Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(7):1107-1116. doi:10.7150/jca.83779 This issue Cite
Research Paper
Sex Differences in the Impact of Metabolic Dysfunction-associated Fatty Liver Disease on the of Patients with Hepatocellular Carcinoma After Radical Resection
1. Department of Hepatobiliary, Pancreas and Spleen Surgery, Guangxi Academy of Medical Sciences, People's Hospital of Guangxi Zhuang Autonomous Region, Nanning 530021, Guangxi, China.
2. Department of Scientific Cooperation of Guangxi Academy of Medical Sciences, People' s Hospital of Guangxi Zhuang Autonomous Region, Nanning 530021, Guangxi, China.
3. Department of Colorectal and Anal Surgery, The People's Hospital of Guangxi Zhuang Autonomous Region & Guangxi Academy of Medical Sciences, Guangxi, China.
4. Institute of Oncology, Guangxi Academy of Medical Sciences, the People's Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, People's Republic of China.
5. Department of Hepatobiliary, Pancreas and Spleen Surgery, Guangxi Academy of Medical Sciences, People's Hospital of Guangxi Zhuang Autonomous Region, No.6 Taoyuan Road, Qingxiu District, Nanning 530021, Guangxi, China.
#These authors contributed equally to this work.
Received 2023-2-23; Accepted 2023-4-8; Published 2023-4-17
Abstract
Background: International experts have put forward a new definition for metabolic dysfunction-associated fatty liver disease (MAFLD). Nonetheless, sex differences in MAFLD function in hepatocellular carcinoma (HCC) survival is still unknown. Therefore, the current work focused on exploring the gender-specific association of MAFLD effect on prognosis after radical resection of liver cancer.
Methods: The long-term prognostic outcomes of 642 HCC patients undergoing hepatectomy were analyzed retrospectively. To calculate overall survival (OS) and recurrence-free survival (RFS), Kaplan-Meier (KM) curve was plotted. Further, using Cox proportional model to explore the prognostic factors. Sensitivity analysis was performed using propensity score matching (PSM) to balance the confounding bias.
Results: For MAFLD patients, median OS and RFS times were 6.8 years and 6.1 years, respectively, compared to 8.5 years and 2.9 years in non-MAFLD patients. KM curve shown that compare with non-MAFLD patients, MAFLD patients had a higher survival rate in men, but had a lower survival rate in women (P<0.05). Multivariate analysis showed that MAFLD was significantly risk factor with mortality in the female (HR = 5.177, 95%CI: 1.475-18.193). However, MAFLD was not related to RFS This correlation was consistent after PSM analysis.
Conclusions: MAFLD can improve the mortality of women undergoing radical resection for liver cancer, which independently estimate disease prognosis but is not related to recurrence-free survival.
Keywords: Hepatocellular carcinoma, Metabolic syndrome, Radical resection, Sex
Introduction
Hepatocellular carcinoma (HCC) ranks third among cancer-associated mortalities and sixth among all causes of cancer-related deaths across the world, with malignant tumors. In accordance with the statistics, about 906,000 recently diagnosed patients and 830,000 primary liver cancer death cases were reported [1]. HCC increases mortality by approximately 2-3% per year compared with the reduction in mortality from other common tumors, including lung and breast cancer [2]. Based on prior epidemiological information, alcohol consumption and hepatitis B/C virus (HBV/HCV) infection are major reasons for HCC. Nonalcoholic fatty liver disease (NAFLD) has been increasingly determined to become a risk factor for HCC over the past five years and is diagnosed in around 1/4 of the world's adults [3, 4]. This illustrates the significantly increased risk of NAFLD on the incidence of HCC and the need to analyze the NAFLD-HCC prognosis.
NAFLD indicates steatosis in over 5% of the liver cells after the exclusion of hepatic steatosis caused by heavy alcohol consumption or other toxic substances and drugs [3, 5-7]. Recently, MAFLD is redefined as a fatty liver disease by 30 international experts from 22 countries in 2020 [5]. MAFLD can be diagnosed on the basis of liver steatosis, obesity, prediabetes, hypertension, dyslipidemia, type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation [5, 8]. Defining diagnosis-related criteria help to identify individuals with metabolic hepatopathy. In addition, it is possible to confirm the MAFLD diagnosis in people of normal weight. As shown in a recent study, a liver biopsy was carried out on 1000 patients with MAFLD. The study suggested that hepatic steatosis severity was approximately identical in patients with a BMI <23 kg/m2 and a BMI >25 kg/m2 [9]. Therefore, metabolic health is more vital than is reflected in the definition. Additionally, MAFLD is significantly associated with 10 types of cancer, including liver cancer [10, 11].
Surgery for liver cancer remains a key approach for obtaining long-term survival of liver cancer patients [12, 13]. However, the recurrence rate of HCC after liver cancer resection is high. Recently, a systematic review of large sample data demonstrated that the 5-year overall survival (OS) rates of patients with intermediate or advanced HCC were 42% and 33%, respectively [14, 15]. Thus, the relationship between the clinical etiology and HCC prognosis after radical resection of liver cancer needs to be studied in depth. Furthermore, tumor staging and treatment measures are critical factors influencing the survival of HCC patients. Additionally, the effect of MAFLD on HCC prognosis based on cancer and clinical features is poorly understood. Therefore, the current work concentrated on comparing the functions of MAFLD in the survival of HCC patients following curative resection.
Patients and Methods
Study type and subjects
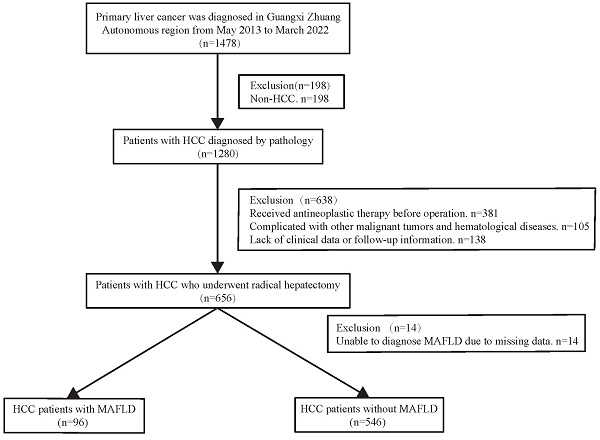
The present work had a retrospective cohort design. Consecutive patients with HCC diagnosed by pathology between May 2013 and March 2022 were treated with radical resection, following the Chinese guidelines for the diagnosis and treatment of HCC. Liver cancer cases were identified using a big data platform and health management platform from the People's Hospital of Guangxi Zhuang Autonomous Region. We used the present active health management platform to conduct a retrospective cohort study (Registration site: http://www.chictr.org.cn/index.aspx; registration number: ChiCTR2200062446). In brief, an active health management platform used an advanced medical data management system to manage patients, and connected and indexed all diagnostic and treatment records at the hospital. It contained outpatient, inpatient, and physical examination data concerning patient diagnosis. Additionally, treatment data, test reports, examination reports, electronic medical records, and other medical data related to outpatient, inpatient, and physical examinations were also recorded. All medical information can be accessed from this platform, and when a patient arrives at the clinic, the information is automatically integrated into the platform. The following are the inclusion criteria for this study: Patients who met the indications for hepatectomy underwent radical hepatectomy and were histopathologically confirmed to have HCC were contained in the study. Subjects with the following characteristics will be excluded: 1) Those with incomplete clinical data. 2) Individuals diagnosed with hepatobiliary cell carcinoma, mixed hepatocyte-cholangiocarcinoma, or combined with other system malignant tumors (such as lung cancer, colorectal cancer, etc.). 3) Patients with severe cardiac and pulmonary organ dysfunction. 4) Patients with severe infection. 5) Individuals who have not undergone preoperative transcatheter arterial chemoembolization (TACE) or radiofrequency ablation (RFA) for the first time. 6) Patients who are unable to complete the follow-up. The flowchart for patient selection is presented in Figure 1. All operations were performed according to the 1975 Helsinki Declaration. The patients did not provide written informed consent due to the retrospective nature of the present study. The approval of the current work was obtained by the institutional committee of the People's Hospital of Guangxi Zhuang Autonomous Region.
Definition
Based on the consensus reached by international experts, MAFLD is, diagnosed based on hepatic steatosis (through imaging, liver biopsy, or by using scores and blood biomarkers), combined with one or more of the listed conditions including obesity or overweight, T2DM, or metabolic disorder (those with at least two risk factors for metabolic abnormalities: hypertension, plasma triglyceride ≥ 1.70 mmol/L) [5]. The plasma high density lipoprotein-cholesterol (HDL-C) of <1.3 mmol/L and <1.0 mmol/L for women and men, respectively, prediabetes (fasting blood glucose of 5.6-6.9 mmol/L, plasma high sensitivity C-reactive protein (CRP) levels > 2 mg/L, and glycosylated hemoglobin A1c (Hb-A1c) of 5.7%-6.4% are the criteria for defining metabolic risk factors [5]. Hypertension condition is determined as diastolic blood pressure (DBP) ≥ 90 mmHg, systolic blood pressure (SBP) ≥140 mmHg or the use of hypertensive medication. Subjects with glycosylated hemoglobin ≥ 6.5, fasting blood glucose (FBG) level ≥7.0 mmol/l, or diabetes was determined as prediabetes. Dyslipidemia-diagnostic code plus drug or laboratory value (LDL cholesterol > 100 mg/dL or triglyceride > 150 mg/dL). Radical resection was defined as no tumor thrombus in the great vein and bile duct, no adjacent organ invasion, and a liver cutting edge ≥ 1cm from the tumor boundary; if the cutting edge was < 1cm, the resection margin of the liver tissue was negative, and no tumor focus was found by imaging examination 1-2 months after the operation [16, 17]. Using imaging or histopathological reports, liver steatosis and cirrhosis were identified directly. OS was regarded as the duration between randomization and all-cause mortality. RFS represents the duration from randomization to disease relapse or all-cause mortality.
Flow chart for the selection of the study population. Abbreviations: HCC: hepatocellular carcinoma; MAFLD: metabolic dysfunction-associated fatty liver disease.
Data collection and outcomes
Using the hospital big data and health management platforms, this study obtained the following patients related data: age, sex, body mass index (BMI), Hypertension, T2DM, liver cirrhosis, tumor number, tumor size, Child-Pugh liver function grade, Barcelona clinical liver cancer (BCLC) stage, macrovascular invasion, microvascular invasion, alanine aminotransferase (ALT), plasma triglyceride (TC), MAFLD, total bilirubin (TB) and Triglycerides.
The principal outcome was OS between hepatectomy and all-cause mortality or the final follow-up. None of the patients in this cohort received a living transplant. The secondary endpoints were RFS, perioperative mortality, and morbidity. This study utilized telephone calls, hospital electronic medical records, or outpatient follow-up, with the last follow-up period up to June 30, 2022. The non-recurrence survival period was determined as the period between hepatectomy and disease relapse or all-cause mortality. This study evaluated HCC relapse according to postoperative imaging data (B-ultrasound, CT, and MRI). HCC recurrence is defined as a new lesion after radical resection that fully meets the diagnostic criteria for HCC [18, 19].
Statistical analysis
Data were explored through SPSS 18.0 (BM Corp, Armonk, NY, USA) and R software (version 3.4.1, The R Project for Statistical Computing). Continuous data were represented by median and interquartile ranges (IQRs), while comparisons among groups were conducted with the Kruskal-Wallis or Mann-Whitney U tests as appropriate. Categorical data were represented by totals and frequencies. In addition, the chi-square test was adopted for comparison. Kaplan-Meier (KM) analysis was conducted with the aim of analyzing OS and RFS, which were compared with the use of the log-rank test. Factors showing significant association with survival (OS and RFS) were identified using multivariate Cox regression to evaluate hazard ratios (HR) and 95% confidence intervals (95% CI) after adjustment for possible confounding factors. Propensity score matching (PSM) analysis with a nearest-neighbor 1:1 matching scheme and a caliper size of 0.05 was used for sensitivity analysis. A P-value <0.05 was regarded to be of statistical significance.
Results
Basic demographic and clinical features of cases
From May 2013 to March 2022, 1478 patients were diagnosed with primary liver cancer. Finally, 642 patients were enrolled in the current analysis, containing 96 patients diagnosed with MAFLD. The mean age of the enrolled patients was 53 (44-62) years, and 18.1% were female. Compared with men, women were older (58.0 vs. 52.0 years old, P<0.05) and had high blood pressure (47.4% vs. 36.7%, P<0.05). However, liver synthesis function (measured by total bilirubin and alanine aminotransferase), dyslipidemia, and overweight/obesity in female patients with HCC were better than those in male patients. In addition, microvascular infiltration in females markedly decreased compared with males (29.3% vs. 44.5%; P<0.05). However, there were no statistically significant differences in type 2 diabetes, Child-Pugh grade, BCLC grade, macrovascular invasion rate, and tumor size and diameter between women and men (Table 1).
The patients were classified into two subgroups according to MAFLD diagnosis: MAFLD (15.0%) and non-MAFLD (546) groups. This study compared the clinical features of MAFLD and non-MAFLD groups (Table 2). It was shown that the MAFLD group had higher proportions of type 2 diabetes (18.8%) and hypertension (54.2%) than the non-MALFD group (8.6% and 31.9%, respectively; P<0.05). The median BMI in the MALFD group (25.6) increased in relative to the non-MAFLD group (21.6) (P<0.05). The MALFD group had increased measured triglyceride value relative to the non-MAFLD group (1.2 vs. 1.9 mmol/L; P<0.05).
Clinicopathological characteristics of HCC patients.
| Variables | Overall | Men | Women | P-value |
|---|---|---|---|---|
| n=642 | (526, 81.9%) | (116, 18.1%) | ||
| Age, year | 53.0 (44.0-62.0) | 52.0 (44.0-61.0) | 58.0 (48.0-65.0) | 0.004 |
| Body mass index, kg/m2 | 23.4 (21.3-25.6) | 23.5 (21.5-25.7) | 22.5 (20.2-24.9) | 0.004 |
| Triglycerides, mmol/L | 1.0 (0.8-1.3) | 1.0 (0.8-1.3) | 1.0 (0.7-1.2) | 0.198 |
| Alanine aminotransferase, U/L | 33.0 (23.0-52.0) | 35.0 (24.0-54.0) | 26.0 (17.0-36.5) | <0.001 |
| Total bilirubin, mmol/L | 3.2 (2.3-4.5) | 3.3 (2.4-4.6) | 2.8 (1.9-3.8) | <0.001 |
| Hypertension, n (%) | 248 (38.6%) | 193 (36.7%) | 55 (47.4%) | 0.032 |
| Type 2 diabetes mellitus, n (%) | 65 (10.1%) | 52 (9.9%) | 13 (11.2%) | 0.669 |
| Dyslipidemia, n (%) | 214 (33.3%) | 194 (36.9%) | 20 (17.2%) | <0.001 |
| Child-Pugh grade | 0.181 | |||
| A | 584 (91.0%) | 480 (91.3%) | 104 (89.7%) | |
| B | 58 (8.7%) | 46 (8.6%) | 12(9.5%) | |
| BCLC, stage | 0.203 | |||
| 0/A | 284 (44.2%) | 54 (46.6%) | 230 (43.7%) | |
| B | 31 (4.8%) | 4 (3.4%) | 27 (5.1%) | |
| C | 318 (49.5%) | 57 (49.1%) | 261 (49.6%) | |
| D | 9 (1.4%) | 1 (0.9%) | 8 (1.5%) | |
| Liver cirrhosis, n (%) | 248 (38.6%) | 209 (39.7%) | 39 (33.6%) | 0.221 |
| MAFLD, n (%) | 96 (15.0%) | 79 (15.0%) | 17 (14.7%) | 0.921 |
| Tumor size, cm | 5.0 (3.0-7.7) | 5.0 (3.2-7.6) | 5.0 (2.8-7.8) | 0.879 |
| Tumor number, n (%) | 0.559 | |||
| Single | 542 (84.4%) | 100 (86.2%) | 442 (84.0%) | |
| Multiple | 100 (15.6%) | 16 (13.8%) | 84 (16.0%) | |
| Macrovascular invasion, n (%) | 73 (11.4%) | 61 (11.6%) | 12 (10.3%) | 0.701 |
| Microvascular invasion, n (%) | 268 (41.7% | 234 (44.5%) | 34 (29.3%) | 0.003 |
Data are mean ± standard deviation, median (IQR) or N (%).
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; MAFLD, metabolic dysfunction-associated fatty liver disease.
Baseline demographic and clinical characteristics of patients with MAFLD or non-MAFLD.
| Variables | All patients | P-value | Men | P-value | Women | P-value | |||
|---|---|---|---|---|---|---|---|---|---|
| MAFLD | non-MAFLD | MAFLD | non-MAFLD | MAFLD | non-MAFLD | ||||
| (n =96) | (n =546) | (n =79) | (n =447) | (n =17) | (n =99) | ||||
| Age, year | 55.5 (45.0-64.0) | 53.0 (44.0-62.0) | 0.146 | 55.0 (44.5-61.5) | 52.0 (43.5-61.0) | 0.183 | 60.0 (51.0-66.0) | 57.0 (48.0-64.0) | 0.423 |
| BMI, kg/m2 | 25.6 (24.0-27.7) | 22.9 (21.0-24.9) | <0.001 | 25.7 (24.1-27.8) | 23.1 (21.2-25.0) | <0.001 | 25.3 (23.5-26.9) | 22.1 (19.9-24.0) | <0.001 |
| Triglycerides, mmol/L | 1.2 (1.0-1.6) | 0.9 (0.7-1.2) | <0.001 | 1.2 (1.0-1.6) | 0.9 (0.7-1.2) | <0.001 | 1.1 (0.8-1.4) | 1.0 (0.7-1.2) | 0.224 |
| ALT, U/L | 37.0 (23.5-56.5) | 33.0 (23.0-50.0) | 0.181 | 37.0 (24.0-58.0) | 34.0 (24.0-53.0) | 0.469 | 35.0 (19.0-50.0) | 25.0 (17.0-35.0) | 0.139 |
| Total bilirubin, mmol/L | 13.0 (10.2-17.2) | 13.6 (10.3-18.8) | 0.250 | 13.0 (10.5-17.1) | 14.2 (10.6-19.4) | 0.130 | 12.3 (9.7-17.1) | 11.9 (9.3-14.5) | 0.737 |
| Albumin, g/L | 38.7 (36.1-41.0) | 37.9 (34.9-40.7) | 0.131 | 38.4 (35.8-40.9) | 38.0 (35.0-40.8) | 0.436 | 39.4 (36.9-41.3) | 37.3 (34.8-40.0) | 0.055 |
| Hypertension, n (%) | 52 (54.2%) | 196 (35.9%) | <0.001 | 40 (50.6%) | 153 (34.2%) | 0.005 | 12 (70.6%) | 43 (43.4%) | 0.038 |
| T2DM, n (%) | 18 (18.8%) | 47 (8.6%) | 0.002 | 14 (17.7%) | 38 (8.5%) | 0.011 | 4 (23.5%) | 9 (9.1%) | 0.081 |
| Dyslipidemia, n (%) | 35 (36.5%) | 179 (32.8%) | 0.481 | 35 (44.3%) | 159 (35.6%) | 0.138 | 0 (0.0%) | 20 (20.2%) | 0.042 |
| Liver cirrhosis, n (%) | 34 (35.4%) | 214 (39.2%) | 0.483 | 29 (36.7%) | 180 (40.3%) | 0.551 | 5 (29.4%) | 34 (34.3%) | 0.691 |
| Child-Pugh grade, n (%) | 0.518 | 0.410 | 0.835 | ||||||
| A | 89 (92.7%) | 495 (90.7%) | 74 (93.7%) | 406 (90.8%) | 15 (88.2%) | 89 (89.9%) | |||
| B | 7 (7.3%) | 51 (9.3%) | 5 (6.3%) | 41 (9.2%) | 2 (11.8%) | 10 (10.1%) | |||
| BCLC stage, n (%) | 0.107 | 0.144 | 0.219 | ||||||
| 0/A | 37 (38.5%) | 247 (45.2%) | 29 (36.7%) | 201 (45.0%) | 8 (47.1%) | 46 (46.5%) | |||
| B | 8 (8.3%) | 23 (4.2%) | 6 (7.6%) | 21 (4.7%) | 2 (11.8%) | 2 (2.0%) | |||
| C | 48 (50.0%) | 270 (49.5%) | 41 (51.9%) | 220 (49.2%) | 7 (41.2%) | 50 (50.5%) | |||
| D | 3 (3.1%) | 6 (1.1%) | 3 (3.8%) | 5 (1.1%) | 0 (0.0%) | 1 (1.0%) | |||
| Tumor size, cm | 4.7 (3.0-6.6) | 5.0 (3.1-7.7) | 0.237 | 4.5 (2.8-6.6) | 5.1 (3.2-7.6) | 0.129 | 5.3 (4.1-6.7) | 4.9 (2.6-7.8) | 0.809 |
| Tumor number, n (%) | 0.124 | 0.260 | 0.208 | ||||||
| Single | 76 (79.2%) | 466 (85.3%) | 63 (79.7%) | 379 (84.8%) | 13 (76.5%) | 87 (87.9%) | |||
| Multiple | 20 (20.8%) | 80 (14.7%) | 16 (20.3%) | 68 (15.2%) | 4 (23.5%) | 12 (12.1%) | |||
| Macrovascular invasion, n (%) | 3 (3.1%) | 70 (12.8%) | 0.006 | 3 (3.8%) | 58 (13.0%) | 0.019 | 0 (0.0%) | 12 (12.1%) | 0.130 |
| Microvascular invasion, n (%) | 33 (34.4%) | 235 (43.0%) | 0.112 | 32 (40.5%) | 202 (45.2%) | 0.440 | 1 (5.9%) | 33 (33.3%) | 0.022 |
Data are mean ± standard deviation, median (IQR) or N (%).
Abbreviations: ALT, Alanine aminotransferase; T2DM, type 2 diabetes mellitus; BMI, body mass index; BCLC, Barcelona Clinic Liver Cancer; MAFLD, metabolic dysfunction-associated fatty liver disease.
Moreover, the macrovascular infiltration rate in the non-MAFLD group was notably higher than that in the MAFLD group (12.8 vs. 3.1; P<0.05). The other basic parameters were comparable between groups (P>0.05). In the male and female subgroup analysis, the same results were obtained by comparing the two groups.
RFS and OS in HCC cases
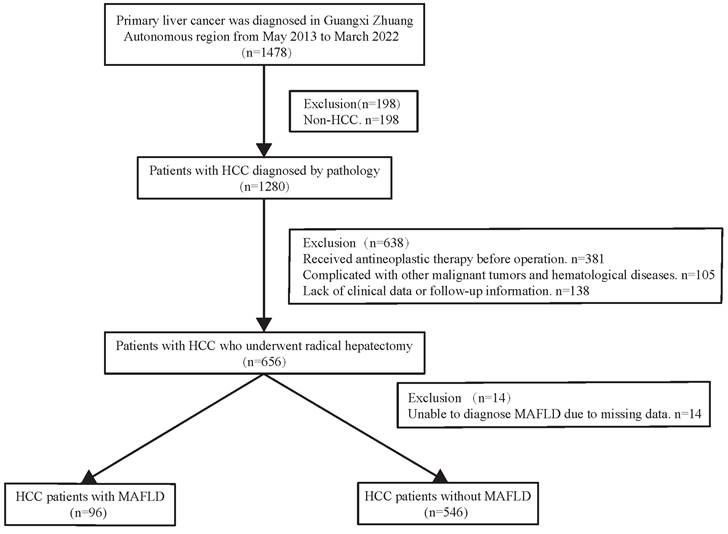
During a median 1.8-year follow-up period [IQR (0.92-3.47) years], 142 (22.12%) of the 642 patients died. In the total population (n =642), the overall survival rates for MAFLD patients at 1, 3, and 5 years were 94.45%, 80.69%, and 77.33%, respectively and in the non-MAFLD group, were 89.06%, 74.88%, and 64.51%, respectively, while the RFS rates for MAFLD patients at 1, 3, and 5 years were 72.68%, 57.92%, and 52.13%, whereas 67.47%, 49.87%, and 39.78%, respectively for non-MAFLD cases. The OS and RFS rates were comparable between the two groups (P> 0.05). The KM results demonstrated comparable OS and RFS in both groups for the overall population (P>0.05) (Figure 2C, Figure 3C). However, in the male population, the survival rate of the MAFLD group elevated in relative to the non-MAFLD group (P<0.05) (Figure 2A). By contrast, in the female population, the survival rate of the MAFLD group decreased compared to the non-MAFLD group (P<0.05) (Figure 2B). Moreover, RFS was not statistically significant. among MAFLD and non-MAFLD cases (P>0.05) (Figure 3).
Univariate as well as multivariate regression on RFS and OS-related factors in HCC patients
Tables 3 and 4 summarize the univariate and multivariate analyses of mortality and RFS on the basis of the gender of the patients. MAFLD was risk factor to mortality (HR = 5.177,95%CI1.475-18.193) in female patients, but not statistically significant in male patients and RFS rate.
Sensitivity analysis using PSM
To minimize the possible confounding bias, we performed PSM in MAFLD group and non-MAFLD group, the characteristics of the two group after PSM was showed in Table S1. Kaplan-Meier analysis performed in the after PSM data showed that the relationship between MAFLD and the OS and RFS in patients with hepatocellular carcinoma after radical resection was consistent to that prior to PSM, which validated our results (Figure S1, Figure S2).
Discussion
We used a large database from the People's Hospital of Guangxi Zhuang Autonomous Region in order to explore how MAFLD affects patient prognosis after radical resection of liver cancer in a well-matched cohort. In the current observational study, it was found that MAFLD significantly increased mortality in female HCC patients but had no significant effect on RFS and male death. MAFLD increased overall survival and relapse-free survival, but the difference was not of statistical significance.
Kaplan-Meier analysis of overall survival for each counterpart. (A) Survival rate of men group (MAFLD vs Non-MAFLD, P=0.035); (B) Survival rate of women group (MAFLD vs Non-MAFLD, P=0.030); (C) Survival rate of all patients (MAFLD vs Non-MAFLD, P=0.270). Abbreviations: MAFLD, metabolic dysfunction-associated fatty liver disease.
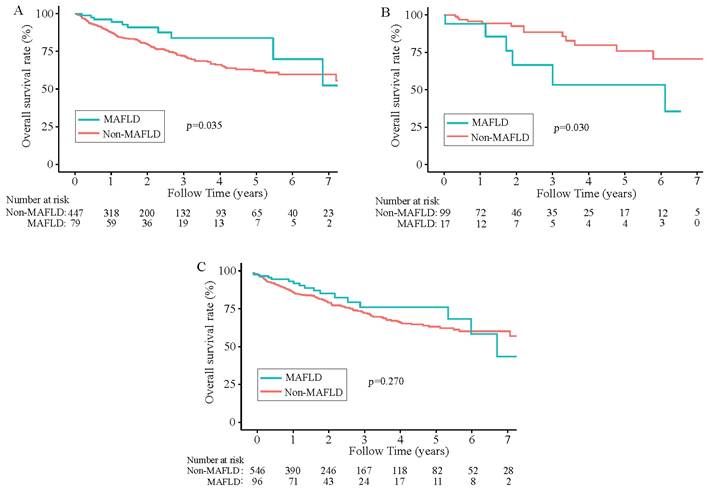
Kaplan-Meier analysis of recurrence-free survival for each counterpart. (A) Recurrence-free survival of men group (MAFLD vs Non-MAFLD, P=0.290); (B) Recurrence-free survival of women group (MAFLD vs Non-MAFLD, P=0.700); (C) Recurrence-free survival of all patients (MAFLD vs Non-MAFLD, P=0.430). Abbreviations: MAFLD, metabolic dysfunction-associated fatty liver disease.
Univariate and multivariable analyses of mortality according to sex.
| Variables | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, year | 1.01 (0.98, 1.05) | 0.514 | 1.020 (0.979, 1.062) | 0.343 | 0.98 (0.96, 1.00) | 0.013 | 0.984 (0.967, 1.000) | 0.052 |
| ALT, U/L | 1.00 (0.99, 1.01) | 0.985 | 1.00 (1.00, 1.00) | 0.582 | ||||
| Albumin, g/L | 0.88 (0.81, 0.97) | 0.008 | 0.892 (0.775, 1.027) | 0.111 | 0.98 (0.94, 1.02) | 0.350 | 0.983 (0.940, 1.028) | 0.450 |
| Hypertension | ||||||||
| No | 1 | 1.000 | ||||||
| Yes | 1.87 (0.75, 4.68) | 0.180 | 0.84 (0.58, 1.23) | 0.374 | ||||
| BMI, kg/m2 | 0.94 (0.81, 1.10) | 0.455 | 0.91 (0.86, 0.97) | 0.003 | ||||
| T2DM | ||||||||
| No | 1 | 1.000 | ||||||
| Yes | 2.07 (0.59, 7.21) | 0.255 | 1.01 (0.56, 1.84) | 0.961 | ||||
| Liver cirrhosis | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.27 (0.48, 3.37) | 0.627 | 0.88 (0.59, 1.30) | 0.515 | ||||
| Child-Pugh grade | ||||||||
| A | 1 | 1 | 1 | 1 | ||||
| B | 4.12 (1.33, 12.77) | 0.014 | 2.910 (0.426, 19.891) | 0.276 | 1.35 (0.70, 2.59) | 0.368 | 0.791 (0.359, 1.741) | 0.560 |
| BCLC stage | ||||||||
| 0/A | 1 | 1 | ||||||
| B | 4.24 (0.51, 35.30) | 0.181 | 0.86 (0.31, 2.37) | 0.769 | ||||
| C | 2.50 (0.95, 6.61) | 0.064 | 1.32 (0.91, 1.93) | 0.143 | ||||
| D | 0.00 (0.00, Inf) | 0.999 | 0.73 (0.10, 5.29) | 0.755 | ||||
| Tumor size, cm | 1.14 (1.02, 1.27) | 0.019 | 1.117 (0.945, 1.320) | 0.195 | 1.14 (1.09, 1.19) | <0.0001 | 1.091 (1.029, 1.158) | 0.004 |
| Tumor number | ||||||||
| Single | 1 | 1 | 1 | 1 | ||||
| Multiple | 2.85 (0.91, 8.94) | 0.073 | 3.656 (1.006, 13.283) | 0.049 | 1.52 (0.94, 2.44) | 0.085 | 1.505 (0.927, 2.441) | 0.098 |
| Microvascular invasion | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 2.47 (0.98, 6.23 | 0.055 | 2.840 (0.893, 9.031) | 0.077 | 2.63 (1.82, 3.79) | <0.0001 | 2.042 (1.375, 3.030) | 0.0004 |
| Macrovascular invasion | ||||||||
| NO | 1 | 1 | 1 | 1 | ||||
| Yes | 1.45(0.33, 6.34) | 0.619 | 1.091 (0.175, 6.802) | 0.926 | 2.62 (1.62, 4.22) | <0.0001 | 1.145 (0.649, 2.002) | 0.640 |
| MAFLD | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 2.80 (1.06, 7.39) | 0.038 | 5.177 (1.475, 18.193) | 0.010 | 0.51 (0.26, 0.97) | 0.039 | 0.569 (0.292, 1.089) | 0.088 |
Abbreviations: ALT, Alanine aminotransferase; BMI, body mass index; T2DM, type 2 diabetes mellitus; BCLC, Barcelona Clinic Liver Cancer; MAFLD, metabolic dysfunction-associated fatty liver disease.
Univariate and multivariable analyses of recurrence-free survival according to sex.
| Variables | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, year | 0.99 (0.97, 1.01) | 0.408 | 0.989(0.965-1.012) | 0.3431 | 0.98 (0.97, 0.99) | 0.004 | 0.988(0.976, 0.999) | 0.042 |
| ALT, U/L | 1.00 (0.99, 1.00) | 0.331 | 1.00 (1.00, 1.00) | 0.935 | ||||
| Albumin, g/L | 0.98 (0.91, 1.04) | 0.456 | 0.974(0.895-1.061) | 0.5454 | 0.97 (0.95, 1.00) | 0.040 | 0.986(0.954,1.020) | 0.412 |
| Hypertension | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.02 (0.58, 1.82) | 0.936 | 0.75 (0.57, 0.98) | 0.037 | ||||
| BMI, kg/m2 | 1.03 (0.95, 1.12) | 0.463 | 0.99 (0.95, 1.04) | 0.793 | ||||
| T2DM | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.22 (0.48, 3.09) | 0.681 | 0.90 (0.58, 1.38) | 0.624 | ||||
| Liver cirrhosis | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.32 (0.72, 2.43) | 0.369 | 0.98 (0.75, 1.28) | 0.880 | ||||
| Child-Pugh grade | ||||||||
| A | 1 | 1 | ||||||
| B | 1.12 (0.40, 3.16) | 0.825 | 0.953(0.256-3.551) | 0.9428 | 1.71 (1.13, 2.59) | 0.012 | 0.931(0.550,1.575) | 0.790 |
| BCLC stage | ||||||||
| 0A | 1 | 1 | ||||||
| B | 2.11 (0.48, 9.23) | 0.320 | 1.78 (1.05, 3.02) | 0.032 | ||||
| C | 1.95 (1.05, 3.62) | 0.034 | 1.02 (0.78, 1.33) | 0.903 | ||||
| D | 0.00 (0.00, Inf) | 0.998 | 0.58 (0.14, 2.36) | 0.450 | ||||
| Tumor size, cm | 1.09 (1.02, 1.17) | 0.013 | 1.094(0.988,1.212) | 0.0833 | 1.13 (1.09, 1.16) | <0.0001 | 1.097(1.050,1.147) | <0.0001 |
| Tumor number | ||||||||
| Single | 1 | 1 | ||||||
| Multiple | 1.64 (0.76, 3.54) | 0.203 | 1.921(0.808,4.567) | 0.1393 | 1.67 (1.20, 2.32) | 0.002 | 1.510(1.080,2.113) | 0.0161 |
| Microvascular invasion | ||||||||
| No | 1 | 1 | ||||||
| Yes | 2.49 (1.39, 4.46) | 0.002 | 2.416(1.212,4.820) | 0.0123 | 2.18 (1.68, 2.83) | <0.0001 | 1.777(1.345,2.349) | <0.0001 |
| Macrovascular invasion | ||||||||
| NO | 1 | 1 | ||||||
| Yes | 2.12 (0.94, 4.74) | 0.069 | 1.148(0.437,3.017) | 0.7793 | 2.16 (1.48, 3.14) | <0.0001 | 1.067(0.678,1.678) | 0.780 |
| MAFLD | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.16 (0.54, 2.50) | 0.698 | 1.736(0.720-4.183) | 0.219 | 0.82 (0.56, 1.19) | 0.295 | 0.991(0.674, 1.459) | 0.965 |
Abbreviations: ALT, Alanine aminotransferase; BMI, body mass index; T2DM, type 2 diabetes mellitus; BCLC, Barcelona Clinic Liver Cancer; MAFLD, metabolic dysfunction-associated fatty liver disease.
In the current work, 15% of the HCC cases satisfied the diagnostic criteria for MAFLD, similar to other cohort studies [20]. Of the MAFLD-associated HCC patients, 64.6% did not have cirrhosis, suggesting that patients with NAFLD or MAFLD may develop MAFLD and NAFLD-associated HCCs without cirrhosis [21, 22], which highlights the importance of monitoring MAFLD-associated HCC among patients with cirrhotic. Surprisingly, the OS and RFS of MAFLD-HCC cases were superior to those of non-MAFLD-HCC cases in this study, but the difference was not of statistical significance (P>0.05). Similar observations were made in a recent study that retrospectively re-analyzed 6882 HCC patients enrolled consecutively in 23 liver cancer centers in Italy from 2002 to 2019 using diagnostic criteria for MAFLD. The study reported that median overall survival (23.8 months) among HCC patients without MAFLD was lower than that of HCC patients with single-cause MAFLD (28.1 months) and HCC patients with mixed causes of MAFLD (27.1 months) [23]. Additionally, individuals diagnosed with MAFLD typically exhibit a higher BMI. Conversely, lower BMI values are often associated with conditions such as malnutrition [24]. In recent studies, it has been discovered that metabolic factors such as MAFLD [25, 26], obesity [27], and diabetes [28, 29] do not have an effect on the mortality of HCC cases, and MAFLD has been found to increase the surgical rate and liver failure rate after hepatectomy and have a positive impact on long-term prognosis [30], with no significant difference in gender. In this present study, we found that the effect of MAFLD in the prognosis in patients with HCC after radical resection was significantly different between men and women.
According to our results, despite a higher male predominance, the prevalence of diabetes and hypertension in women was still higher than that in men. The World Health Organization estimates that diabetes is more frequently associated with global deaths among women than among men (3.1% vs. 2.3%) [31]. The correlation between metabolic syndrome and tumor mortality across South Korea showed that hypertension and blood pressure were obvious risk factors for cancer-associated mortality in women [32]. A Japanese cohort study reported results similar to those of our study. During the 18.5-year follow-up period, 473 men and 297 women died due to cancer, and metabolic syndrome showed positive relation to cancer-associated death among females; the opposite was true for men [33]. In addition, our data were supported by a cohort study conducted in Canada. The study suggested that metabolic syndrome increases mortality risk among males and females (including 331 cancer cases); however, females were at a higher risk than males [34]. Similarly, a recent cohort study conducted in Switzerland suggested that MAFLD leads to a gradually elevated HCC prevalence in women [35]. In recent years, a large-scale study in the USA observed that MAFLD cases were related to a 17% [36] higher risk of all-cause death in a median 23-year follow-up period. Based on several prior studies, it can be concluded that metabolic syndrome is strongly related to a higher mortality risk among females than in males [28, 37, 38]. The MAFLD definition suggests that metabolic dysfunction plays a crucial role in disease prognosis [5, 8]. The above-mentioned studies support our findings that females with MAFLD developing hepatoma are associated with increased mortality compared with males. The relationship between MAFLD and the heightened risk of cancer death remains unclear. However, it may be attributed to factors such as obesity, insulin resistance, and the insulin-like growth factor (IGF) system [39]. Obesity can trigger inflammation, which, in turn, can lead to insulin resistance and increase the risk of cancer death [40-42]. Insulin can also stimulate the production of IGF-1, which promotes tumor growth [43]. This present study found that MAFLD heightens mortality rates in women with HCC, but not in men. BMI is a reliable indicator of overall obesity, and the link between visceral adipose tissue (VAT) and metabolic risk factors is stronger in women than in men [44, 45]. Moreover, women tend to release more excess free fatty acids than men, which heightens the risk of the triglyceride/FFA cycle and, in turn, increases the risk of obesity-mediated cancer [46, 47]. Consequently, further experiments are needed to analyze the possible heterogeneities in MAFLD and HCC prognosis according to gender.
However, the current work still has the following limitations. First, the data set was obtained from a single institution. Second, this is a retrospective cohort study. Third, the study contained only patients undergoing surgery. Consequently, it is unclear whether our findings are consistent with those of patients receiving other treatments. Furthermore, the sample size was moderate, and the proportion of women was lower than that in other similar studies. In addition, the correlation between MAFLD and HCC survival has not been completely understood because the MAFLD case number examined was relatively small and there was a potential selection bias. Therefore, it is necessary to carry out further careful research with larger sample sizes. Despite the above-mentioned limitations, we believe that the present study offers new and interesting information about gender differences in prognostic effects in patients with MAFLD-related HCC from a retrospective cohort.
In summary, MAFLD can improve the mortality rate of women undergoing radical resection for liver cancer, which independently estimate poor prognosis and is not related to recurrence-free survival.
Supplementary Material
Supplementary figures and table.
Acknowledgements
This study was supported by the Major Project of Science and Technology of Guangxi Zhuang Autonomous Region (grant number: Guike-AA22096018), the Specific Research Project of Guangxi for Research Bases and Talents (grant number: Guike-AD21220042), the National Natural Science Foundation of China (grant number 82160589), the Nanning Qingxiu District Science and Technology Bureau key research and development plan (grant number 2020035) and the National Natural Science Foundation of China (grant number 82260558).
Ethics approval
All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the institutional committee of the People's Hospital of Guangxi Zhuang Autonomous Region.
Author contributions
JH and SW: Conceptualization, Methodology, Software; JH, SW, HL and WH: Data curation, Writing- Original draft preparation. JH, SW, HL and WH: Visualization, Investigation; JY, YT, ZT, HL, QZ, XD and YT: Supervision; JH and SW: Software, Validation; JH and SW: Writing- Reviewing and Editing. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021;71:209-49
2. Wang W, Wei C. Advances in the early diagnosis of hepatocellular carcinoma. Genes & diseases. 2020;7:308-19
3. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology. 2018;15:1-20
4. Pais R, Fartoux L, Goumard C, Scatton O, Wendum D, Rosmorduc O. et al. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Alimentary pharmacology & therapeutics. 2017;46:856-63
5. Eslam M NP, Sarin SK, Anstee QM, Targher G, Romero-Gomez M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. Journal of hepatology. 2020;73:202-9
6. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of hepatology. 2016;64:1388-402
7. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M. et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md). 2018;67:328-57
8. Eslam M, Sanyal AJ, George J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1
9. Rastogi A, Shasthry SM, Agarwal A, Bihari C, Jain P, Jindal A. et al. Non-alcoholic fatty liver disease - histological scoring systems: a large cohort single-center, evaluation study. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2017;125:962-73
10. Liu Z, Lin C, Suo C, Zhao R, Jin L, Zhang T. et al. Metabolic dysfunction-associated fatty liver disease and the risk of 24 specific cancers. Metabolism: clinical and experimental. 2022;127:154955
11. Parra-Soto S, Cowley ES, Rezende LFM, Ferreccio C, Mathers JC, Pell JP. et al. Associations of six adiposity-related markers with incidence and mortality from 24 cancers-findings from the UK Biobank prospective cohort study. BMC medicine. 2021;19:7
12. Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. Journal of hepatology. 2020;72:262-76
13. Zhong JH, Ke Y, Wang YY, Li LQ. Liver resection for patients with hepatocellular carcinoma and macrovascular invasion, multiple tumours, or portal hypertension. Gut. 2015;64:520-1
14. Labgaa I, Taffé P, Martin D, Clerc D, Schwartz M, Kokudo N. et al. Comparison of Partial Hepatectomy and Transarterial Chemoembolization in Intermediate-Stage Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver cancer. 2020;9:138-47
15. Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS. et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology (Baltimore, Md). 2018;68:977-93
16. Kuo MJ, Mo LR, Chen CL, Chen CL. Factors predicting long-term outcomes of early-stage hepatocellular carcinoma after primary curative treatment: the role of surgical or nonsurgical methods. BMC cancer. 2021;21:250
17. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary surgery and nutrition. 2020;9:452-63
18. Harding-Theobald E, Louissaint J, Maraj B, Cuaresma E, Townsend W, Mendiratta-Lala M. et al. Systematic review: radiomics for the diagnosis and prognosis of hepatocellular carcinoma. Alimentary pharmacology & therapeutics. 2021;54:890-901
19. Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T. et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2018;29:iv238-iv55
20. Lin BZ, Lin TJ, Lin CL, Liao LY, Chang TA, Lu BJ. et al. Differentiation of clinical patterns and survival outcomes of hepatocellular carcinoma on hepatitis B and nonalcoholic fatty liver disease. Journal of the Chinese Medical Association: JCMA. 2021;84:606-13
21. Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C. et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology (Baltimore, Md). 2016;63:827-38
22. Xu L, Li L, Wang P, Zhang M, Zhang Y, Hao X. et al. Novel Prognostic Nomograms for Hepatocellular Carcinoma Patients with Microvascular Invasion: Experience from a Single Center. Gut and liver. 2019;13:669-82
23. Vitale A, Svegliati-Baroni G, Ortolani A, Cucco M, Dalla Riva GV, Giannini EG. et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002-2033: the ITA.LI.CA database. Gut. 2023;72:141-52
24. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. Journal of hepatology. 2019;70:172-93
25. Xiong KG, Ke KY, Chen LF, Kong JF, Lin TS, Lin QB. et al. et al. The impact of metabolic dysfunction-associated fatty liver disease on the prognosis of patients with hepatocellular carcinoma after radical resection. Hepatobiliary & pancreatic diseases international: HBPD INT. 2022Apr;6:S1499-3872 (22)00051-0
26. Liu L, Xie S, Teng YX, Deng ZJ, Chen K, Liu HT. et al. Outcomes of Liver Resection for Metabolic Dysfunction-Associated Fatty Liver Disease or Chronic Hepatitis B-Related HCC. Frontiers in oncology. 2021;11:783339
27. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. The Relation between Obesity and Survival after Surgical Resection of Hepatitis C Virus-Related Hepatocellular Carcinoma. Gastroenterology research and practice. 2013;2013:430438
28. Liang J, Ariizumi SI, Nakano M, Yamamoto M. Diabetes Mellitus and/or Nonalcoholic Steatohepatitis-related Hepatocellular Carcinoma Showed Favorable Surgical Outcomes After Hepatectomy. Anticancer research. 2019;39:5639-43
29. Wang YY, Huang S, Zhong JH, Ke Y, Guo Z, Liu JQ. et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. PloS one. 2014;9:e113858
30. Koh YX, Tan HJ, Liew YX, Syn N, Teo JY, Lee SY. et al. Liver Resection for Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma. Journal of the American College of Surgeons. 2019;229:467-78.e1
31. WHO. Global Health Estimates: Life expectancy and leading causes of death and disability. World Health Organization.
32. Lee JS, Cho SI, Park HS. Metabolic syndrome and cancer-related mortality among Korean men and women. Annals of oncology: official journal of the European Society for Medical Oncology. 2010;21:640-5
33. Watanabe J, Kakehi E, Kotani K, Kayaba K, Nakamura Y, Ishikawa S. Metabolic syndrome is a risk factor for cancer mortality in the general Japanese population: the Jichi Medical School Cohort Study. Diabetology & metabolic syndrome. 2019;11:3
34. Kuk JL, Ardern CI. Age and sex differences in the clustering of metabolic syndrome factors: association with mortality risk. Diabetes care. 2010;33:2457-61
35. Myers S, Neyroud-Caspar I, Spahr L, Gkouvatsos K, Fournier E, Giostra E. et al. NAFLD and MAFLD as emerging causes of HCC: A populational study. JHEP reports: innovation in hepatology. 2021;3:100231
36. Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. Journal of hepatology. 2021;75:1284-91
37. Vishram JK, Borglykke A, Andreasen AH, Jeppesen J, Ibsen H, Jørgensen T. et al. Impact of age and gender on the prevalence and prognostic importance of the metabolic syndrome and its components in Europeans. The MORGAM Prospective Cohort Project PloS one. 2014;9:e107294
38. Wang WS, Wahlqvist ML, Hsu CC, Chang HY, Chang WC, Chen CC. Age- and gender-specific population attributable risks of metabolic disorders on all-cause and cardiovascular mortality in Taiwan. BMC public health. 2012;12:111
39. Mendonca FM, de Sousa FR, Barbosa AL, Martins SC, Araujo RL, Soares R. et al. Metabolic syndrome and risk of cancer: which link? Metabolism. 2015;64:182-9
40. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H. et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4-2
41. Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev. 2015;95:727-48
42. Ben-Shmuel S, Rostoker R, Scheinman EJ, LeRoith D. Metabolic Syndrome, Type 2 Diabetes, and Cancer: Epidemiology and Potential Mechanisms. Metabolic Control. 2015 p. 355-72
43. Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328-36
44. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY. et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39-48
45. Tanaka S, Togashi K, Rankinen T, Perusse L, Leon AS, Rao DC. et al. Sex differences in the relationships of abdominal fat to cardiovascular disease risk among normal-weight white subjects. Int J Obes Relat Metab Disord. 2004;28:320-3
46. Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. Journal of Clinical Investigation. 2004;113:1582-8
47. Gong Y, Dou LJ, Liang J. Link between obesity and cancer: role of triglyceride/free fatty acid cycling. Eur Rev Med Pharmacol Sci. 2014;18:2808-20
Author contact
![]() Corresponding authors: Xiaofeng Dong, Department of Hepatobiliary, Pancreas and Spleen Surgery, Guangxi Academy of Medical Sciences, People's Hospital of Guangxi Zhuang Autonomous Region, Nanning530021, Guangxi, China. E-mail: gandanyingcaicom. Jianrong Yang, Department of Hepatobiliary, Pancreas and Spleen Surgery, Guangxi Academy of Medical Sciences, People's Hospital of Guangxi Zhuang Autonomous Region, No.6 Taoyuan Road, Qingxiu District, Nanning530021, Guangxi, China. E-mail: yjrorg.cn.
Corresponding authors: Xiaofeng Dong, Department of Hepatobiliary, Pancreas and Spleen Surgery, Guangxi Academy of Medical Sciences, People's Hospital of Guangxi Zhuang Autonomous Region, Nanning530021, Guangxi, China. E-mail: gandanyingcaicom. Jianrong Yang, Department of Hepatobiliary, Pancreas and Spleen Surgery, Guangxi Academy of Medical Sciences, People's Hospital of Guangxi Zhuang Autonomous Region, No.6 Taoyuan Road, Qingxiu District, Nanning530021, Guangxi, China. E-mail: yjrorg.cn.

 Global reach, higher impact
Global reach, higher impact