Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(6):966-980. doi:10.7150/jca.80456 This issue Cite
Review
Advances in the Mechanism of Luteolin against Hepatocellular Carcinoma Based on Bioinformatics and Network Pharmacology
1. The Affiliated People's Hospital of Inner Mongolia Medical University/Inner Mongolia Autonomous Region Cancer Hospital, Hohhot 010050, China.
2. Department of Pharmacy, Inner Mongolia Medical University, Hohhot 010110, China.
3. Department of Pharmacy, Traditional Chinese Medicine Hospital of Inner Mongolia Autonomous Region, Hohhot 010020, China.
4. Department of Medicine, Ordos Institute of Technology, Inner Mongolia Autonomous Region, Ordos 017000, China.
5. Department of Urology, The Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010050, China.
# These authors are equal contributors to this review.
Received 2022-11-3; Accepted 2023-3-3; Published 2023-4-9
Abstract
As one of the most common malignant tumors, hepatocellular carcinoma (HCC) has a rising incidence rate and also seriously endangers human life and health. According to research reports, hepatitis B, hepatitis C, intake of aflatoxin in the diet, and the effects of alcohol and other chemicals can induce an increase in the incidence of liver cancer. However, in the current clinical treatment of HCC, most of the drugs are chemical drugs, which have relatively large side effects and are prone to drug resistance. Therefore, the development of natural compounds to treat HCC has become a new treatment strategy. Several studies have shown that flavonoids have shown outstanding effects and exhibit strong tumor growth inhibitory effects in vivo experimental studies. Luteolin, as a natural flavonoid, has anti-tumor, anti-inflammatory, anti-viral, anti-oxidation, immune regulation, and other pharmacological effects. The anti-cancer mechanism of luteolin mainly directly acts on tumor cells to inhibit their growth, induce cell apoptosis, reduce tumor tissue angiogenesis, regulate long non-coding RNA, affect immunogenic cell death, and regulate autophagy. As well as improving the curative effect of radiotherapy and chemotherapy and chemoprevention. In this study, we evaluated the function of luteolin in regulating cancer cell proliferation, migration, and invasion will summarize and analyze luteolin and its mechanism of regulating HCC to improve the role of luteolin in the clinical prevention and treatment of HCC.
Keywords: Luteolin, Hepatocellular carcinoma, Flavonoids, Mechanism
Introduction
Hepatocellular carcinoma is the deadliest and most common type of liver cancer, ranking sixth in the world in incidence and second in mortality [1,2]. According to the GLOBOCAN 2020 database, it is estimated that there are 905,677 new liver cancer cases and about 830,180 death [3]. This also shows that the mortality rate of liver cancer is gradually approaching the incidence rate. At this stage, the clinical early treatment of hepatocellular carcinoma (HCC) mainly consists of immunotherapy, surgical resection, radiofrequency ablation, and targeted therapy [4-7]. Patients with early liver cancer can also be effectively treated by surgery, but many patients lose the best opportunity for surgical treatment because hepatocellular carcinoma is an invasive disease with a poor prognosis [8]. It's worth noting that, flavonoids play an excellent anti-tumor effect, participate in and regulate the expression of a variety of tumor miRNAs, inhibit tumor cell mitosis, induce apoptosis, participate in immune responses, as well as inhibit the process of tumorigenesis through a variety of signal pathways [9-11]. For example, luteolin, a derivative of flavonoids, is mainly found in fruits, vegetables, and natural plants and can be isolated from a variety of traditional Chinese medicines [12]. Luteolin also can regulate miRNA expression in different cancer to affect the cancer progression. At present, LUT has a good anti-cancer application prospect, and its derivatives have potential anti-cancer effects, and Ma Jun, Yoo Ho Soo, and others have proved that luteolin can affect the survival of a variety of cancer cells such as gastric cancer and colon cancer [13,14]. Nevertheless, more studies are needed to provide a better understanding of the mechanism of cancer treatment using luteolin. For this reason, based on the structure and pharmacological effects of luteolin, this article explored and reviewed the mechanism of luteolin's anti-growth, proliferation, apoptosis, anti-oxidative stress, angiogenesis, and related molecular signaling pathways of hepatocellular carcinoma, to provide new strategies for the treatment of hepatocellular carcinoma.
The pharmacological activity of luteolin
The structure of flavonoids
Flavonoids are a class of planting metabolites with pharmacological activity. It is a compound with 2-phenyl chromone as the core. It generally refers to a series of compounds formed by connecting two benzene rings (A and B rings) with phenolic hydroxyl groups through the central 3 carbon. The basic skeleton is C6-C3-C6, mainly found in the natural plant kingdom [15]. According to the characteristics of the core structure, the structure of flavonoids can be modified by different substituents to obtain discrete derivatives, resulting in different properties [7,16]. The main structural types of its derivatives are: including flavones, flavonols, isoflavones, flavanones, flavanonols, dihydro isoflavones Dihydro-isoflavones, flavan-3-ols, flavan-3,4-diol, anthocyanidins, chalcones (chalcones) and dihydrochalcones (dihydrochalcones), etc. (Table 1).
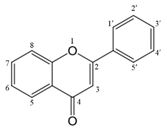
The chemical structure of luteolin (3',4',5,7-tetrahydroxyflavonoids, Luteolin, LUT) (Figure 1), which is a representative derivative of dihydroflavonoids, mainly found in plants such as Verbenaceae, Apiaceae and other natural medicinal materials and vegetables [17]. In recent years, research on the structure of Luteolin has shown that the structure of LUT contains two aromatic rings (ring A and ring B) connected through three carbon atoms in the center [18]. Therefore, the structural characteristics of LUT also determine the nature of its action. Several pharmacological studies have proved that LUT has a wide range of biological activities, including anti-inflammatory, anti-allergic, anti-cancer, antioxidant, or pro-oxidant. In recent years, flavonoids have played a major role in tumor treatment, and luteolin as a flavonoid derivative has also received research attention [19]. Just as Kapoor S [20] and Lee EJ [21] proposed that luteolin can inhibit EGF-mediated MAPP, ERK, and AKT signal pathways by reducing the expression of epidermal growth factor (EGF) mRNA.
The chemical structure of luteolin. It is a compound with 2-phenyl chromone as the core. It refers to a series of compounds formed by connecting two benzene rings (A and B rings) with phenolic hydroxyl groups through the central 3 carbon. The basic skeleton is C6-C3 -C6.

The pharmacological effects of luteolin
The effect of LUT on scavenging free radicals and anti-oxidation
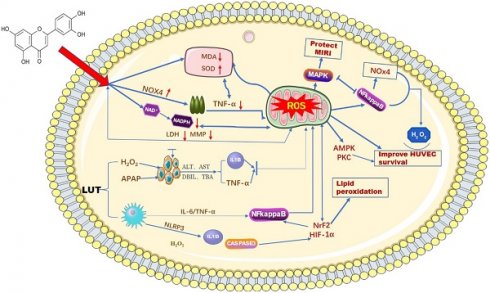
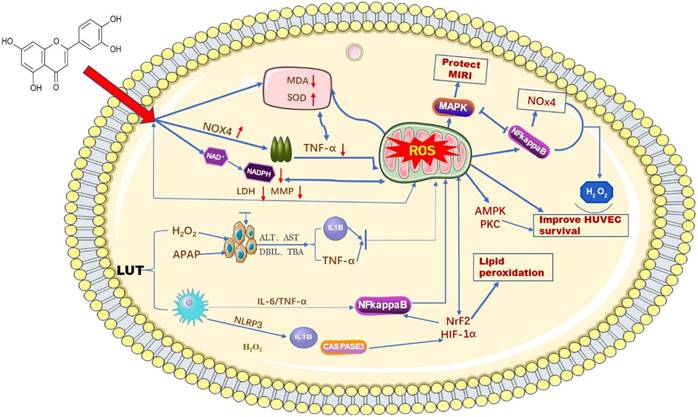
ROS, as important molecules that induce oxidative stress in the body, can be generated in hepatocytes and macrophages when cells undergo an inflammatory response [22]. The imbalance of stromal cells, the immune microenvironment, and some other biological pathways are also closely associated with ROS [23]. Luteolin, as a natural flavonoid compound, contains a certain number of phenolic hydroxyl groups in its structure, which provides it with strong reducibility and antioxidant properties. Luteolin exerts an antioxidant effect on biological systems by directly inhibiting the formation of reactive oxygen species, activating antioxidant enzymes, and promoting antioxidant defenses [24,25]. Balanchine [26] et al. demonstrated that luteolin can stabilize the leakage of intracellular antioxidant defense systems GSH, CAT, and SOD and reduce the activity of MDA, thus reducing the production of ROS and ultimately protecting mitochondria from damage in cardiac myocytes. According to reports. H2O2 can induce oxidative stress in human umbilical vein endothelial cells (HUVECs), and the production of ROS superoxide can be reduced after an intervention by luteolin [27]. At the same time, luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation through its effects on Nox4/ROS-NF-κB and MAPK pathways [28]. Research data show that luteolin has been found to reduce the generation of ROS by reducing the leakage of LDH and the reduction of mitochondrial membrane potential, thereby alleviating OTA-induced oxidative stress and lipid peroxidation through NrF2/HIF-1α [29]. Prateheshkumar [30]. Showed that luteolin inhibits ROS production, NADPH oxidase (NOX) activation, glutathione consumption, and lipid peroxidation in a dose-dependent manner (Figure 2).
The structure of flavonoids and their derivatives
| Flavonoid core | Modification site | Substituent type | Derivative structure | Derivative name |
|---|---|---|---|---|
 | 3 | 3=-OH |  | Flavonols |
| 3 | 3=  | 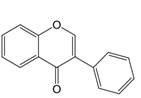 | Isoflavones | |
| 2,3 | 2=H 3=H | 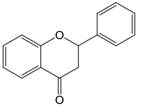 | Dihydroflavonoids | |
| 2,3 | 2=H 3=-OH | 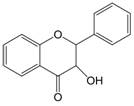 | Dihydroflavonol | |
| 2,3 | 2=H 3= 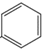 | 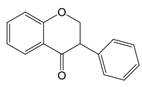 | Dihydroisoflavones | |
| 3,4 | 3=-OH 4=C=O→CH | 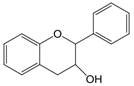 | Flavan-3-ols | |
| 3,4 | 3=-OH 4=C=0→C-OH | 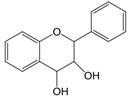 | Flavan-3,4-diols | |
| 3,4 | 3=-OH 4=C=0→CH= | 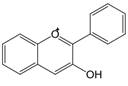 | Anthocyanins | |
| 1 | 1=-O-→-OH |  | Chalcones | |
| 1,2 | 1=-O-→-OH 2=CH2 |  | Dihydrochalcones |
Schematic diagram of the influence of LUT on oxygen free radicals. Luteolin has a wide range of anti-cancer effects. Among them, it can affect the content of ROS in mitochondria by reducing various biochemical indicators and signaling factors in liver cancer cells.
Influence of LUT on inflammation
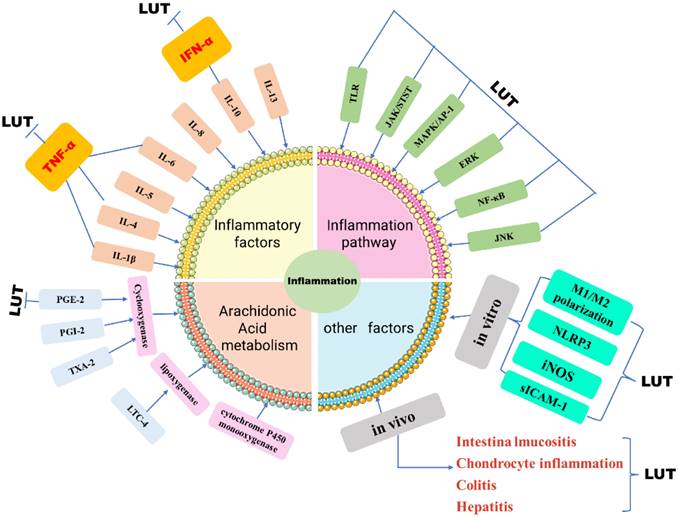
Part of the anti-inflammatory effect of luteolin is achieved by regulating inflammatory mediators, and it has been demonstrated to regulate inflammatory factors and inflammatory mediators in a variety of in vivo and in vitro models [31] (Figure 3). Luteolin can inhibit IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, TNF-α, interferon (IFN)-β and granulocyte-macrophages Cell colony-stimulating factor (GM-CSF), and can increase the level of anti-inflammatory cytokine IL-10 [32]. In addition, luteolin can also inhibit chemokines that can control the migration and localization of immune cells such as CCL2, CXCL2, CXCL8, and CXCL9 [33]. And by regulating diverse signaling pathways, such as nuclear transcription factor NF-κB, MAPK/AP-1, JAK-STAT, and TLR signaling pathways, etc [34]. At the same time, Cho Young [35]. Concluded that luteolin can reduce the production of NO by reducing the synthesis of nitric oxide synthase (iNOS). In macrophages induced by lipopolysaccharide plus interferon-γ stimulation and interleukin 4 (IL-4) stimulation, luteolin changed the M1/M2 polarization of macrophages and was down-regulated by p-STAT3 the up-regulation of p-STAT6 exerts anti-inflammatory effects [36]. Studies have also shown that in the bone marrow-derived macrophages of SD rats, Luteolin inhibits the production of TNF-α and IL-6 in a dose-dependent manner, and shortens the half-life of TNF-α and IL-6mRNA [37]. In addition, luteolin can also inhibit inflammation by changing the activities of histone decarboxylase (HDAC) and acetylene (HAT) [38]. Luteolin-mediated Coxsackie virus B3 (CVB3) can be achieved. Luteolin inhibits the phosphorylation of p38 MAPK, JNK, and ERK in CVB3, thereby inhibiting NF-κB nuclear translocation and subsequently reducing inflammation in CVB3-infected cells and the expression of cytokines [39]. In IL-1β-induced rat chondrocyte inflammation, Luteolin inhibits the phosphorylation of NF-kappaB in vivo and attenuates the pathogenesis of osteoarthritis model rats [40]. In the rat model of acute pneumonia, luteolin treatment reduced the dry-to-wet weight ratio of lung tissue and decreased the total number of serum white blood cells in a dose-dependent manner. These studies proved that luteolin partially inhibited the neutrophil ring Adenosine phosphate (cAMP-PDEs) or PDE4 activity and the expression of vascular cell adhesion molecule (VCAM-1) and intracellular cell adhesion molecule (sICAM-1) in microvascular endothelial cells to inhibit inflammation [41]. Arachidonic acid acts as the direct precursor of prostaglandin (PGI-2), thromboxane A2 (TXA-2), and leukopenia (LTC-4) [42]. In the inflammation reaction, PGE2 can play a pro-inflammatory effect by regulating the differentiation of immune cells or increasing the expression of related cytokines, thereby exacerbating the inflammatory response [43]. However, studies have confirmed that luteolin has varying degrees of influence on the two pathways of cyclooxygenase (COX) and lipoxygenase (LOX), among which the mechanism of action is to regulate PGE-2, IFN-α, and β [44].
Schematic diagram of LUT regulating inflammation. The role of inflammatory response in tumors cannot be underestimated. Luteolin reduces the expression of inflammatory factors in tumor cells by influencing inflammatory factors, inflammatory signal pathways, and arachidonic acid, in vivo and in vivo.
Experimental study of luteolin compounds on tumor cells
| Cancer types | Subjects cells | Mechanisms | The target gene | Have an effect | Reference |
|---|---|---|---|---|---|
| Breast cancer | MCF-7 | EGFR pathway | PI3K AKT mTOR | Inhibitory signaling pathway | [45,46] |
| Colon cancer | SW620 | ERK/FOXO3a pathway | ERK1/2 FOXO3a caspase-3 | Cell apoptosis | [47] |
| Pancreatic cancer | PANC-1 | MicroRNAs | miR-301-3p caspase-3 | Gene expression | [48] |
| Lung cancer | A549 | JAK/STAT1 pathway | IFN-α IFN-β JAK STAT1 MicroRNA-155 | Influencing cytokines Signaling pathways Gene expression | [49,50] |
| NSCLC | Oxidative stress | MicroRNA-34a-5p Caspase-3 Caspase-9 Bcl-2 MDM4 | Cell apoptosis Signaling pathways | [51] | |
| Kidney cancer | 786-O | AKT pathway | JNK p38MAPK Ask1 PP2a | Inhibit cell proliferation Induce apoptosis Factor signaling | [52] |
| Gastric cancer | AGS BGC823 SGC7901 | MEK pathyway | MEK ERK1/2 P21 P53 miRNA-34a | Gene expression Gene expression Signaling pathways | [53] |
| Prostate cancer | 22Rv1 | MicroRNAs | miR-8080 AR-V7 AR-FL Caspase-3 Caspase-7 | Gene expression | [54] |
The impact of LUT on tumor
Luteolin is present as a flavonoid in vegetables, plants, and fruit. Take part in the fight against various human malignant tumors. In the mechanism studies published so far, most of the effects of luteolin in alleviating breast cancer, colon cancer, pancreatic cancer, lung cancer, kidney cancer, gastric cancer, and other tumors are by inhibiting the proliferation of tumor cells. Reduce the stimulation of carcinogens, activate cell cycle arrest, etc. to play a role. In addition, luteolin is also involved in the regulation of genes and proteins to induce tumor cells to induce apoptosis through distinct signal pathways and block the development of cancer in vivo and in vivo (Table 2).
The effect of LUT on HCC
Luteolin's anti-hepatocellular carcinoma activity is linked to its influence on various signal transduction pathways and cytokines in liver cancer cells. Several experimental studies have proved that luteolin can prevent the spread, metastasis, and cell cycle arrest of hepatocellular carcinoma, promote cell differentiation and angiogenesis, and also promote the apoptosis of malignant cells. A large number of in vivo and in vivo evaluations show that luteolin promotes tumor cell apoptosis, inhibits tumor cell growth cycle, tumor cell migration and invasion, etc., and its anti-tumor development potential is huge.
The effect of LUT on the growth of different HCC cells. Note: The figure above illustrates the effect of different concentrations of luteolin on inhibiting the proliferation of different types of liver cancer cells such as Huh7, HepG2, Hep3B, SK-Hep-1, PC-3, LCSLCs, etc. Luteolin 11.54μM can promote cell apoptosis by influencing the polarization of Huh7 cells in the G0/G1, G2/M phase and regulating the expression of miR-6809-5P gene; oxidative stress is another important part of tumor development. On the one hand, it directly or indirectly affects the proliferation of tumor cells. Luteolin has the effect of affecting ROS, and finally induces ER stress and oxidative stress; the self-renewal of cancer cells plays a key role in the occurrence and metastasis of tumor cells. Luteolin can regulate the self-renewal of LCSLCs.
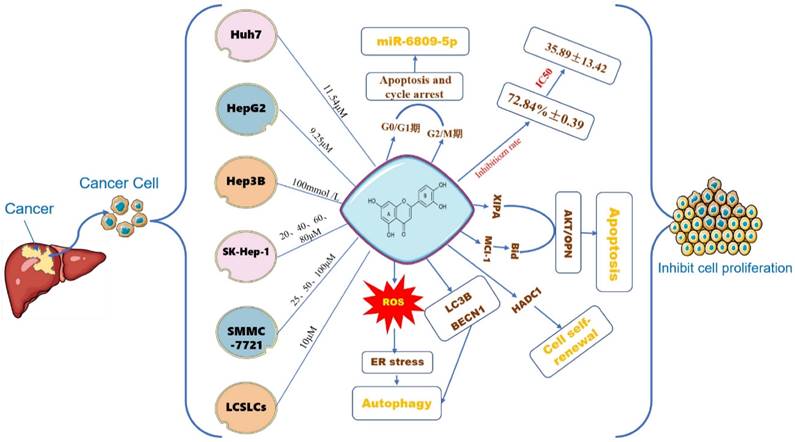
The effect of LUT on the proliferation of HCC cells
Cell proliferation is one of the important physiological functions of cells, and an essential life characteristic of organism growth, development, reproduction, and heredity [55]. Similarly, the proliferation of cancer cells is another important step in tumorigenesis and development. The antitumor activity of luteolin has been investigated in various cancer cells. Luteolin can induce cell cycle arrest and cell apoptosis, and inhibit proliferation and metastatic progression. Based on the rapid progress of flavonoids, their effects on tumor cell proliferation have also been extensively studied (Figure 4). Yang P W [56] et al, treated HuH7 and HepG2 hepatoma cells with different concentrations of luteolin for 24-120h, the results showed that luteolin inhibited the proliferation of hepatoma cells in a dose-dependent manner, and the result of HepG2 was more obvious than that of HuH7. Luteolin 9.25 ± 1.67 μM can achieve half the inhibitory effect, while in Huh7 cells 11.54 ± 2.32 μM can achieve half of the inhibitory effect. Experimental studies by Chang J [57] and others suggest that luteolin has an inhibitory rate of 72.84% ± 0.39 on human liver cancer cells Hep3B at a concentration of 100mmol/L, which has a significant inhibitory effect on cell proliferation. Hepatocyte growth factor (HGF), also known as scattering factor (SF) and its receptor c-Met tyrosine kinase is in charge of the proliferation of a variety of cancer cells. Researchers have found that luteolin and other flavonoids can regulate HGF factors it reduces the survival rate of HepG2 cells [58]. 100μmol/L luteolin inhibits the proliferation of liver cancer cells by down-regulating the miRNA expression of the proliferation-related genes LETM1, URG11, PICK1, and CyclinD1 in HepG2 liver cancer cells, suggesting that luteolin may inhibit cell proliferation [59]. Im Eunji [60] et al, used 20, 40, 60, and 80 µM luteolin to interfere with human hepatocellular carcinoma SK-Hep-1 cells and mouse normal hepatocyte AML12 cells, and the results showed that luteolin significantly reduced SK-The viability of Hep-1 cells is dose-dependent.
To detect the effect of luteolin on SMMC-7721 cell autophagy, the formation of autophagosomes was observed using transmission electron microscopy, the number of intracellular autophagosomes after treatment with 25,50, or 100 µM luteolin for 48 h increased compared with cells treated with 0 µM luteolin [61]. Furthermore, luteolin increased the number of autophagosomes within cells, promoted the conversion of LC3B-I to LC3B-II, and increased the expression of Beclin 1, a phenomenon that, finally, was altered by the addition of an autophagosome inhibitor. 10μM luteolin can inhibit the activity and expression of histone deacetylase-1 (HADC1) in liver cancer stem-like cells (LCSLCs), thereby influencing the self-renewal of LCSLCs [62]. 5- 10 µmol/L luteolin induces oxidative stress and ER stress in p53-null Hep3B cells, and only induces autophagy in Hep3B cells, enhancing cell viability [63].
The effect of LUT on HCC cell apoptosis
Cell cycle regulation requires the cooperation of a large number of intracellular and extracellular signals, without proper signals, cells will be unable to move from one stage to the next, this phenomenon is called cell cycle arrest [64]. Cell cycle arrest helps to maintain the stability of genes, and gene mutations that regulate the cell cycle plays a major role in tumorigenesis. When the cell cycle is normal, if DNA damage occurs, the cell cycle stops at the corresponding checkpoint, and the cell cycle block provides extra time for the cell to repair the damage, thereby reducing the occurrence of mutations and avoiding the occurrence of tumors [65], studies have demonstrated that treatment with luteolin and apoptosis-inducing ligand (TRAIL) has a synergistic effect and mechanism on Huh7 cells [66]. This is consistent with the study of Wu B [67] that luteolin induces autophagic flux of human liver cancer cells, significantly inhibits the expression of death receptor 5 (DR5) in the process of tumor apoptosis, and effectively enhances Apoptosis induced by TRAIL. CyclinD1 protein is a key protein for cells to transform from the G1 phase to the S phase. Studies have shown that luteolin regulates cell cycle arrest by down-regulating the expression of CyclinD1 gene mRNA in liver cancer cells [68], this is in line with the discovery of Shi Dongdong et al. that luteolin-blocked MCF-7 cells in S phase [69]. Luteolin can promote the apoptosis of hepatoma cells by up-regulating the expression of p-JNK protein in HepG2 cells, and it can also induce mitochondrial autophagy in HepG2 cells by down-regulating the expression of Bcl-2 [56]. Studies have shown that treatment with 40 μmol/L luteolin for 2 hours can promote the activity of caspase-3 and -8 by degrading the x-linked apoptosis protein inhibitor (XIAP) and inhibiting the activity of protein kinase C (PKC) [70]. Pretreatment of SMMC-7721 and Bel-7402 liver cancer cells with 50μM luteolin showed that the level of apoptosis factor Bax was up-regulated, the anti-apoptotic factor Bcl-2 was down-regulated, caspase-3 enzyme was activated, and mitochondrial membrane potential was reduced and induced Liver cancer cells to undergo apoptosis and exert their anti-liver cancer function [71]. In addition, the combination of metformin and luteolin sympathetically protects liver toxicity induced by carbon tetrachloride, and its effect may be related to the anti-apoptotic pathway Nrf2/HO-1 [72].
The effect of LUT on HCC angiogenesis
Angiogenesis, the process of forming new blood vessels and blood supply structures, is one of the important mechanisms of tumor growth and metastasis [73]. Vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), angiotensin-converting enzyme (Ang) and fibroblast growth factor (FGF) are key factors in angiogenesis. High levels of circulating vascular endothelial growth factor in patients with hepatocellular carcinoma are closely associated with tumor angiogenesis [74]. Therefore, preventing/blocking the rapid formation of blood vessels is an effective way to prevent hepatocellular carcinoma. Rat microvascular endothelial cells have been shown to express high levels of cAMP-PDEs, especially PDE4, and further studies have shown that lignocaine has a dose-dependent inhibitory effect on the activity of endothelial cAMP-PDEs or PDE4 [41]. Luteolin can regulate the mRNA expression of pro-proliferative genes, pro-apoptotic genes, and angiogenic molecules Uba2, VEGF, Fra-1, HIF-1α, and Rac1 in hepatocellular carcinoma HepG2 cells, thereby inhibiting the proliferative activity and angiogenesis of hepatocellular carcinoma cells [75]. Studies have reported that luteolin downregulates lymphocyte function-related molecules (LFA-3) and PCNA and upregulates intercellular adhesion molecule-1 (ICAM-1) in a way that inhibits tumor angiogenesis and tumor cell proliferation to achieve the anti-tumor effect of LUT [76] (Figure 5A). SRC and EGFR can be considered the main genes to prevent angiogenesis and inhibit the growth of tumor cells. Zhulin Wu [77] et al. studied 207 patients with hepatocellular carcinoma and found that luteolin and quercetin could play a therapeutic role through MAPK, JAK-STAT, and other pathways, and the key targets included SRC, EGFR, VEGFA, PIK3R1 and so on. Based on the above research results, we verified by network pharmacology technology the potential targets of luteolin in the treatment of hepatocellular carcinoma angiogenesis include AKT1, SRC, EGFR, ESR1, MMP9, and PTGSR (Figure 5B).
A. The effect of LUT on the angiogenesis of liver cancer. The production of VEGF (vascular endothelial growth factor, vascular endothelial growth factor) is reduced under the action of luteolin and further down-regulates the cyclic adenosine monophosphate (cAMP-PDEs) of neutrophils to inhibit tumor angiogenesis; B. LUT regulates tumor cells A variety of gene expressions and DNA inhibit tumor angiogenesis; research on the potential targets of luteolin for hepatocellular carcinoma angiogenesis, PPI shows that luteolin can inhibit angiogenesis through targeted regulation of AKT1, SRC, EGFR, ESR1, etc.;
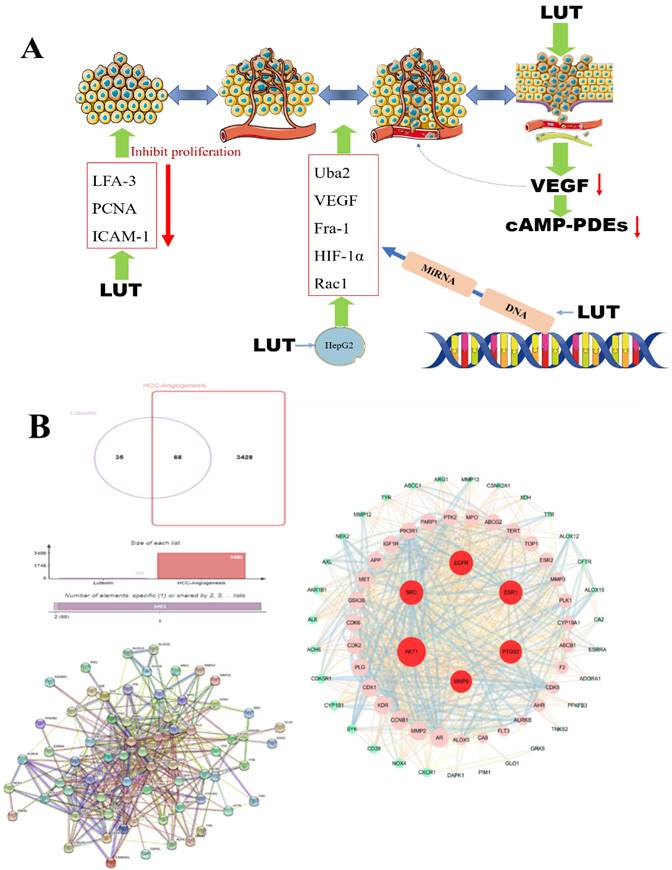
Mechanism and clinical application of luteolin in the treatment of HCC
Bioinformatics study of luteolin in the treatment of HCC
In recent years, bioinformatics technology has been widely used to study the mechanism of various diseases. Network pharmacology and bioinformatics can not only systematically combine the multi-targets of drugs and diseases, but also meet the problem of few therapeutic targets and research targets in the process of clinical research through genetic research [78]. Through the data mining of network pharmacology and bioinformatics analysis, we found that luteolin can interfere with the occurrence and development of hepatocellular carcinoma through multi-target, multi-gene, and multi-pathway (Figure 6). After visualization, it is concluded that the main core targets of mignonette in the treatment of hepatocellular carcinoma (HCC) are SRC, EGF, AKT1, ESR1, PI3KR1, AR, CDK1, and so on. Among them, SRC and ESR1 affect the trend of the survival curve of patients with hepatocellular carcinoma and are significantly correlated with the pathological stage of patients with HCC. In addition, immune infiltration analysis showed that SRC and ESR1 were significantly correlated with six kinds of immune cells infiltrated by hepatocellular carcinoma. GO and KEGG enrichment analysis proved that the potential pathways of luteolin in the treatment of hepatocellular carcinoma include JAK-STAT, AMPK, and NF-κB.
Signal pathway pathways of LUT treatment of HCC
When a tumor cell has a special response, the signal transmits information from outside to inside the cell. Cells respond to this kind of information. However, in the study of flavonoids, LUT plays an anti-hepatoma effect through signal transduction and protein modification. The nuclear factor-kappa B (NF-κB) signal pathway is the most important transcriptional pathway. The regulation of NF-κB can control the expression of a variety of pro-inflammatory cytokines, including cytokines, chemokines, and adhesion molecules [79]. As a key effector of the Hippo pathway, YAP is activated by transport from the cytoplasm to the nucleus, which regulates gene expression and promotes tumorigenesis. Fas bind to the receptor Fas and initiate death signal transduction, which leads to apoptosis of cells expressing Fas. It has been found that luteolin can affect the survival of hepatocellular carcinoma by regulating NF- κ B, YAP, AKT/OPN, NRF2/HO-1, Fas and Fas ligand, p53, AMPK, and other signal pathways (Table 3).
The effect of LUT on HCC biological process and gene transcription
Cancer has become one of the leading causes of death because of the difficulty in treating it. Both direct and indirect risk factors can contribute to an outbreak of cancer. The emergence of gene transcription has led to significant advances in Epigenetics, with the differential expression of genes determining outcomes in cancer patients. Genome-wide screening and the discovery of high-throughput genomics have promoted the further development of proteomics, which is a breakthrough in the diagnosis and prediction of liver cancer [83]. Yang Pei [56] et al. demonstrated that luteolin upregulates MIR-6809-5P expression, overexpression of miR-6809-5p inhibits HCC cell growth, and knockdown of miR-6809-5p can reverse the anti-cancer effect of luteolin. FLOT1, an essential protein for plasma membrane transport, cell division, T cell activation, and cell surface receptor signaling, also has some effects on tumor cells, and MiR-6809-5p directly targets FLOT1 in hepatoma cells [84]. FLOT1 is highly expressed in a variety of tumor cells, but the down-regulation of FLOT1 by luteolin directly inhibits the growth of hepatoma cells. In most biological processes of liver cancer, multiple signaling pathways, including ERK1/2, p38, JNK, and NF-ΚB/P65, are overexpressed by miR-6809-5p or downregulated and inactivated by Flot1 [85]. However, in the luteolin study, extracts from Scutellaria baicalensis Georgi and Hedyotis diffusa were found to inhibit HCC cell growth and Hepatitis B virus activity in vivo and in vivo by altering circRNA-miRNA gene expression, the efficacy of these extracts may be consistent with that of Luteolin in the presence of apigenin [86]. Based on network pharmacology and molecular docking techniques, Liu et al. [87] found that luteolin, as the main component of Polygonum hydropiper, was successfully combined with AKT1 to treat liver cancer, and the therapeutic effect of luteolin was verified by experiments, the levels of P-AKT mirnas are reduced in low-grade liver cancer cells and have regulatory effects on various genes such as AKT1, JUN, MAPK1, RELA, IL6, and MAPK14. In HCC cells, luteolin targets the expression of the THOC1 gene and induces DNA damage to prevent the proliferation of HCC Cells [88].
Bioinformatics analysis of potential targets of luteolin in the treatment of hepatocellular carcinoma. (A) Potential targets of luteolin in the treatment of hepatocellular carcinoma. (B) Protein interactions at potential core targets. (C) Seven core genes screened. (D) Multigene comparative analysis of candidate biomarkers based on TCGA database. (E)Survival heat map of core genes in hepatocellular carcinoma. (F) Expression of core genes based on GEPIA database. (G) Correlation analysis of differential genes in the pathological stage of hepatocellular carcinoma. (H) Influence of differential gene on survival curve of patients. (I) Correlation of differential gene SRC, ESR1 with immune cells infiltrated by hepatocellular carcinoma. (J) KEGG enrichment analysis pathway of potential core genes. (K-M) GO enrichment analysis of potential targets for luteolin in the treatment of hepatocellular carcinoma includes biological process (BP), cell composition (CC), and molecular function (MF)
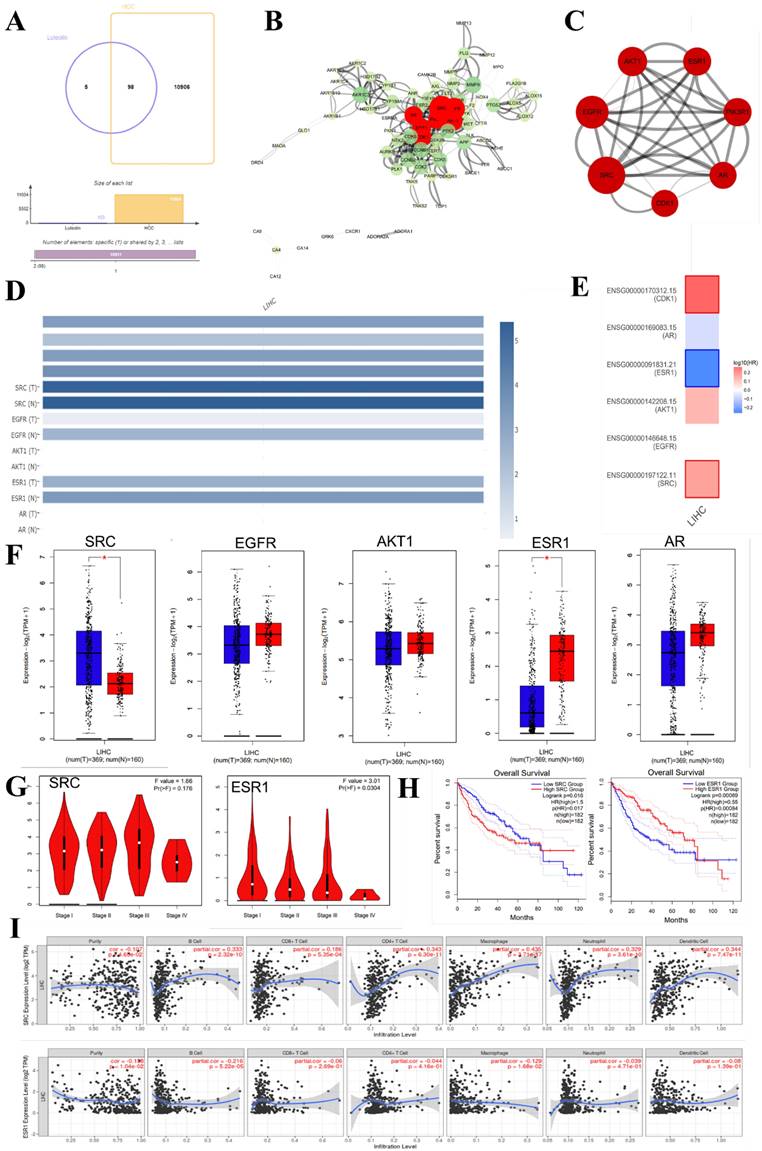
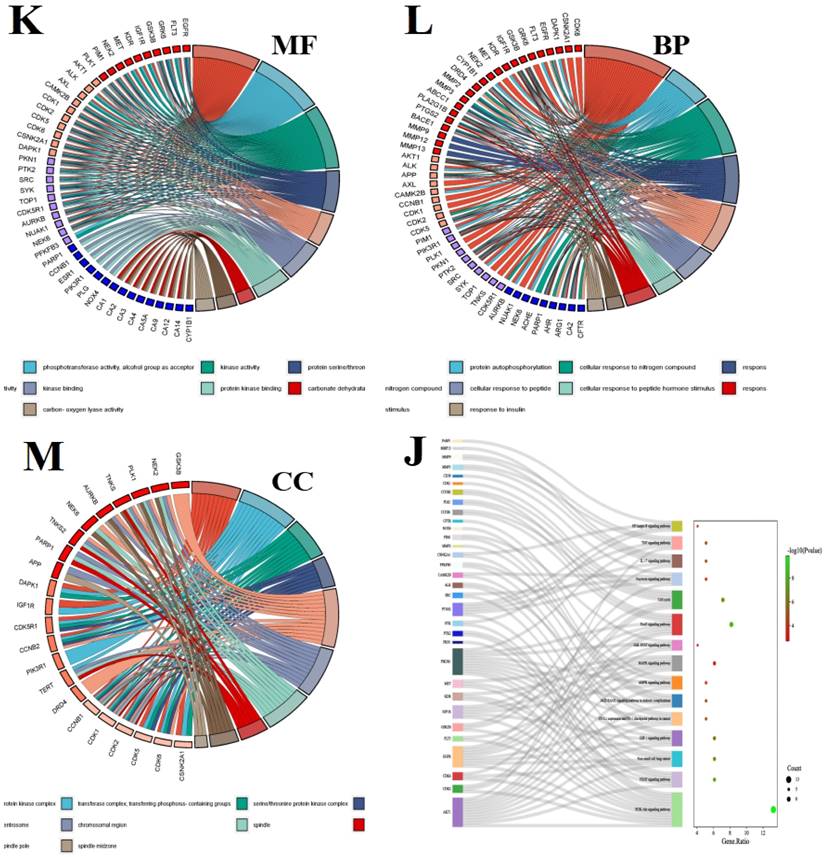
Clinical significance and application of luteolin
With the development of natural medicine, the research on luteolin has made a great breakthrough, and the clinical significance of luteolin has been confirmed by a lot of reliable data [89]. Sorafenib, a small-molecule multi-kinase inhibitor, has been approved by the US Food and Drug Administration as an oral drug for the treatment of hepatocellular carcinoma and renal cell carcinoma. But to the best of our knowledge, the combination of luteolin and sorafenib has been shown to kill cancer cells, with the study claiming that the combination increased the expression of the phosphorylated form of JNK, sP600125, a JNK inhibitor, effectively attenuated the cell death induced by combination therapy. Thus, when combined with sorafenib and luteolin to synergistically kill HCC cells through JNK-mediated apoptosis, luteolin may be an ideal drug [90]. As a tyrosine kinase inhibitor, lapatinib inhibits the activation of downstream signaling pathways by blocking the activation of HER1 and HER2 tyrosine kinases, thereby inhibiting the survival and proliferation of tumors. These data suggest that the combination of lapatinib and luteolin may inhibit HER2+ human breast cancer by significantly increasing the expression of FOXO3a and NQO1 [91]. The results of metabolomics showed that the combination of luteolin and resveratrol could decrease the production of Glucuronic acid metabolites in patients with liver disease and increase the bioavailability of luteolin [92]. In recent years, the development of key therapeutic targets of natural drugs has become an important method for the clinical treatment of tumor diseases, and the combination of luteolin, a flavonoid derivative, with clinical drugs is also of great significance for patients with hepatocellular carcinoma.
Luteolin regulates the signal pathway of HCC
| Pathway | Action factor | Result | Biological process | References |
|---|---|---|---|---|
| NF-κB | IκBα p65 COX-2 | Decreased NF-κB expression | HepG2 apoptosis | [80] |
| YAP | CXCR-4 UBTD-1 | Decreased UBTD1 expression | Hep3B and Huh7 signal transduction and abnormal gene expression | [81] |
| AKT/OPN | AKT P-AKT OPN P-OPN Caspase-3 | Decreased AKT/OPN expression | SK-Hep-1 apoptosis | [60] |
| NRF2/HO-1 | TNF-α IL-6 NRF2 HO-1 ROS | Decreased NRF2/HO-1 expression ROS reduction | Activation of NF-κB signaling pathway in RAW264.7 | [80] |
| AMPK | AMPK P-AMPK NF-κB | Decreased P-AMPK/AMP K expression | AMPK activation in HepG2 activates the NF-κB inflammatory pathway | [81] |
| p53, Fas-Fas ligand | TGF-β1 p21WAF1/CIP1 p27KIP1 Smad4 | Up-regulatio of TGF-β1, p21WAF1/CIP, p27KIP1, and Smad4 gene expression | G1 arrest in Hep3B cells leads to apoptosis | [82] |
Summary and Outlook
The effect of exploring effective compounds from natural sources for the prevention and treatment of tumors or other cancer diseases has been well proven. Flavonoids are the most widely studied anti-tumor organic compounds at this stage, and their results have provided a solid basis for clinical practice [93]. In this context, luteolin, as a derivative structure of flavonoids, has received extensive attention. It promotes tumor cell apoptosis by inhibiting tumor cell growth, migration, invasion, gene expression, protein modification, etc. [94], and through the anti-tumor effects of different signal transduction processes and biological processes are becoming more and more optimistic.
Hepatocellular carcinoma is a primary malignancy tumor of the liver, and the mechanism of hepatocellular carcinogenesis is mainly concentrated in the process of hepatitis and cell regeneration [95]. Liver cancer has been documented to develop in a vicious cycle of viral inflammation, alcohol-induced hepatocyte damage, or chronic liver damage caused by oxidative stress, this increases genomic instability and the risk of liver cancer [96]. However, flavonoids can inhibit tumor cells through classical signaling pathways such as NF-ΚB, AKT, and AMPK, and it has been demonstrated that luteolin can achieve in vivo and in vivo anti-tumor activity by affecting levels of inflammatory factors, with no apparent toxicity to normal cells and low side effects, further validating its clinical effect [97]. Non-coding RNAs, mirnas, and increase are not only involved in the initiation and development of disease but are also potential targets for drug intervention, which is consistent with studies by Yang P W et al. [86]. Therefore, the next step is to search for reliable biomarkers in terms of the effect of luteolin on hepatocellular carcinoma miRNAs and lncRNAs. Based on the above data, the potential biomarkers SRC and ESR1 derived from our bioinformatics analysis may also be potential targets for luteolin in the treatment of hepatocellular carcinoma, this not only expands our hepatocellular carcinoma of luteolin treatment but also provides insight into the pathogenesis of hepatocellular carcinoma, This is consistent with the results of many previous investigators, suggesting the efficacy of already existing therapeutic targets and that new therapeutic targets may provide new strategies for the treatment of HCC. Although luteolin has shown excellent results in cancer and animal models of cancer, studies on the role of luteolin in the treatment of pharmacokinetics hepatocellular carcinoma in vivo need to be improved [98]. Mitochondrial energy metabolism also plays an important role in the proliferation of liver cancer and other tumors, luteolin is now known to induce lethal endoparasite reticular responses, stimulus responses, and mitochondrial dysfunction in Glioblastoma multiforme cells by increasing intracellular reactive oxygen species (ROS) levels [99]. As a derivative of flavonoids, luteolin has never been stopped in its research. Using nanotechnology to modify luteolin can not only improve its bioavailability but also, and the bioavailability of luteolin can be better regulated [100]. In conclusion, we describe luteolin as a real source, ensuring its safety and low cost compared to synthetic cancer drugs, and describe whether luteolin can be used to prevent and treat liver diseases through different regulatory mechanisms or molecular features. As an important adjunct to the treatment of cell cancer.
Abbreviations
HCC: Hepatocellular Carcinoma
LUT: Luteolin
miRNAs: Microribonucleic acids
LncRNAs: Long-stranded non-coding ribonucleic acids
ROS: Reactive oxygen species
MAPK: Mitogen-activated protein kinase
ERK: Extracellular regulated protein kinases
AKT: Protein kinase B, PKB
LDH: Lactate dehydrogenase
SOD: Superoxide dismutase
MDA: Malondialdehyde
GSH: Glutathione, r-glutamyl cysteine +glycine
FOXO3a: Forkhead box O3
NQO1: NAD(P)H:quinone oxidoreductase 1
SRC: Sarcoma
ESR1: Estrogen receptor 1
GO: Gene Ontology Consortium
KEGG: Kyoto Encyclopedia of Genes and Genomes
Acknowledgements
This study was supported by the Natural Science Foundation of Inner Mongolia Autonomous Region (2020LH08031, 2021MS08021,2020MS08117); Chunhui of the Ministry of education project (Cui Hongwei 20180056); The Health Technology Plan Project of Inner Mongolia Autonomous Region Health Committee (202202156, 202201112); Inner Mongolia Medical University Zhiyuan Talent Program (Good Learning Talent Program) (ZY0202031); Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (NJYT23050); and Inner Mongolia Autonomous Region "Grassland Talent" project youth innovation and entrepreneurship talent project (Cui Hongwei, Yu Lei).
Ethical Approval
This paper has passed ethical review.
Author contributions
All authors participated in the conception and design of the study. YQ Han, YF Xiao and L Yu, J Chen, XD Yang, HW Cui, JQ Liang and initiated this study. YQ Han, YF Xiao, and L Yu wrote the first draft of the manuscript. All authors contributed to the manuscript revision, and read, and approved the submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Craig AJ, von Felden J, Garcia-Lezana T. et al. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17(3):139-152
2. Baby J, Devan AR, Kumar AR. et al. Cogent role of flavonoids as key orchestrators of chemoprevention of hepatocellular carcinoma: A review. J Food Biochem. 2021;45(7):e13761
3. Yang JD, Hainaut P, Gores GJ. et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589-604
4. Anwanwan D, Singh SK, Singh S. et al. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314
5. Pinato DJ, Guerra N, Fessas P. et al. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020;39(18):3620-3637
6. Llovet JM, Kelley RK, Villanueva A. et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6
7. Liao CY, Lee CC, Tsai CC. et al. Novel Investigations of Flavonoids as Chemopreventive Agents for Hepatocellular Carcinoma. Biomed Res Int. 2015;2015:840542
8. Luo Y, Ding G S, Dong J Y. Advances in translational treatment strategies for unresectable advanced hepatocellular carcinoma. Journal of Liberation Army Medicine. 2022;47(07):731-738 https://kns-cnki-net(Chinese)
9. Hamaoka M, Kobayashi T, Kuroda S. et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: A retrospective cohort study. Int J Surg. 2017;44:223-228
10. Yan ZQ, Tan J, Guo K. et al. Phytotoxic mechanism of allelochemical liquiritin on root growth of lettuce seedlings. Plant Signal Behav. 2020;15(10):1795581
11. Al-Ishaq RK, Overy AJ, Busselberg D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules. 2020 10(1)
12. Wang S, Cao M, Xu S. et al. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. Inflammation. 2020;43(1):95-108
13. Ma J, Chen X, Zhu X. et al. Luteolin potentiates low-dose oxaliplatin-induced inhibitory effects on cell proliferation in gastric cancer by inducing G(2)/M cell cycle arrest and apoptosis. Oncol Lett. 2022;23(1):16
14. Yoo HS, Won SB, Kwon YH. Luteolin Induces Apoptosis and Autophagy in HCT116 Colon Cancer Cells via p53-Dependent Pathway. Nutr Cancer. 2022;74(2):677-686
15. Fu Lin. First total synthesis of a novel flavonoid natural product and its conformational relationship: M.S, Shandong University of Science and Technology; 2021. https://kns-cnki-net(Chinese)
16. Qing W X. Construction of lignocaine and lignan nano-drug delivery system: PhD, Henan University; 2016. https://kns-cnki-net(Chinese).
17. Xiao J, Capanoglu E, Jassbi AR. et al. Advance on the Flavonoid C-glycosides and Health Benefits. Crit Rev Food Sci Nutr. 2016;56(Suppl 1):S29-S45
18. Wang J, Zhang ZZ, Mei XZ. et al. Advances in the antibacterial effects and mechanisms of flavonoids. Jiangsu Agricultural Science. 2023;51(01):1-8 https://kns-cnki-net(Chinese)
19. Imran M, Salehi B, Sharifi-Rad J. et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules. 2019 24(12)
20. Kapoor S. Luteolin and its inhibitory effect on tumor growth in systemic malignancies. Exp Cell Res. 2013;319(6):777-778
21. Lee EJ, Oh SY, Sung MK. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Toxicol. 2012;50(11):4136-4143
22. Jakubczyk K, Dec K, Kaldunska J. et al. Reactive oxygen species - sources, functions, oxidative damage. Pol Merkur Lekarski. 2020;48(284):124-127
23. Chen Y, Luo X, Zou Z. et al. The Role of Reactive Oxygen Species in Tumor Treatment and its Impact on Bone Marrow Hematopoiesis. Curr Drug Targets. 2020;21(5):477-498
24. Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035-1042
25. Ravishankar D, Rajora AK, Greco F. et al. Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell Biol. 2013;45(12):2821-2831
26. Balamurugan K, Karthikeyan J. Evaluation of Luteolin in the Prevention of N-nitrosodiethylamine-induced Hepatocellular Carcinoma Using Animal Model System. Indian J Clin Biochem. 2012;27(2):157-163
27. Pratheeshkumar P, Son YO, Budhraja A. et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Plos One. 2012;7(12):e52279
28. Xia F, Wang C, Jin Y. et al. Luteolin protects HUVECs from TNF-alpha-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-kappaB and MAPK pathways. J Atheroscler Thromb. 2014;21(8):768-783
29. Liu M, Cheng C, Li X. et al. Luteolin alleviates ochratoxin A induced oxidative stress by regulating Nrf2 and HIF-1alpha pathways in NRK-52E rat kidney cells. Food Chem Toxicol. 2020;141:111436
30. Pratheeshkumar P, Son YO, Divya SP. et al. Luteolin inhibits Cr(VI)-induced malignant cell transformation of human lung epithelial cells by targeting ROS mediated multiple cell signaling pathways. Toxicol Appl Pharmacol. 2014;281(2):230-241
31. Baek KS, Yi YS, Son YJ. et al. Comparison of anticancer activities of Korean Red Ginseng-derived fractions. J Ginseng Res. 2017;41(3):386-391
32. Choi EM. Modulatory effects of luteolin on osteoblastic function and inflammatory mediators in osteoblastic MC3T3-E1 cells. Cell Biol Int. 2007;31(9):870-877
33. Kim TW, Kim YJ, Seo CS. et al. Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-ss and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine. 2016;23(4):331-339
34. Park CM, Song YS. Luteolin and luteolin-7-O-glucoside protect against acute liver injury through regulation of inflammatory mediators and antioxidative enzymes in GalN/LPS-induced hepatitic ICR mice. Nutr Res Pract. 2019;13(6):473-479
35. Cho YC, Park J, Cho S. Anti-Inflammatory and Anti-Oxidative Effects of luteolin-7-O-glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation. Int J Mol Sci. 2020 21(6)
36. Wang S, Xu S, Zhou J. et al. Luteolin transforms the polarity of bone marrow-derived macrophages to regulate the cytokine storm. J Inflamm (Lond). 2021;18(1):21
37. Shi F, Zhou D, Ji Z. et al. Anti-arthritic activity of luteolin in Freund's complete adjuvant-induced arthritis in rats by suppressing P2X4 pathway. Chem Biol Interact. 2015;226:82-87
38. Scafuri B, Bontempo P, Altucci L. et al. Molecular Docking Simulations on Histone Deacetylases (HDAC)-1 and -2 to Investigate the Flavone Binding. Biomedicines. 2020 8(12)
39. Wu S, Wang HQ, Guo TT. et al. Luteolin inhibits CVB3 replication through inhibiting inflammation. J Asian Nat Prod Res. 2020;22(8):762-773
40. Fei J, Liang B, Jiang C. et al. Luteolin inhibits IL-1beta-induced in fl ammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother. 2019;109:1586-1592
41. Kong X, Huo G, Liu S. et al. Luteolin suppresses inflammation through inhibiting cAMP-phosphodiesterases activity and expression of adhesion molecules in microvascular endothelial cells. Inflammopharmacology. 2019;27(4):773-780
42. Kang OH, Choi JG, Lee JH. et al. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and MAPKs activation pathways in HMC-1 cells. Molecules. 2010;15(1):385-398
43. Li YC, Yeh CH, Yang ML. et al. Luteolin Suppresses Inflammatory Mediator Expression by Blocking the Akt/NFkappaB Pathway in Acute Lung Injury Induced by Lipopolysaccharide in Mice. Evid Based Complement Alternat Med. 2012;2012:383608
44. Anilkumar K, Reddy GV, Azad R. et al. Evaluation of Anti-Inflammatory Properties of Isoorientin Isolated from Tubers of Pueraria tuberosa. Oxid Med Cell Longev. 2017;2017:5498054
45. Sui JQ, Xie KP, Xie MJ. Inhibitory effect of luteolin on the proliferation of human breast cancer cell lines induced by epidermal growth factor. Sheng Li Xue Bao. 2016;68(1):27-34
46. Wu HT, Liu YE, Hsu KW. et al. MLL3 Induced by Luteolin Causes Apoptosis in Tamoxifen-Resistant Breast Cancer Cells through H3K4 Monomethylation and Suppression of the PI3K/AKT/mTOR Pathway. Am J Chin Med. 2020;48(5):1221-1241
47. Potocnjak I, Simic L, Gobin I. et al. Antitumor activity of luteolin in human colon cancer SW620 cells is mediated by the ERK/FOXO3a signaling pathway. Toxicol In vitro. 2020;66:104852
48. Moeng S, Son SW, Seo HA. et al. Luteolin-regulated MicroRNA-301-3p Targets Caspase-8 and Modulates TRAIL Sensitivity in PANC-1 Cells. Anticancer Res. 2020;40(2):723-731
49. Wang S, Ling Y, Yao Y. et al. Luteolin inhibits respiratory syncytial virus replication by regulating the MiR-155/SOCS1/STAT1 signaling pathway. Virol J. 2020;17(1):187
50. Masraksa W, Tanasawet S, Hutamekalin P. et al. Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway. Nutr Res Pract. 2020;14(2):127-133
51. Jiang ZQ, Li MH, Qin YM. et al. Luteolin Inhibits Tumorigenesis and Induces Apoptosis of Non-Small Cell Lung Cancer Cells via Regulation of MicroRNA-34a-5p. Int J Mol Sci. 2018 19(2)
52. Ou YC, Kuan YH, Li JR. et al. Induction of apoptosis by luteolin involving akt inactivation in human 786-o renal cell carcinoma cells. Evid Based Complement Alternat Med. 2013;2013:109105
53. Ribeiro V, Ferreres F, Macedo T. et al. Activation of caspase-3 in gastric adenocarcinoma AGS cells by Xylopia aethiopica (Dunal) A. Rich. fruit and characterization of its phenolic fingerprint by HPLC-DAD-ESI(Ion Trap)-MS(n) and UPLC-ESI-QTOF-MS(2). Food Res Int. 2021;141:110121
54. Naiki-Ito A, Naiki T, Kato H. et al. Recruitment of miR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer. Carcinogenesis. 2020;41(8):1145-1157
55. Jarrett AM, Lima E, Hormuth DN. et al. Mathematical models of tumor cell proliferation: A review of the literature. Expert Rev Anticancer Ther. 2018;18(12):1271-1286
56. Yang PW, Lu ZY, Pan Q. et al. MicroRNA-6809-5p mediates luteolin-induced anticancer effects against hepatoma by targeting flotillin 1. Phytomedicine. 2019;57:18-29
57. Chang J, Hsu Y, Kuo P. et al. Increase of Bax/ Bcl-XL ratio and arrest of cell cycle by luteolin in immortalized human hepatoma cell line. Life Sci. 2005;76(16):1883-1893
58. Lee WJ, Wu LF, Chen WK. et al. Inhibitory effect of luteolin on hepatocyte growth factor/scatter factor-induced HepG2 cell invasion involving both MAPK/ERKs and PI3K-Akt pathways. Chem Biol Interact. 2006;160(2):123-133
59. Hasebe Y, Egawa K, Yamazaki Y. et al. Specific inhibition of hypoxia-inducible factor (HIF)-1 alpha activation and of vascular endothelial growth factor (VEGF) production by flavonoids. Biol Pharm Bull. 2003;26(10):1379-1383
60. Im E, Yeo C, Lee EO. Luteolin induces caspase-dependent apoptosis via inhibiting the AKT/osteopontin pathway in human hepatocellular carcinoma SK-Hep-1 cells. Life Sci. 2018;209:259-266
61. Cao Z, Zhang H, Cai X. et al. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell Physiol Biochem. 2017;43(5):1803-1812
62. Cao X, Zou H, Cao J. et al. A candidate Chinese medicine preparation-Fructus Viticis Total Flavonoids inhibits stem-like characteristics of lung cancer stem-like cells. BMC Complement Altern Med. 2016;16:364
63. Lee Y, Kwon YH. Regulation of apoptosis and autophagy by luteolin in human hepatocellular cancer Hep3B cells. Biochem Biophys Res Commun. 2019;517(4):617-622
64. Qian XP, Zhang XH, Sun LN. et al. Corosolic acid and its structural analogs: A systematic review of their biological activities and underlying mechanism of action. Phytomedicine. 2021;91:153696
65. Zhang Y, Chen HG, Zhao C. et al. [Research progress on anti-hepatocellular carcinoma mechanism of active ingredients of traditional Chinese medicine]. Zhongguo Zhong Yao Za Zhi. 2020;45(14):3395-3406
66. Nazim UM, Park SY. Luteolin sensitizes human liver cancer cells to TRAIL-induced apoptosis via autophagy and JNK-mediated death receptor 5 upregulation. Int J Oncol. 2019;54(2):665-672
67. Wu B, Xiong J, Zhou Y. et al. Luteolin enhances TRAIL sensitivity in non-small cell lung cancer cells through increasing DR5 expression and Drp1-mediated mitochondrial fission. Arch Biochem Biophys. 2020;692:108539
68. Zheng L. Luteolin Stimulates Proliferation and Inhibits Late Differentiation of Primary Rat Calvarial Osteoblast Induced by High-dose Dexamethasone via Sema3A /NRP1/Pleixin A1. Curr Pharm Biotechnol. 2021;22(11):1538-1545
69. Sun DW, Zhang HD, Mao L. et al. Luteolin Inhibits Breast Cancer Development and Progression In vitro and In vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell Physiol Biochem. 2015;37(5):1693-1711
70. Shi RX, Ong CN, Shen HM. Protein kinase C inhibition and x-linked inhibitor of apoptosis protein degradation contribute to the sensitization effect of luteolin on tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in cancer cells. Cancer Res. 2005;65(17):7815-7823
71. Ding S, Hu A, Hu Y. et al. Anti-hepatoma cells function of luteolin through inducing apoptosis and cell cycle arrest. Tumour Biol. 2014;35(4):3053-3060
72. Yan Y, Jun C, Lu Y. et al. Combination of metformin and luteolin synergistically protects carbon tetrachloride-induced hepatotoxicity: Mechanism involves antioxidant, anti-inflammatory, antiapoptotic, and Nrf2/HO-1 signaling pathway. Biofactors. 2019;45(4):598-606
73. Shinkaruk S, Bayle M, Lain G. et al. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2003;3(2):95-117
74. Morse MA, Sun W, Kim R. et al. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25(3):912-920
75. Selvendiran K, Koga H, Ueno T. et al. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66(9):4826-4834
76. Niu JX, Guo HP, Gan HM. et al. Effect of luteolin on gene expression in mouse H22 hepatoma cells. Genet Mol Res. 2015;14(4):14448-14456
77. Wu Z, Wei C, Wang L. et al. Determining the Traditional Chinese Medicine (TCM) Syndrome with the Best Prognosis of HBV-Related HCC and Exploring the Related Mechanism Using Network Pharmacology. Evid Based Complement Alternat Med. 2021;2021:9991533
78. Ahsan MA, Liu Y, Feng C. et al. Bioinformatics resources facilitate understanding and harnessing clinical research of SARS-CoV-2. Brief Bioinform. 2021;22(2):714-725
79. Lee JK, Kim SY, Kim YS. et al. Suppression of the TRIF-dependent signaling pathway of Toll-like receptors by luteolin. Biochem Pharmacol. 2009;77(8):1391-1400
80. Witkowska-Banaszczak E, Krajka-Kuzniak V, Papierska K. The effect of luteolin 7-glucoside, apigenin 7-glucoside and Succisa pratensis extracts on NF-kappaB activation and alpha-amylase activity in HepG2 cells. Acta Biochim Pol. 2020;67(1):41-47
81. Yang N, Chen T, Wang L. et al. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics. 2020;10(13):5790-5801
82. Yee SB, Choi HJ, Chung SW. et al. Growth inhibition of luteolin on HepG2 cells is induced via p53 and Fas/Fas-ligand besides the TGF-beta pathway. Int J Oncol. 2015;47(2):747-754
83. Nia A, Dhanasekaran R. Genomic Landscape of HCC. Curr Hepatol Rep. 2020;19(4):448-461
84. Zhang SH, Wang CJ, Shi L. et al. High Expression of FLOT1 Is Associated with Progression and Poor Prognosis in Hepatocellular Carcinoma. Plos One. 2013;8(6):e64709
85. Yang PW, Chen TT, Zhao WX. et al. Scutellaria barbata D.Don and Oldenlandia diffusa (Willd.) Roxb crude extracts inhibit hepatitis-B-virus-associated hepatocellular carcinoma growth through regulating circRNA expression. J Ethnopharmacol. 2021;275:114110
86. Yang PW, Chen TT, Zhao WX. et al. Scutellaria barbata D.Don and Oldenlandia diffusa (Willd.) Roxb crude extracts inhibit hepatitis-B-virus-associated hepatocellular carcinoma growth through regulating circRNA expression. J Ethnopharmacol. 2021;275:114110
87. Liu M, Wu X, Li E N. et al. Exploring the biomolecular mechanism of Tiger Cane in the treatment of hepatocellular carcinoma based on network pharmacology and molecular docking. Shanxi TCM. 2021;37(11):44-48 https://kns-cnki-net(Chinese)
88. Cai S, Bai Y, Wang H. et al. Knockdown of THOC1 reduces the proliferation of hepatocellular carcinoma and increases the sensitivity to cisplatin. J Exp Clin Cancer Res. 2020;39(1):135
89. Atwa G, Omran G, Elbaky AA. et al. The antitumour effect of galangin and luteolin with doxorubicin on chemically induced hepatocellular carcinoma in rats. Contemp Oncol (Pozn). 2021;25(3):174-184
90. Feng XQ, Rong LW, Wang RX. et al. Luteolin and sorafenib combination kills human hepatocellular carcinoma cells through apoptosis potentiation and JNK activation. Oncol Lett. 2018;16(1):648-653
91. Zhang L, Liu Q, Huang L. et al. Combination of lapatinib and luteolin enhances the therapeutic efficacy of lapatinib on human breast cancer through the FOXO3a/NQO1 pathway. Biochem Biophys Res Commun. 2020;531(3):364-371
92. Wu W, Li K, Zhao C. et al. A rapid HPLC-MS/MS method for the simultaneous determination of luteolin, resveratrol and their metabolites in rat plasma and its application to pharmacokinetic interaction studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2022;1191:123118
93. Roy A. Plumbagin: A Potential Anti-cancer Compound. Mini Rev Med Chem. 2021;21(6):731-737
94. Shi ML, Chen YF, Wu WQ. et al. Luteolin inhibits the proliferation, adhesion, migration and invasion of choroidal melanoma cells in vitro. Exp Eye Res. 2021;210:108643
95. Ding WX, Sancho-Bru P. SOX9 acts downstream of YAP to decide liver cell fate and tumor types. J Hepatol. 2022;76(3):503-505
96. Yang C, Huang X, Liu Z. et al. Metabolism-associated molecular classification of hepatocellular carcinoma. Mol Oncol. 2020;14(4):896-913
97. Elsayed M, Okda TM, Atwa G. et al. Design and Optimization of Orally Administered Luteolin Nanoethosomes to Enhance Its Anti-Tumor Activity against Hepatocellular Carcinoma. Pharmaceutics. 2021 13(5)
98. Ying Y, Wan H, Zhao X. et al. Pharmacokinetic-Pharmacodynamic Modeling of the Antioxidant Activity of Quzhou Fructus Aurantii Decoction in a Rat Model of Hyperlipidemia. Biomed Pharmacother. 2020;131:110646
99. Wang Q, Wang H, Jia Y. et al. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother Pharmacol. 2017;79(5):1031-1041
100. Wang L, Lu S, Deng Y. et al. Pickering emulsions stabilized by luteolin micro-nano particles to improve the oxidative stability of pine nut oil. J Sci Food Agric. 2021;101(4):1314-1322
Author contact
![]() Corresponding authors: Cui Hongwei, E-mail: cuihw2001423com. Liang Junqing, E-mail: liang_junqingcom
Corresponding authors: Cui Hongwei, E-mail: cuihw2001423com. Liang Junqing, E-mail: liang_junqingcom

 Global reach, higher impact
Global reach, higher impact