3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(6):952-965. doi:10.7150/jca.77199 This issue Cite
Research Paper
The Real-world Therapeutic Analysis of First-line Immunotherapy in Chinese Patients with Drive Gene Positive for Advanced Non-Small Cell Lung Cancer
1. Department of Oncology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
2. The Clinical Medical College, Shandong First Medical University (Shandong Academy of Medicine), Jinan, Shandong, China
3. Department of Oncology, The Second Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
4. Department of Oncology, Shandong Provincial Hospital Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
5. Department of Clinical Laboratory, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
6. Department of Pathology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
7. Department of Pathology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
* These authors have contributed equally to this work and share first authorship
Abstract
Background: Immune checkpoint inhibitors (ICIs) are widely used for treating advanced non-small cell lung cancer (NSCLC). However, some studies indicate that patients with genetic mutations do not benefit from immunotherapy. Hence, this study explored the efficacy of anti-programmed death-1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) antibodies in the first-line treatment of advanced NSCLC with driver gene mutations in real-world settings.
Methods: We retrospective analyzed patients with advanced NSCLC who treated with first-line anti-PD-1/PD-L1 antibodies at Shandong Provincial Hospital between May 2019 and October 2020. The patient's driver gene mutation status was identified using amplification refractory mutation system PCR (ARMS-PCR). The basic clinical characteristics, objective response rate (ORR), progression free survival (PFS), and other clinical data of patients were collected to evaluate the clinical efficacy and potential prognostic factors of treatment for patients with driver gene mutations.
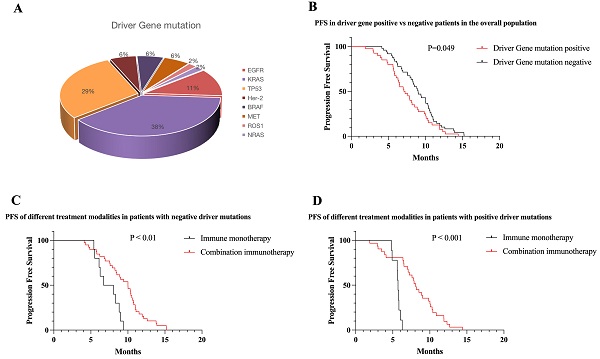
Results: A total of 430 patients' information was counted during this period, finally, 89 patients with NSCLC were enrolled in the study. The main pathological subtype of patients was adenocarcinoma (62.9%). The overall mutation rate was 44.9% (n = 40) and included following mutations: KRAS (n = 20), TP53 (n = 18), EGFR (n = 6), BRAF (n = 3), Her-2 (n = 3), MET (n = 3), ROS1 (n = 1), and NRAS (n = 1). The overall ORR was 44.30% and the disease control rate (DCR) was 82.23%. At the time of follow-up cut-off, the median PFS of all patients was 8.2 month. In NSCLC patients treated with ICI, median PFS was longer in mutation-negative patients than in mutation-positive patients (8.98 vs 7.07 months, P < 0.05). Survival benefit varied across mutational subgroups: KRAS patients could benefit from first-line immunotherapy (10.1 months, P < 0.05), patients with EGFR mutations have poor first-line immunotherapy outcomes, with a median PFS of only 3.0 months (P < 0.01), and patients with other mutation types having no significant difference in response from mutation-negative patients. In most mutation subgroups, immune combination therapy had longer PFS than immune monotherapy, and PD-L1 expression levels were positively correlated with clinical benefit in patients.
Conclusion: In the real world, patients with KRAS mutations benefit from first-line immunotherapy, immune-combination modalities are more effective, and immune efficacy is positively correlated with PD-L1 expression; Patients with other driver mutations (BRAF, NRAS, Her2, MET, ROS1) benefit similarly to mutation-negative patients in first-line immunotherapy, and immunotherapy is recommended for first-line therapy; Immunotherapy is worse effective in patients with EGFR mutations, immunotherapy is not recommended in first-line therapy even patients with high PD-L1 expression.
Keywords: Non-small cell lung cancer, Driver Gene mutation, Immune checkpoint inhibitor, Survival analysis

 Global reach, higher impact
Global reach, higher impact