3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(4):634-645. doi:10.7150/jca.81069 This issue Cite
Research Paper
A mechanism study of DUSP1 in inhibiting malignant progression of endometrial carcinoma by regulating ERK/AP-1 axis and dephosphorylation of EPHA2
1. Department of Gynecology and Obstetrics, Central Laboratory & Institute of Clinical Molecular Biology, Peking University People's Hospital, No.11 Xizhimen South Street, Beijing 100044, China.
2. Department of Gynecology and Obstetrics, The Third Affiliated Hospital of Zhengzhou University, No.3 Kangfu Middle Street, Zhengzhou 450052, China.
Abstract
Background: Endometrial carcinoma is one of the most common female malignancies worldwide. Based on our preliminary investigation, DUSP1 was identified as a potential biomarker for endometrial carcinoma prognosis, but its function and mechanism remained unclear.
Methods: In this study, genes highly correlated with DUSP1 in endometrial cancer were found through correlation analysis, and the promoter sequence of DUSP1 was analyzed by PROMO program. Next-generation phosphorylation mass spectrometry was used to explore new downstream target proteins and pathways of DUSP1 in endometrial carcinoma. The mRNA and protein expression levels were detected by real-time quantitative PCR, immunohistochemistry and Western blotting. The cell survival and proliferation were analyzed by CCK8 assay, cell apoptosis was analyzed by Annexin-V-APC and PI dual staining assay, and the cell invasion was analyzed by Transwell method.
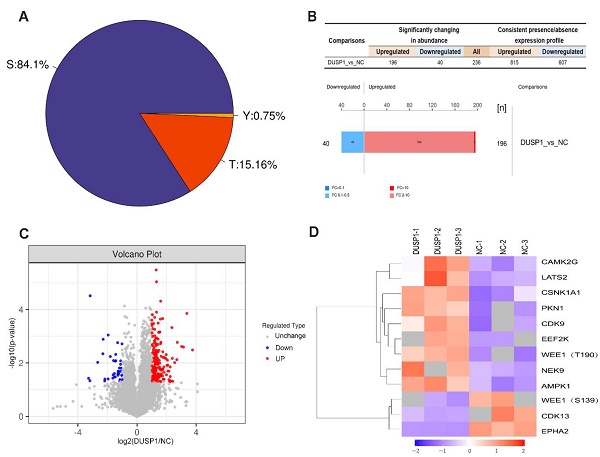
Results: (1) There was a high correlation between the expression of DUSP1 and the genes involved in AP-1 complex and its co-expression network. (2) Promoter sequence analysis predicted that the members of AP-1 complex might be the upstream transcriptional regulators of DUSP1. (3) Transfection experiments proved DUSP1 can inhibit tumor growth and invasion, and promote apoptosis by regulating ERK pathway. (4) The results of phosphorylation mass spectrometry showed that overexpression of DUSP1 mainly dephosphorylated EPHA2 in endometrial carcinoma, and co-immunoprecipitation verified the protein interaction between DUSP1 and EPHA2. (5) Overexpression or knockdown of EPHA2 significantly changed the phosphorylation level of EPHA2. (6) The expression of EPHA2 protein was high in patients with more aggressive endometrial cancer. (7) Using EPHA2 inhibitor could significantly slow down the growth rate of tumor cells.
Conclusion: (1) There exists a mutual regulation relationship between DUSP1 and AP-1 co-expression network in endometrial carcinoma. (2) It is reported for the first time that DUSP1 phosphatase acts on the ser899 site of EphA2 in endometrial carcinoma. (3) DUSP1 can inhibit tumor growth and invasion, and promote apoptosis by regulating MAPK pathway through directly dephosphorylating ERK, or by dephosphorylating EPHA2.
Keywords: Endometrial carcinoma, DUSP1, AP-1 complex, MAPK pathway, EPHA2.

 Global reach, higher impact
Global reach, higher impact