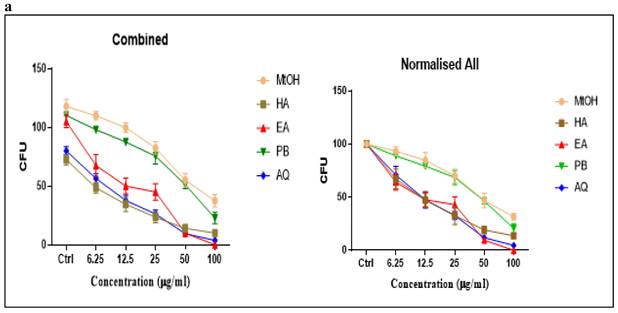
The reduction in absorbance caused by plant antioxidants was used to test the DPPH radical scavenging activity of Petroleum benzene, ethyl acetate, methanol, aqueous, and hydroalcoholic extracts of M. chamomilla flowers. The inhibition of the DPPH free radical was obtained according to dose-dependent manner. The antioxidant activity of all extracts showed an increase as the concentrations increased. When the DPPH radical scavenging activity of M. chamomilla extracts were compared with respect to solvent, reduction ability was found to be much higher in ethyl acetate followed by aqueous extract and there was a slight difference in suppression among hydro alcoholic and petroleum benzene and finally methanolic extract with inhibition reaching up to 48.693 percent, 46.803 percent, 45.903 percent, 46.596 percent, 43.85 percent respectively (Table 6 and Figure 3a, b).
Graphical representation of (a) DPPH radical scavenging activity of Ascorbic acid, PB, EA, MeOH, HA and AQ extracts of Matricaria chamomilla L. flowers. (b) IC50 values of Ascorbic acid, PB, EA, MeOH, HA and AQ extracts of Matricaria chamomilla L.
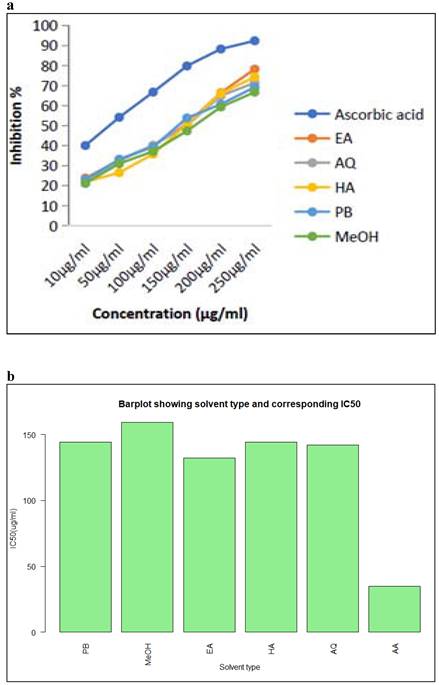
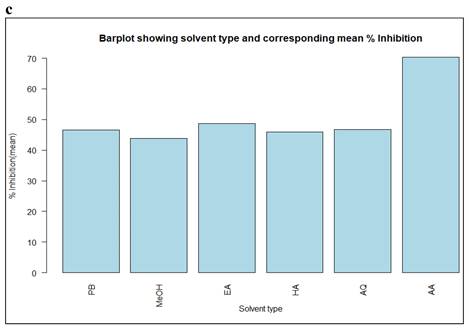
DPPH based % inhibition and IC50 value of Matricaria chamomilla L. flower extracts using different solvents
| Concentration (µg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Solvent type | % Inhibition | Mean | IC50 (µg/ml) | |||||
| 10 | 50 | 100 | 150 | 200 | 250 | |||
| Petroleum benzene | 23.09 | 33.23 | 39.39 | 53.87 | 60.75 | 69.25 | 46.596 | 144.34 |
| Methanolic extract | 21.29 | 31.06 | 37.19 | 47.40 | 59.37 | 66.79 | 43.85 | 159.077 |
| Ethyl acetate extract | 23.84 | 32.59 | 40.02 | 50.94 | 66.50 | 78.27 | 48.693 | 132 |
| Hydro alcoholic extract | 21.90 | 26.57 | 35.82 | 50.69 | 66.15 | 74.29 | 45.903 | 144.241 |
| Aqueous extract | 21.20 | 32.93 | 40.19 | 50.12 | 65.01 | 71.40 | 46.803 | 141.901 |
| Ascorbic acid (Standard) | 40.11 | 54.21 | 66.78 | 79.87 | 88.34 | 92.43 | 70.29 | 34.755 |
Proliferation and Migration studies
Colony Forming Unit Assay (CFU)
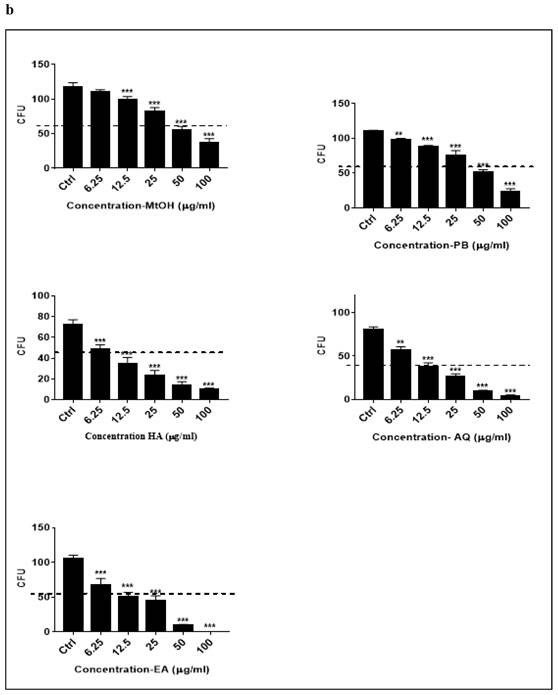
The plant extracts (Petroleum benzene, ethyl acetate, methanol, aqueous and hydroalcoholic) of M. chamomilla flowers inhibited colony-forming units with increasing concentration (6.25µg/ml, 12.5µg/ml, 25µg/ml, 50µg/ml, 100µg/ml). Ethyl acetate (p<0.001) showed best results in C4-2 cell line followed by aqueous extract (p<0.001) then hydro alcoholic (p<0.001), petroleum benzene (p<0.001) respectively and methanolic extract had less effect as compared to others as tabulated in Table 7. Comparison of the inhibition potential of five extracts (different concentrations) on CFU in C4-2 was observed in which ethyl acetate, aqueous extract and hydroalcoholic extracts showed more inhibition with increasing concentrations as shown in Figure 4 (a, b). Dunnett's multiple comparisons test for the effect of different extracts of M. chamomilla flower with control on inhibition of CFU in C4-2 cells is tabulated in Table 8.
Anti-cancer activity of different extracts of Matricaria chamomilla L. flowers by using CFU method
| Concentration (µg/ml) | |||||
|---|---|---|---|---|---|
| Solvent type | CFU quantification | ||||
| 6.25 | 12.5 | 25 | 50 | 100 | |
| Petroleum benzene | 88.903 ± 2.265 | 79.371 ± 2.313 | 68.527 ± 6.801 | 46.478 ± 2.979 | 20.638 ± 4.431 |
| Ethyl acetate extract | 64.193 ± 7.361 | 47.720 ± 7.245 | 42.980 ± 7.561 | 9.560 ± 1.154 | 0 ± 0 |
| Methanolic extract | 93.329 ± 4.133 | 84.779 ± 7.136 | 70.072 ± 6.181 | 46.848 ± 6.814 | 31.675 ± 3.039 |
| Hydro alcoholic extract | 67.244 ± 9.328 | 47.371 ± 6.182 | 32.750 ± 8.516 | 19.187 ± 3.653 | 13.749 ± 1.302 |
| Aqueous extract | 70.886 ±8.181 | 47.397 ± 7.587 | 32.800 ± 3.737 | 11.944 ± 2.136 | 4.686 ± 2.056 |
| DMSO (Control) | - | - | - | - | 100 ± 0.0 |
Dunnett's Multiple Comparison test
| Extracts | Pair Comparison | Mean difference | Significance (P value) |
|---|---|---|---|
| PB | Ctrl vs 6.25 | 12.25 | Yes (0.002) |
| Ctrl vs 12.5 | 22.75 | Yes (<0.001) | |
| Ctrl vs 25 | 34.75 | Yes (<0.001) | |
| Ctrl vs 50 | 59 | Yes (<0.001) | |
| Ctrl vs 100 | 87.5 | Yes (<0.001) | |
| EA | Ctrl vs 6.25 | 37.5 | Yes (0.001) |
| Ctrl vs 12.5 | 55 | Yes (0.001) | |
| Ctrl vs 25 | 60 | Yes (0.001) | |
| Ctrl vs 50 | 95 | Yes (0.001) | |
| Ctrl vs 100 | 105 | Yes (0.001) | |
| Ctrl vs 6.25 | 8 | No (0.13) | |
| Ctrl vs 12.5 | 18.25 | Yes (<0.001) | |
| Ctrl vs 25 | 35.5 | Yes (<0.001) | |
| Ctrl vs 50 | 63 | Yes (<0.001) | |
| MeOH | Ctrl vs 100 | 80.5 | Yes (<0.001) |
| Ctrl vs 6.25 | 24 | Yes (0.0001) | |
| Ctrl vs 12.5 | 38 | Yes (0.0001) | |
| Ctrl vs 25 | 49 | Yes (0.0001) | |
| Ctrl vs 50 | 58.5 | Yes (0.0001) | |
| HA | Ctrl vs 100 | 62.5 | Yes (0.0001) |
| Ctrl vs 6.25 | 23.5 | Yes (<0.001) | |
| Ctrl vs 12.5 | 42.25 | Yes (<0.001) | |
| Ctrl vs 25 | 53.75 | Yes (<0.001) | |
| Ctrl vs 50 | 70.5 | Yes (<0.001) | |
| AQ | Ctrl vs 100 | 76.25 | Yes (<0.001) |
p <0.05, p<0.01, p<0.001, p>0.05 will be considered as significant, highly significant, extremely significant and insignificant respectively compared with control.
Showing Compound formulations of Matricaria Chamomilla (Babuna) with their Ingredients. [ Arzani HMA, NFUM-II, NFUM-VI]
| S. No | Compound Formulation | Ingredients used in compound formulation with their Dosage | Babuna Used as a | Action | Dosage |
|---|---|---|---|---|---|
| Majoon-i-Falasfa | 1. Zanjabeel 35g 2. Filfil 35g 3. Dar-i- Filfil 35g 4. Darchini 35g 5. Amla 35g 6. Post-i-Halela 35g 7. Shitraj Hindi 35g 8. Zarawand-i-Mudhiraj 35g 9. Khussiyatul Salab 35g 10. Maghz-i-chilghoza 35g 11. Bekh-i-babuna 35g 12. Narjeel 35g 13. Gul-i-Babuna 28g 14. Makveez-i-Munaqqa 105g 16. Shehad Musaffa Twice or Thrice of all drugs | 1. Bekh-i-babuna 35g 2. Gul-i-Babuna 28g | 1. Falij (Paralysis) 2. Nisyan (Dementia) | 9-18g Orally | |
| Ayarij-i-loghaziya | 1. Babuna Pahadi 7g 2. Zarawand Mudharaj 7g 3. Bahar Kunda Mushawwa 10.5g 4. Alwa 10.5g 5. Farfiyoon 10.5g 6. Zafran 10.5g 7. Juntiyana 10.5g 8. Fatrasaliyoon 10.5g 9. Ushaq 10.5g 10. Jawsheer 10.5g | Babuna 7g | 1. Dard-i-Khussiyatain (Testicular Pain) 2. Dard-i-Pusht (Backache) | 18g Orally | |
| Majoon-i-Hafiz-ul-Ajsad | 1. Darchini 20g. 2. Post-e-Beikh Kibr 20g. 3. Bisfaij 20g. 4. Izkhar 20g. 5. Zafran 10g. 6. Sumbul-ut-Teeb 40 g. 7. Asaroon 15g. 8. Rewand Chini 15g. 9. Qust Shireen 15g. 10. Majeeth 15g. 11. Nagar Motha 15g. 12. Raughan-e-Babuna 15ml. 13. Qand Safaid 600g. | Roghan-e-Babuna 15ml | 1.Muhallil-i-Waram (Anti-inflammatory) 2.Mudirr-i-Bawl (Diuretic) | 5-10g Orally | |
| Raughan Samaat Kusha Jadeed | 1. Marzanjosh 40g 2. Mako Khushk 40g 3. Lehsun 40g 4. Gul-i-Babuna 40g 5. Barg-e-Neeb 80g 6. Barg-e-Sukhdarshan 80g 7. Barg-e-Tambaku 20g 8. Kundur 20g 9. S indoor 10g 10. Raughan-e-Talkh 40ml 11. Sirka Naishkar 80ml | Gul-i-babuna 40g | 1.Muhallil (Resolvent) 2.Musakkin-i-Dard-i-Uzn (Earache Sedative) | 2 drops in each ear | |
| Qairooti-e-Babuna Wali | 1. Gul-e-Banafsha 100g. 2. Iklil-e-Malik 100g. 3. Babuna 100g. 4. Aab Q.S. 5. Loab-e-Aspghol 50ml. 6. Loab-e-Gul-e-Khatmi 50ml. 7. Raughan-e-Badam Shireen Q.S. 8. Mom Safaid Q.S. | Babuna 100g | 1.Muhallil-i-Waram (Anti-inflammatory) 2.Musakkin (Sedative) | Local application | |
| Qairooti-e-Arad-e-Baqla | 1. Banafsha 100g. 2. Suboos-e-Gandum 100g. 3. Arad-e-Jau 100g. 4. Arad-e-Baqla 100g. 5. Babuna 100g. 6. Gul-i-Khatmi 100g. 7. Iklil-ul-Malik 100g. 8. Raughan-e-Mom 100ml. 9. Katan 100g. 10. Hulba 100g. 11. Aab-e-Karnab Q.S. | Babuna 100g | 1.Muhallil-i-Waram | Local application |
Dose-dependent inhibition of prostate cancer colony formation unit by of Matricaria chamomilla in C4-2 cells. (a) Effect of Matricaria chamomilla on inhibition of CFU in C4-2 cells by quantification of CFU. (b) Comparison of the effect of M. chamomilla extracts on inhibition of colony-forming units in C4-2 cells. Dashed line depicts IC50 value.
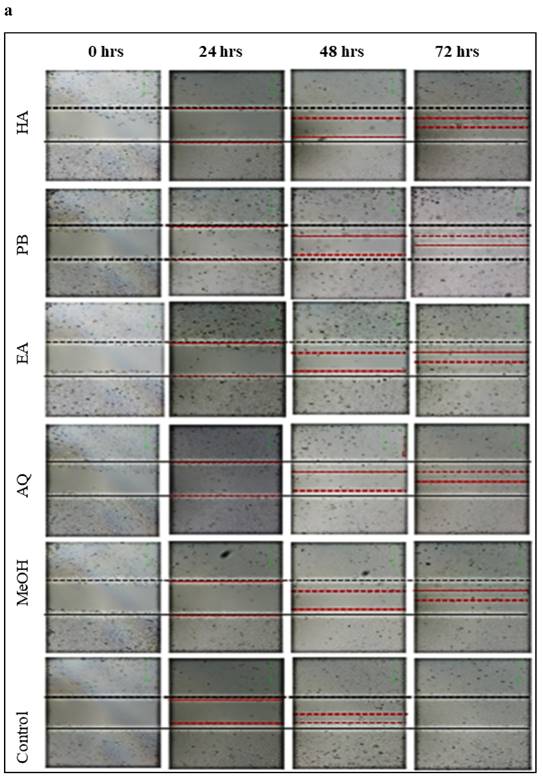
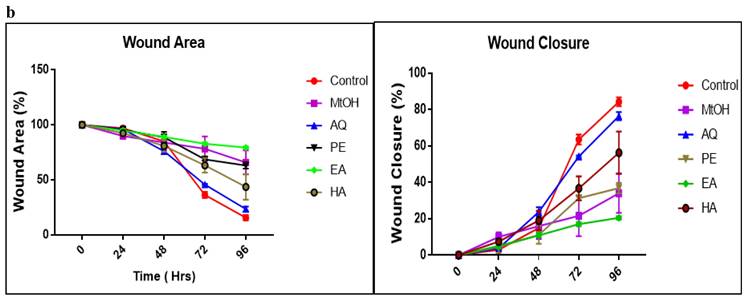
Effect of M. chamomilla flower extracts on prostate cancer cells migration using wound healing assay
On comparing with control group as shown in Figure 5 (a,b), Babuna extracts reduced C4-2 tumor cell showed migration by 70 per-cent at a dosage of 50µg/ml. Gul-i-Babuna (Matricaria chamomilla L.) ethyl acetate extract produced significant results, followed by methanolic extract, with no significant difference between the effects of methanolic extract and petroleum benzene. Whereas the hydroalcoholic and aqueous extracts showed less effect as compared to others. Analysis of variance (ANOVA) in which three replications were carried out for inhibition of wound healing in C4-2 cells tested with plant extracts is tabulated in Table S1 and Dunnett's multiple comparisons test for inhibition of wound healing in C4-2 cells treated with flowers extracts of M. chamomilla is given in Table S2. In this test, at 72 hours all extracts showed a significant effect (p<0.001) except aqueous extract (p< 0.05) which showed less effect as compared to others. The results suggest that the extracts suppress tumor growth in cultured C4-2 cells and their effect does not promote prostate cancer cell migration. After 72 hours, there was no substantial wound healing in the treated cell line, whereas wound closure was close to complete in the untreated cell line (control) Figure 5 (a, b) Table S3-S4. The compound formulations of M. chamomilla viz; Majoon-i-Falasfa, Ayarij-i-loghaziya, Majoon-i-Hafiz-ul- Ajsad, Raughan Samaat Kusha Jadeed, Qairooti-e-Babuna Wali, Qairooti-e-Arad-e-Baqla with detailed ingredients used in these ingredients and their dosage and mode of action has been enlisted in Table 9.
Inhibition of cellular migration/wound healing in C4-2 prostate cancer cells treated with Matricaria chamomilla (a) Confluent C4-2 cells were treated with (Matricaria chamomilla) extract (50µg/ml) after wounding across the cell monolayer with a sterile pipette tip and treated for 72hrs. Cells were imaged at 0, 24, 48, 72 hours after treatment and gap widths were measured. (b) Quantification of gap closure.
Discussion
Herbal medicines and their active ingredients have proved to be powerful medicaments against various cancers. Different plants are eaten up as functional foods as well as medicine, and medicine men, physicians, and scientists claim that they improve health. Medicinal plants such as M. chamomilla, Melissa officinalis L. [Lamiaceae], Taraxacum officinale L. [Asteraceae], Hippophae rhamnoides L. [Elaeagnaceae] may have great importance on human health by exerting antioxidant and anti-cancer effects [23, 24]. Very little information on herbal medicines having a potential effect on prostate cancer is available. According to data, prostate cancer and breast cancer account for the majority of cancer cases in both men and women [22, 25]. Various studies have been carried out on M. chamomilla like anti-inflammatory, antimicrobial, anti-anxiety, anti-oxidant but on prostate cancer, few studies were found [26, 27].
Due to their wide range of advantageous effects, many drug formulations and herbal tea of M. chamomilla source has gained extensive popularity. Nevertheless, the biochemical mechanisms involved for this plant's pharmacological efficacy are still unknown. The current research was carried out to explore the physicochemical parameters, phytochemical screening and in vitro antioxidant and anti-cancer effects present in M. Chamomilla flower extracts. For antioxidant activity, DPPH assay was used and for anti-cancer activity CFU and wound healing assays were used. The results demonstrate that plant extracts possess antioxidant and anticancer activities and this may provide a novel approach to prostate cancer therapeutic strategies.
Antioxidant activity of chamomile extracts
The potential of essential oils to function as free radical scavengers was assessed using the change in absorbance caused by lower DPPH. The extracts' potential to inhibit the DPPH free radical was found to be concentration-dependent. The antioxidant activity of ethyl acetate, aqueous, hydroalcoholic, petroleum benzene, and finally methanol was found to be much higher, with IC50 values of 132, 141.901, 144.241, 144.34, and 159.077, respectively. The anti-free radical and antioxidant action of Chamomile extract (0.2-0.8 mg/mL) was discovered in experiments using sunflower oil as a model system [28].
Anticancer activity of chamomile extracts
For anticancer activity CFU assay was used and the experiment was carried out on Prostate cancer cell line (C4-2) because this cell line is the most carcinogenic and shows high migration rates. Petroleum benzene, ethyl acetate, methanol, hydroalcoholic and aqueous extracts of M. chamomilla flowers were tested on C4-2 which showed a notable decrease in CFU's in a concentration-dependent manner. Ethyl acetate showed significant results in C4-2 cell line followed by aqueous extract then hydroalcoholic, petroleum benzene respectively and methanolic extract has less effect as compared to others. The active constituents of the extracts, that promote inhibition of cancer survival protein expression, are responsible for the decrease in CFUs. To verify the anti-migratory effect of the flower extract of M. chamomilla by the means of the scratch assay, the lowest concentration of 50µg/ml was tested on the C4-2 cell line. It can be observed in Figure 4 that, the extracts at the concentration of 50µg/ml, exhibited an anti-migratory effect on C4-2 cells. In this test at 72 hours, all extracts showed a significant effect (p<0.001) except the aqueous extract (p 0.05) that showed less effect as compared to others, p value range from 0 to 1, represents the statically significance of the results and probability of accepting or rejecting null hypothesis, ability of rejecting null hypothesis occurs and thus results are said to be statically significant. These results are in complete agreement with the studies that showed induction of cell growth inhibition and apoptosis when human prostate cancer PC-3 cells were exposure to aqueous and methanolic chamomile extract [29]. Chamomile exerts selective dose-dependent cytotoxic response towards target cancer cells was also observed by other researchers [30]. The major component of chamomile essential oil (αBisabolol), induced a reduction in cell proliferation and viability in pancreatic cancer cell lines (KLM1, KP4, Panc1, MIA, Paca2). A sesquiterpene (a-Bisabolol) present in chamomile has shown apoptosis in case of carcinoma cell line HepG2 in human liver [31].
Numerous bioactive constituents had been examined in countless medicinal plants due to their usefulness against different cancers such as colorectal cancers. The growth inhibitory activity of chamomile aqueous extract in virally transformed normal human prostate epithelium PZ-HPV-7 cells and other human prostate cancer cells, such as PC-3 and LNCaP cells, have been reported from multiple investigations. The viability of PZ-HPV-7 cells was reduced when they were exposed to chamomile [32]. Another study found that natural compounds from M. chamomilla have anti-proliferative actions against MCF-7 in a dose-dependent manner [30]. In pancreatic cancer cell lines (KP4, MIA, KLM1), α-Bisabolol, a significant component of chamomile essential oil, reduced cell proliferation and viability. The apoptotic impact of -Bisabolol, a sesquiterpene found in chamomile, on the human liver cancer cell line HepG2 was also discovered [33]. Cell growth inhibition in several solid tumours and haematological malignancies by Apigenin has been shown. Its anti-cancer capabilities have been researched in vitro and in vivo extensively. It suppresses human prostate cancer and leukaemia development, arrests the cell cycle, and induces apoptosis through enhancing gap junction intercellular communication [13].
Phytochemical evaluation
In the phytochemical evaluation, it was observed that Matricaria chamomilla L.flowers contain alkaloids, polyphenols (flavonoids and tannins), terpenoids, sterols etc. which can be the reason for cytotoxic and antioxidant properties in this wonderful herb. Flavonoids are primarily responsible for antioxidant and cytotoxic properties that help in protecting the cells from genetic mutation, oxidative damage, and eventually cancer. Many researchers have found that flavonoids suppress NF-B expression, which is important for cancer progression, angiogenesis, and proliferation. [11] thus, presence of flavonoids in this herb makes it a potential source for drug designing in various cancers. The ability of plant polyphenols to inhibit the growth of tumor cells due to interference with proteins found in tumor cells was also noted. Polyphenol can influence acetylation, methylation, or phosphorylation by interacting directly with cancer agents was reported by researchers [9, 10] thus favoring the exploration of M. chamomilla in cancer treatments.
Conclusion
Based on the findings from the current study, the conclusion can be drawn that M. chamomilla plant extracts and formulations possess in vitro antioxidant and anticancer activities on prostate cancer cell line (C4-2) which might be useful in preventing oxidative stress during cancer treatment. The current study also validates the claims of Unani physicians that Gul-e-Babuna (M. chamomilla) can be used in prostate cancer treatment, but it needs further investigation and clinical studies for validation of anticancer effect in humans. In future, if phytoconstituents responsible for the anticancer effect on prostate cancer cell line are identified and isolated it could lead to the development of a novel natural remedy against prostate cancer. Therefore, Babuna flowers appear to be a rich source of a drug candidate that can restrict growth of prostate cancer cells. In a nutshell, the presented results support further investigation of Babuna flowers as an evolving remedial agent with high efficiencies for treatment of various cancerous conditions.
Supplementary Material
Supplementary tables.
Abbreviations
DPPH: 2,2 Diphenyl-1-picryl hydrazyl; CFU: Colony Forming Units; DMSO: Dimethyl Sulfoxide; TLC: Thin Layer Chromatography; ROS: Reactive Oxygen Species; GFP: Green Fluorescent Protein; AR: Androgen Receptor.
Acknowledgements
The authors thank SKUAST-Kashmir, Shalimar for providing facilities for the current investigation. The authors thank CCRUM, New Delhi for providing research grant No. F.No.8-1/2017-CCRUM/Estt. The study was also funded by Science and Engineering Research Board, Department of Science and Technology, Government of India under SERB project no. ECR/2016/000025 (2016-2019) as Early Career Research Award (ECRA) awarded to Dr. Khalid Z. Masoodi (K-Lab, SKUAST-Kashmir). Moreover, the instruments used in the study were procured under SERB research grant no. EMR/2016/005598 (K-Lab). The current work was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R91), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia and South African Medical Research Council (SAMRC) Grant Number 23108, and the National Research Foundation (NRF) Grant Number 138139.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Saad B. Greco-Arab and Islamic diet therapy: Tradition, research and practice. Arabian Journal of Medicinal and Aromatic Plants. 2015;1:2-23
2. Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ali B, Shah SA. et al. Plant-derived anticancer agents: A green anticancer approach. Asian Pacific Journal of Tropical Biomedicine. 2017;7:1129-50
3. Kalim MD, Bhattacharyya D, Banerjee A, Chattopadhyay S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complementary and alternative medicine. 2010;10:1-11
4. Njeru SN, Matasyoh J, Mwaniki CG, Mwendia CM, Kobia K. A Review of some phytochemicals commonly found in medicinal plants. Int J Med Plant. 2013;105:135-40
5. Bourhia M, Bari A, Ali SS, Benbacer L. Phytochemistry and toxicological assessment of Bryonia dioica roots used in north-African alternative medicine. Open Chemistry. 2019;17:1403-11
6. Amrati FE-Z, Bourhia M, Saghrouchni H, Slighoua M, Grafov A, Ullah R. et al. Caralluma europaea (Guss.) NE Br.: Anti-inflammatory, antifungal, and antibacterial activities against nosocomial antibiotic-resistant microbes of chemically characterized fractions. Molecules. 2021;26:636
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7-30
8. Rawla P. Epidemiology of prostate cancer. World journal of oncology. 2019;10:63
9. Azmi AS, Bhat SH, Hanif S, Hadi S. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for anticancer properties. FEBS letters. 2006;580:533-8
10. Siriwatanametanon N, Fiebich BL, Efferth T, Prieto JM, Heinrich M. Traditionally used Thai medicinal plants: in vitro anti-inflammatory, anticancer and antioxidant activities. Journal of ethnopharmacology. 2010;130:196-207
11. Wen L, Wu D, Jiang Y, Prasad KN, Lin S, Jiang G. et al. Identification of flavonoids in litchi (Litchi chinensis Sonn.) leaf and evaluation of anticancer activities. Journal of functional foods. 2014;6:555-63
12. Singh O, Khanam Z, Misra N, Srivastava MK. Chamomile (Matricaria chamomilla L.): an overview. Pharmacognosy reviews. 2011;5:82
13. Fajemiroye J, Ferreira N, de Oliveira L, Elusiyan C, Pedrino G, da Cunha L. et al. Matricaria recutita and its isolate-apigenin: economic value, ethnopharmacology and chemico-biological profiles in retrospect. J Pharma Phytochem. 2016;4:17-31
14. Radhika B, Begum N, Srisailam K. Pharmacognostic and preliminary phytochemical evaluation of the leaves of Bixa orellana. Pharmacognosy Journal. 2010;2:132-6
15. Organization WH. Quality control methods for herbal materials: World Health Organization; 2011
16. Gupta AK, Khan M, Khan D. Physicochemical study of Delphinium denudatum Wall (Ranunculales: Ranunculaceae) and their antioxidant activity. Brazilian Journal of Biological Sciences. 2019;6:161-9
17. Chase Jr CR, Pratt R. Fluorescence of powdered vegetable drugs with particular reference to development of a system of identification. Journal of the American Pharmaceutical Association. 1949;38:324-31
18. Chaudhari R. Herbal drug industry. Eastern Publication. 1996:498-9
19. Pawar H, Karde M, Mundle N, Jadhav P, Mehra K. Phytochemical evaluation and curcumin content determination of turmeric rhizomes collected from Bhandara District of Maharashtra (India). Med Chem. 2014;4:588-91
20. Silva IK, Soysa P. Evaluation of phytochemical composition and antioxidant capacity of a decoction containing Adenanthera pavonina L. and Thespesia populnea L. Pharmacognosy Magazine. 2011;7:193
21. Haq SAU, Mir MA, Lone SM, Banoo A, Shafi F, Mir SA, et al. Explicating genetic diversity based on ITS characterization and determination of antioxidant potential in sea buckthorn (Hippophae spp.). Molecular Biology Reports. 2021: 1-12
22. Masoodi KZ, Wani W, Dar ZA, Mansoor S, Anam-ul-Haq S, Farooq I. et al. Sea buckthorn (Hippophae rhamnoides L.) inhibits cellular proliferation, wound healing and decreases expression of prostate specific antigen in prostate cancer cells in vitro. Journal of Functional Foods. 2020;73:104102
23. Nahata A. Anticancer agents: a review of relevant information on important herbal drugs. Int J Clin Pharmacol Toxicol. 2017;6:250-5
24. Alam A, Ahmed S, Alam T, Azeez A. Cancer (Sartan) and its management in Unani (Greco-Arab) system of medicine. International journal of pharmamedix India. 2013;1:612-30
25. Hassanpour SH, Dehghani M. Review of cancer from perspective of molecular. Journal of Cancer Research and Practice. 2017;4:127-9
26. Sharma S, Agarwal N. A review on herbs with antidepressant properties. International Journal. 2011;2:2229
27. Domínguez F, Alonso-Castro AJ, Anaya M, González-Trujano ME, Salgado-Ceballos H, Orozco-Suárez S. Mexican Traditional Medicine: Traditions of yesterdey and Phytomedicines for Tomorrow. Therapeutic Medicinal Plants: From Lab to the Market, CRC Press, Boca Raton, Florida. 2015:10-46
28. Sazegar M, Banakar A, Bahrami N, Bahrami A, Baghbani M, Nematolahi P. et al. The antioxidant activity of chamomile (Matricaria chamomilla L.) extract in sunflower oil. World Applied Sciences Journal. 2010;9:873-8
29. Srivastava JK, Gupta S. Extraction, characterization, stability and biological activity of flavonoids isolated from chamomile flowers. Molecular and Cellular Pharmacology. 2009;1:138
30. Matić IZ, Juranić Z, Šavikin K, Zdunić G, Nađvinski N, Gođevac D. Chamomile and marigold tea: Chemical characterization and evaluation of anticancer activity. Phytotherapy research. 2013;27:852-8
31. Chen W, Hou J, Yin Y, Jang J, Zheng Z, Fan H. et al. α-Bisabolol induces dose-and time-dependent apoptosis in HepG2 cells via a Fas-and mitochondrial-related pathway, involves p53 and NFκB. Biochemical pharmacology. 2010;80:247-54
32. Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. Journal of agricultural and food chemistry. 2007;55:9470-8
33. Ali EM. Phytochemical composition, antifungal, antiaflatoxigenic, antioxidant, and anticancer activities of Glycyrrhiza glabra L. and Matricaria chamomilla L. essential oils. Journal of Medicinal Plants Research. 2013;7:2197-207
34. Arzani HMA. Qarabadeen-i-qadri (Urdu Translation). New Delhi. CCRUM Ministry of Health and Family Welfare Government of India; 2009:16, 88.
35. Anonymous. National Formulary of Unani Medicine. Part II, Vol I. New Delhi CCRUM, Ministry of Health and Family Welfare, Government of India. 2007:71-72 136-137
36. Anonymous. National Formulary of Unani Medicine. Part VI. New Delhi CCRUM, Ministry of Health and Family Welfare, Government of India. 2011:89-90
Author contact
![]() Corresponding authors: Dr. Khalid Z. Masoodi, PhD: masoodikzac.in; Dr. Mohd Afsahul Kalam: afsahniumcom; Dr. Shabir Hussain Wani: shabirhussainwanicom; Dr. Zodwa Dlamini: zodwa.dlaminiac.za; Dr. Shahanavaj Khan: sdkhanedu.sa
Corresponding authors: Dr. Khalid Z. Masoodi, PhD: masoodikzac.in; Dr. Mohd Afsahul Kalam: afsahniumcom; Dr. Shabir Hussain Wani: shabirhussainwanicom; Dr. Zodwa Dlamini: zodwa.dlaminiac.za; Dr. Shahanavaj Khan: sdkhanedu.sa

 Global reach, higher impact
Global reach, higher impact