Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(7):1174-1181. doi:10.7150/jca.76977 This issue Cite
Research Paper
Prognostic impact of postoperative neutrophil-to-lymphocyte ratio on survival outcomes of patients treated with radical nephroureterectomy for upper urinary tract urothelial carcinoma: a single institution retrospective analysis using propensity score matching
1. Department of Urology, Dongguk University Ilsan Medical Center, Dongguk University College of Medicine, Goyang-si, Gyeonggi-do, Korea
2. Department of Biostatistics, Dongguk University College of Medicine, Goyang-si, Republic of Korea
3. Department of Urology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
Received 2023-3-30; Accepted 2023-4-19; Published 2023-5-5
Abstract
Purpose: To investigate the prognostic impact of postoperative neutrophil to lymphocyte ratio (NLR) on survival outcomes in upper urinary tract urothelial carcinoma (UTUC).
Materials and methods: Data from 397 patients with UTUC who underwent radical nephroureterectomy (RNU) without a history of neoadjuvant chemotherapy between 2002 and 2017 were retrospectively analyzed. Based on a postoperative NLR cut-off of 3, patients were divided into low NLR (<3) or high NLR (≥ 3) groups. After 2:1 propensity score matching, a Kaplan-Meier with log-rank test was used to compare survival outcomes between the two groups. Univariate and multivariate Cox proportional hazard models were used to investigate the impact of the postoperative NLR on survival outcomes.
Results: The matched cohort (n=176) consisted of 116 low NLR and 60 high NLR patients. The Kaplan-Meier curves showed significant differences in the 3- and 5-year overall and cancer-specific survival rates between the two groups (each p = 0.03). Multivariate Cox regression analysis revealed that a postoperative high NLR was an independent predictor of worse overall survival (hazard ratio [HR]:2.13; 95% confidence interval [CI]:1.18-3.85, p = 0.012) and cancer-specific survival (HR:2.16; 95% CI 1.11-4.21, p = 0.024).
Conclusions: Propensity score matching analysis revealed postoperative high NLR can be considered a potential inflammatory biomarker for predicting survival outcomes of UTUC patients treated with RNU.
Keywords: Carcinoma, transitional cell, nephroureterectomy, neutrophil to lymphocyte ratio, systemic inflammatory response, survival
Introduction
Upper urinary tract urothelial carcinoma (UTUC) is a rare malignant tumor. Although it accounts for only 5-10% of all urothelial carcinomas (UCs), it generally shows aggressive clinical behaviors with a high potential for disease recurrence and eventual death [1, 2]. Radical nephroureterectomy (RNU) with ipsilateral bladder cuff excision has long been the gold standard local treatment modality for high-risk, nonmetastatic UTUC [3]. Despite this aggressive local modality, long-term outcomes usually remain poor because of disease recurrence and distant metastasis. In particular, muscle-invasive disease (pT2 or higher) generally shows a worse prognosis, with a 5-year cancer-specific survival (CSS) rate of < 50% for pT2/pT3 disease and < 10% for pT4 [1, 4]. Such poor outcomes for UTUC suggest a need for risk stratification based on prognostic factors that can predict clinical outcomes so that proper additional treatment modalities, such as systemic chemotherapy in a perioperative setting, could be selected for UTUC patients.
Several prognostic factors have been reported to be associated with UTUC. Tumor-related factors such as pathological tumor stage, tumor grade, carcinoma in situ (CIS), lymphovascular invasion (LVI), and lymph node (LN) involvement (LNI) have been established as primary determinants of postoperative prognosis in patients with UTUC [5-7]. In addition to these tumor-related factors, the systemic inflammatory response (SIR) plays an important role in tumor development, progression, and metastasis. Many SIR-related biomarkers have been evaluated as potential prognostic factors for UTUC [8-10]. Among these inflammatory biomarkers, the neutrophil-to-lymphocyte ratio (NLR) has been vigorously assessed as a potential predictive tool in relation to oncologic outcomes, such as postoperative intravesical recurrence and survival outcomes of patients with UTUC [11-19].
Previous studies have generally suggested that an increased NLR is associated with poor oncological outcomes [11-18]. However, most studies have evaluated the prognostic value of the NLR in the preoperative setting. To the best of our knowledge, few studies have determined the prognostic value of the postoperative NLR in UTUC. Therefore, the objective of this study was to evaluate the prognostic impact of postoperative NLR on survival outcomes of patients with UTUC treated with RNU using propensity score (PS) matching methodology.
Materials and Methods
Selection of study population
The study design and use of patient information were approved by the Institutional Review Board of Seoul National University Hospital. A total of 458 patients who underwent surgery between 2002 and 2017 were initially identified in the UTUC database. After excluding patients who underwent ureterectomy (n = 22), had histology other than UC (n = 6), had a history of neoadjuvant chemotherapy (n = 8), had metastasis at diagnosis (n = 10), and had incomplete blood test data (n = 10), a total of 397 nonmetastatic patients with UTUC who underwent RNU without a history of neoadjuvant chemotherapy were eligible for the final analysis. The patient population included in this study partially overlapped with that of our previous studies [20, 21].
Included variables
The following clinical parameters were included as covariates: age at surgery, sex, body mass index (BMI), previous or concomitant bladder cancer, preoperative urine cytology results, preoperative hydronephrosis, surgical approach (open versus laparoscopic), bladder cuff excision, and adjuvant chemotherapy (ACH). BMI was defined as weight in kilograms divided by the square of height converted to meters. ACH was selectively performed at the physician's discretion for patients with pT2 or higher stage disease and/or who were LN-positive within three months after surgery. Several tumor-related parameters were also considered as covariates, including pathological tumor stage, tumor grade, concomitant CIS, LVI, variant histology, tumor multifocality, tumor location, surgical margin, and LNI. All surgical specimens were read by a urological pathology expert according to the 2010 American Joint Committee on Cancer staging system and the 2004 World Health Organization grading classification. To facilitate the analysis, tumor stage was categorized into localized disease (≤ pT2) and locally advanced disease (pT3/T4). LVI was defined as the movement of cancer cells into either the blood or lymphatic vessels without affecting the underlying muscular walls. Tumor multifocality referred to the synchronous presence of two or more pathologically confirmed tumors at any location. Tumor location was defined as renal pelvic, ureteral, or both.
NLR
To derive the NLR (defined as the absolute neutrophil count divided by the absolute lymphocyte count in peripheral blood), neutrophil and lymphocyte counts were measured using blood tests before and after surgery. Preoperative values were obtained within one month before RNU. Postoperative values were measured 3-4 months after surgery. If ACH was performed, the values measured before ACH were used. In all cases, chart review confirmed that there was no subjective or objective evidence of systemic inflammation at the time of measurement. We classified all patients into high (≥ 3) or low (< 3) NLR groups using a cutoff NLR of 3.0, based on the results of previous studies [11-13].
Postoperative follow-up regimen
The patients were followed-up according to our hospital surveillance protocol. Examinations during postoperative follow-up included history, physical examination, blood chemistry, routine urine analysis, urine cytology, and cystoscopy. Several imaging studies, including chest radiography, abdominal-pelvic computed tomography (CT), chest CT, bone scan, and magnetic resonance imaging, were selectively conducted if clinically indicated. The primary endpoints of this study were overall survival (OS) and cancer-specific survival (CSS). The OS was defined as the period from the date of surgery to the last follow-up or death. The CSS was defined as the period from the time of surgery to the direct death from cancer. The date of death was identified by reviewing medical charts and/or the annual census of the Korea National Statistical Office. The cause of death was determined by the physicians in charge and the death certificates.
Statistical analyses
The chi-square test or Fisher's exact test was used to compare the categorical variables. The Mann-Whitney U-test was used to compare continuous variables between the two NLR groups classified by their NLR values. Continuous variables are expressed as median and interquartile range (IQR). Categorical variables are expressed as absolute numbers with relative percentages. To minimize the risk of selection bias and data heterogeneity resulting from the retrospective design, the PS matching method was used to adjust for other covariates in each NLR group. PS was calculated using a logistic regression model of seven parameters: tumor stage, tumor grade, variant histology, surgical margin, LNI, age, and sex. After PS matching with a 2 (low NLR):1 (high NLR) ratio, a Kaplan-Meier log-rank test was used to compare survival outcomes between the two NLR groups. Univariate and multivariate Cox proportional hazard models were used to evaluate the prognostic impact of NLR on each survival outcome. All statistical analyses were performed using the SAS software (version 9.4; SAS Institute, Cary, NC, USA). All reported p-values were two-sided, and a p-value < 0.05 was considered to indicate a statistically significant difference.
Results
Baseline characteristics of the study cohort
Table 1 shows the baseline characteristics between the two postoperative NLR groups (high NLR and low NLR) classified based on an NLR cutoff of 3. Among the 397 patients, 63 (15.9%) showed a postoperative high NLR. After 2:1 PS matching, 176 patients (low/high NLR group:116/60 patients) were identified. Significant differences between the two groups were observed in variant histology, positive surgical margins, and LNI before PS matching, but no significant differences were observed after PS matching.
Analyzing the impact of postoperative NLR on survival outcomes
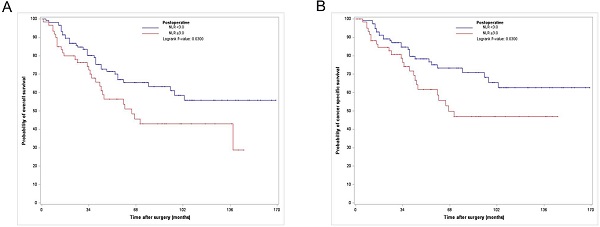
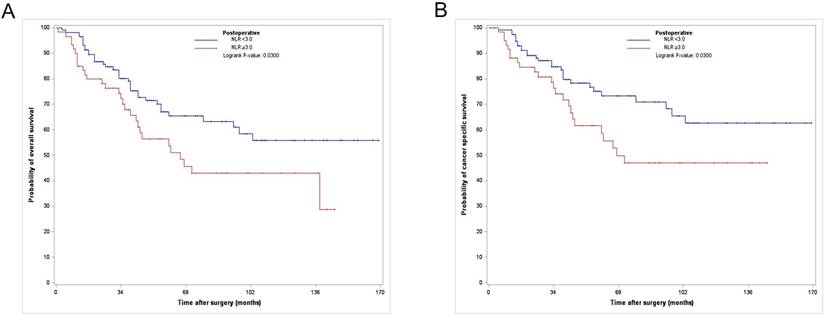
Among the 176 PS-matched patients, 65 (36.9 %) died of all causes, of which 52 (29.5%) died due to UTUC (Table 1) during a median follow-up period of 41.5 months. The Kaplan-Meier analysis showed 3- and 5-year OS rates of 71.7% and 60% and 3- and 5-year CSS rates of 76.7% and 66.7%, respectively, in the postoperative high NLR group, which were significantly lower than those in the postoperative low NLR group (3- and 5-year OS rates of 89.9% and 72.4%, and 3- and 5-year CSS rates of 86.2% and 79.3%, respectively) (Fig. 1).
Kaplan-Meier curves for overall survival (A) and cancer specific survival (B) according to postoperative NLR in the PS matched cohort. NLR, neutrophil-to-lymphocyte ratio; PS, propensity score
Patients' characteristics of the study cohort; Pre and post propensity score matching data.
| Pre-propensity score matching | Post-propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Total (n=397) | NLR < 3 (n=334, 84.1%) | NLR ≥ 3 (n=63, 15.9%) | p-value | Total (n=176) | NLR < 3 (n=116, 65.5%) | NLR ≥ 3 (n=60, 34.5%) | p-value |
| Clinical parameters | ||||||||
| Age, year, median (IQR) | 65.1(58.2, 71.6) | 65.1(58.3, 71.6) | 65.6(58.1, 70.7) | 0.793 | 65.7(58.7, 71.4) | 66 (58.7, 71.5) | 65.4(58.7, 70.5) | 0.935 |
| Gender, n (%) | 0.102 | 0.708 | ||||||
| Male | 302(76.1%) | 249(74.6%) | 53(84.1%) | 144(81.8%) | 94(81%) | 50(83.3%) | ||
| Female | 95(23.9%) | 85(25.4%) | 10(15.9%) | 32(18.2%) | 22(19%) | 10(16.7%) | ||
| BMI (kg/m2), median (IQR) | 24.3(22.9, 25.9) | 24.3(22.3, 26.0) | 24.4(22.1, 25.4) | 0.456 | 24.4(22.3, 25.7) | 24.3(22.5, 25.8) | 24.5(22, 25.2) | 0.483 |
| Previous or concomitant bladder cancer, n (%) | 0.441 | 0.346 | ||||||
| Absent | 320(80.6%) | 267(79.9%) | 53(84.1%) | 146(83%) | 94(81%) | 52(86.7%) | ||
| Present | 77(19.4%) | 67(20.1%) | 10(15.9%) | 30(17%) | 22(19%) | 8(13.3%) | ||
| Previous urine cytology result, n (%) | 0.939 | 0.684 | ||||||
| Negative | 194(48.9%) | 164(49.1%) | 39(47.6%) | 89(50.6%) | 59(50.9%) | 30(50%) | ||
| Positive | 165(41.6%)\ | 139(41.6%) | 26(41.3%) | 71(40.3%) | 49(41.4%) | 23(38.3%) | ||
| Missing/unknown | 38(9.6%) | 31(9.3%) | 7(11.1%) | 16(9.1%) | 9(7.8%) | 7(11.7) | ||
| Preoperative hydronephrosis, n (%) | 0.915 | 0.294 | ||||||
| Absent | 223(56.2%) | 188(56.3%) | 35(55.6%) | 93(52.8%) | 58(50%) | 35(58.3%) | ||
| Present | 174(43.8%) | 146(43.7%) | 28(44.4%) | 83(47.2%) | 58(50%) | 25(41.7%) | ||
| Surgical approach | 0.896 | 0.747 | ||||||
| ORNU | 280(70.5%) | 236(70.7%) | 44(69.8%) | 123(69.9%) | 82(70.7%) | 41(68.3%) | ||
| LRNU | 117(29.5%) | 98(29.3%) | 19(30.2%) | 53(30.1%) | 34(29.3%) | 19(31.7%) | ||
| Bladder cuff excision | 0.452 | 0.704 | ||||||
| Not done | 63(15.9%) | 51(15.3%) | 12(19.0%) | 29(16.5%) | 20(17.2%) | 9(15%) | ||
| Done | 334(84.1%) | 283(84.7%) | 51(81.0%) | 147(83.5%) | 96(82.8%) | 51(85%) | ||
| Pathological parameters | ||||||||
| Tumor stage, n (%) | 0.321 | 0.977 | ||||||
| ≤pT2 | 237(59.7%) | 203(60.8%) | 34(54.0%) | 100(56.8%) | 66(56.9%) | 34(56.7%) | ||
| pT3/T4 | 160(40.3%) | 131(39.2%) | 29(46.0%) | 76(43.2%) | 50(43.1%) | 26(43.3%) | ||
| Tumor grade, n (%) | 0.353 | 0.917 | ||||||
| Low grade | 127(32.0%) | 110(32.9%) | 17(27.0%) | 49(27.8%) | 32(27.6%) | 17(28.3%) | ||
| High grade | 270(68.0%) | 224(67.1%) | 46(73.0%) | 127(72.2%) | 84(72.4%) | 43(71.7%) | ||
| Concomitant CIS, n (%) | 0.640 | 0.699 | ||||||
| Absent | 339(85.4%) | 284(85.0%) | 55(87.3%) | 150(85.2%) | 98(84.5%) | 52(86.7%) | ||
| Present | 58(14.6%) | 50(15.0%) | 8(12.7%) | 26(14.8%) | 18(15.5%) | 9(13.3%) | ||
| LVI, n (%) | 0.147 | 0.901 | ||||||
| Absent | 311(78.3%) | 266(79.6%) | 45(71.4%) | 131(74.4%) | 86(74.1%) | 45(75%) | ||
| Present | 86(21.7%) | 68(20.4%) | 18(28.6%) | 45(25.6%) | 30(25.9%) | 15(25%) | ||
| Variant histology of UC, n (%) | 0.003 | 0.813 | ||||||
| Absent | 361(90.9%) | 310(92.8%) | 51(81.0%) | 148(84.1%) | 97(83.6%) | 51(85%) | ||
| Present | 36(9.1%) | 24(7.2%) | 12(19.0%) | 28(15.9%) | 19(16.4%) | 9(15%) | ||
| Tumor multifocality, n (%) | 0.269 | 0.527 | ||||||
| Unifocal | 328(82.6%) | 279(83.5%) | 49(77.8%) | 148(84.1%) | 99(85.3%) | 49(81.7%) | ||
| Multifocal | 69(17.4%) | 54(16.2%) | 14(22.2%) | 28(15.9%) | 17(14.7%) | 11(18.3%) | ||
| Tumor location, n (%) | 0.064 | 0.605 | ||||||
| Renal pelvis | 192(48.4%) | 164(49.1%) | 28(44.4%) | 82(46.6%) | 54(46.6%) | 28(46.7%) | ||
| Ureter | 148(37.3%) | 128(38.3%) | 20(31.7%) | 65(36.9%) | 45(38.8%) | 20(33.3%) | ||
| Both | 57(14.4%) | 42(12.6%) | 15(23.8%) | 29(16.5%) | 17(14.7%) | 12(20%) | ||
| Surgical margin, n (%) | <0.001 | 0.328 | ||||||
| Negative | 376(94.7%) | 323(96.7%) | 53(84.1%) | 158(89.8%) | 106(91.4%) | 52(86.7%) | ||
| Positive | 21(5.3%) | 11(3.3%) | 10(15.9%) | 18(10.2%) | 10(8.6%) | 8(13.3%) | ||
| LN status, n (%) | 0.042 | 0.497 | ||||||
| pNx | 319(80.4%) | 275(82.3%) | 44(69.8%) | 136(77.3%) | 92(79.3%) | 44(73.3%) | ||
| pN0 | 63(15.9%) | 49(14.7%) | 14(22.2%) | 30(17%) | 17(14.7%) | 13(21.7%) | ||
| pN ≥1 | 15(3.8%) | 10(3.0%) | 5(7.9%) | 10(5.7%) | 7(6%) | 3(5%) | ||
| Postoperative follow up parameters | ||||||||
| ACH, n (%) | 0.152 | 0.477 | ||||||
| Not done | 304(76.5%) | 261(78.1%) | 44(69.8%) | 129(73.3%) | 87(75%) | 42(70%) | ||
| Done | 93(23.5%) | 73(21.9%) | 19(30.2%) | 47(26.7%) | 29(35%) | 18(30%) | ||
| Follow-up duration (mos), median (IQR) | 45(24, 86) | 47.5(24, 87) | 36(18, 71) | 0.058 | 41.5(24, 79) | 42.5(23.5, 84) | 38.5(24, 72) | 0.383 |
| OS result, n (%) | <0.001 | 0.024 | ||||||
| Alive | 272(68.5%) | 241(72.2%) | 31(49.2%) | 111(63.1%) | 80(69%) | 31(51.7%) | ||
| All cause death | 125(31.5%) | 93(27.8%) | 32(50.8%) | 65(36.9%) | 36(31%) | 29(48.3%) | ||
| CSS result, n (%) | <0.001 | 0.029 | ||||||
| Alive or other cause death | 299(75.3%) | 263(78.7%) | 36(57.1%) | 124(70.5%) | 88(75.9%) | 36(60%) | ||
| Cancer-specific death | 98(24.7%) | 71(21.3%) | 27(42.9%) | 52(29.5%) | 28(24.1%) | 24(40%) | ||
Univariate analysis performed in the PS-matched cohort revealed that the postoperative NLR was significantly related to OS and CSS, along with urine cytology, surgical approach, bladder cuff excision, tumor stage, tumor grade, LVI, variant histology, tumor multifocality, surgical margin, and nodal stage (Table 2). When conducting multivariate Cox regression analysis incorporating covariates, a postoperative high NLR (≥3) was an independent predictor of worse OS (hazard ratio [HR]:2.13; 95% confidence interval [CI]:1.18-3.85, p = 0.012) and CSS (HR:2.16; 95% CI 1.11-4.21, p = 0.024) (Tables 2 and 3).
Univariable and multivariable Cox proportional hazard models for overall survival in the propensity score matched cohort (n=176)
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Variables | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
| Clinical parameters | ||||
| Age (continuous) | 1.01(0.98-1.04) | 0.465 | 1.02(0.99-1.05) | 0.139 |
| Gender (female vs. male) | 0.71(0.35-1.43) | 0.334 | 0.51(0.21-1.22) | 0.130 |
| BMI (continuous) | 0.96(0.89-1.04) | 0.344 | 1.03(0.94-1.13) | 0.533 |
| Previous or concomitant bladder cancer (present vs. absent) | 1.61(0.89-2.91) | 0.115 | 1.97(0.88-4.37) | 0.097 |
| Preoperative urine cytology (positive vs. negative) | 1.79(1.06-3.01) | 0.029 | 1.49(0.83-2.70) | 0.184 |
| Preoperative hydronephrosis (present vs. absent) | 1.35(0.83-2.20) | 0.230, | 0.95(0.53-1.71) | 0.876 |
| Surgical approach (LRNU vs. ORNU) | 2.13(1.29-3.52) | 0.003 | 2.21(1.14-4.27) | 0.019 |
| Bladder cuff excision (done vs. not done) | 0.38(0.22-0.66) | <.001 | 0.38(0.19-0.76) | 0.006 |
| ACH (done vs. not done) | 2.34(1.41-3.90) | 0.001 | 0.67(0.29-1.56) | 0.354 |
| Preoperative NLR (continuous) | 1.02(0.92-1.15) | 0.671 | 1.07(0.91-1.27) | 0.419 |
| Postoperative NLR (≥3 vs. <3) | 1.70(1.04-2.78) | 0.033 | 2.13(1.18-3.85) | 0.012 |
| Pathological parameters | ||||
| Tumor stage (pT3/4 vs. ≤pT2) | 2.70(1.64-4.45) | <.001 | 1.50(0.71-3.16) | 0.290 |
| Tumor grade (high vs. low) | 3.10(1.53-6.28) | 0.002 | 1.94(0.79-4.74) | 0.146 |
| Concomitant CIS (present vs. absent) | 0.85(0.40-1.78) | 0.661 | 0.53(0.21-1.36) | 0.188 |
| LVI (present vs. absent) | 1.88(1.12-3.15) | 0.016 | 1.13(0.56-2.29) | 0.738 |
| Variant histology of UC (present vs. absent) | 3.06(1.77-5.31) | <.001 | 2.60(1.20-5.64) | 0.016 |
| Tumor multifocality (multifocal vs. unifocal) | 2.01(1.13-3.59) | 0.018 | 1.95(0.70-5.39) | 0.199 |
| Tumor location (both vs. renal pelvis or ureter only) | 1.56(0.88-2.74) | 0.125 | 1.53(0.59-3.96) | 0.385 |
| Surgical margin (positive vs. negative) | 4.39(2.36-8.16) | <.001 | 3.48(1.51-8.00) | 0.003 |
| Nodal stage (≥pN1 vs. pN0/Nx) | 2.44(1.05-5.68) | 0.038 | 2.71(0.87-8.50) | 0.087 |
Univariable and multivariable Cox proportional hazard models for cancer specific survival in the propensity score matched cohort (n=176)
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Variables | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
| Clinical parameters | ||||
| Age (continuous) | 1.00(0.97-1.03) | 0.834 | 1.01(0.98-1.05) | 0.455 |
| Gender (female vs. male) | 0.57(0.24-1.33) | 0.193 | 0.46(0.17-1.25) | 0.129 |
| BMI (continuous) | 0.96(0.88-1.05) | 0.396 | 1.04(0.93-1.15) | 0.521 |
| Previous or concomitant bladder cancer (present vs. absent) | 1.37(0.69-2.73) | 0.372 | 2.12(0.84-5.39) | 0.114 |
| Preoperative urine cytology (positive vs. negative) | 2.42(1.32-4.43) | 0.004 | 2.05(1.03-4.08) | 0.042 |
| Preoperative hydronephrosis (present vs. absent) | 1.36(0.79-2.34) | 0.271 | 0.95(0.49-1.85) | 0.891 |
| Surgical approach (LRNU vs. ORNU) | 2.40(1.37-4.19) | 0.002 | 1.91(0.91-4.03) | 0.087 |
| Bladder cuff excision (done vs. not done) | 0.30(0.16-0.53) | <.001 | 0.25(0.12-0.52) | <.001 |
| ACH (done vs. not done) | 3.25(1.87-5.65) | <.001 | 0.99(0.38-2.58) | 0.981 |
| Preoperative NLR (continuous) | 1.01(0.89-1.15) | 0.875 | 1.03(0.84-1.27) | 0.749 |
| Postoperative NLR (≥3 vs. <3) | 1.81(1.05-3.12) | 0.033 | 2.16(1.11-4.21) | 0.024 |
| Pathological parameters | ||||
| Tumor stage (pT3/4 vs. ≤pT2) | 2.89(1.65-5.07) | <.001 | 1.14(0.47-2.78) | 0.777 |
| Tumor grade (high vs. low) | 4.76(1.89-12.00) | <.001 | 3.16(0.98-10.16) | 0.054 |
| Concomitant CIS (present vs. absent) | 0.63(0.25-1.58) | 0.32 | 0.33(0.10-1.05) | 0.06 |
| LVI (present vs. absent) | 2.29(1.31-4.02) | 0.004 | 1.07(0.50-2.33) | 0.858 |
| Variant histology of UC (present vs. absent) | 4.02(2.23-7.25) | <.001 | 2.96(1.26-6.94) | 0.013 |
| Tumor multifocality (multifocal vs. unifocal) | 1.79(0.92-3.49) | 0.088 | 2.12(0.65-6.87) | 0.212 |
| Tumor location (both vs. renal pelvis or ureter only) | 1.43(0.75-2.73) | 0.277 | 1.40(0.47-4.16) | 0.548 |
| Surgical margin (positive vs. negative) | 4.39(2.36-8.16) | <.001 | 2.50(0.97-6.41) | 0.057 |
| Nodal stage (≥pN1 vs. pN0/Nx) | 2.56(1.01-6.47) | 0.047 | 2.30(0.65-8.22) | 0.198 |
Discussion
The possible connection between inflammation and cancer was first described by Virchow in 1876, after identifying the presence of leukocytes in tumor tissues. Evidence now clearly supports that SIR plays an important role in the development, progression, metastasis, and survival of malignant cells in most cancers [22]. In most solid tumors, an intrinsic inflammatory response precedes the formation of a protumorigenic microenvironment. In the tumor microenvironment, inflammation promotes processes such as angiogenesis, tumor invasion, and metastasis, which are mediated by several chemokines and cytokines secreted by various immune cells [22, 23]. Based on this background, many clinical studies have been conducted on the prognostic value of inflammatory biomarkers in various solid tumors. Among these biomarkers, the NLR has been vigorously investigated as the most representative inflammatory biomarker [24-26].
The prognostic value of the NLR in UTUC has also been assessed in several studies. However, most of these studies have focused on the prognostic value of the preoperative NLR in relation to oncological outcomes, including intravesical recurrence and survival outcomes [11-18]. Although the threshold to define elevated or high NLR levels was not uniform among studies (ranging from 2.5 to 3), elevated NLR showed close correlations with poor oncologic outcomes, including a higher risk of intravesical recurrence [15. 18] and unfavorable survival outcomes [11-14. 16, 17], regardless of the NLR threshold. Only a few studies have presented results on the prognostic value of the postoperative NLR in UC. In a retrospective analysis of 385 patients who underwent radical cystectomy for bladder UC, an elevated NLR (≥ 2) was significantly associated with unfavorable pathologic outcomes (such as higher disease stage (≥pT3), LVI, and LNI) and poorer OS and CSS [27]. In addition, when the entire population was divided by perioperative (before and after surgery) NLR changes, the group showing pre- and postoperative elevated NLR showed poorer survival results than other groups [27]. A recent study reported the prognostic significance of postoperative NLR in UTUC. In a retrospective analysis of 134 patients with UTUC who underwent RNU, a postoperative high NLR, defined as an NLR of ≥ 2.5, showed significant associations with an advanced pathologic T stage and LVI. Five-year OS (33.7% vs. 70.2%) and CSS rates (22.7% vs. 80.7%) were also significantly lower in the postoperative high-NLR group [19].
Similar to results reported in previous studies [19, 27], our study showed that a postoperative high NLR in UTUC was associated with lower survival rates after surgery. However, our study and related studies reported so far have inherent limitations in that they were all retrospective and observational rather than a prospective, randomized design. To overcome the possible selection bias and data heterogeneity resulting from the retrospective design, we used PS-matching methodology [28]. In observational studies, PS matching is a commonly performed statistical analysis technique. When PS matching is performed, the distribution of covariates between two groups is balanced, and the possibility of bias is reduced [28]. In this study, there was a significant difference in the distribution of variant histology, surgical margins, and LNI between the NLR groups, but there was no difference between the two groups in the variables after PS matching. Based on Cox regression analysis performed in the cohort created after PS matching, postoperative high NLR, which was defined as ≥ 3, was identified as an independent factor predicting poor OS and CSS. Unlike previous studies, the strength of our study is that it was conducted using PS matching, which in turn can contribute to the generalizability of the results derived from this study. Furthermore, our study results may help determine how to follow-up patients and whether additional treatment, such as adjuvant chemotherapy, is needed after surgery in patients with UTUC.
However, the results derived from this study should be interpreted with caution because of the following limitations. First, although the PS matching method was used, the possibility of inevitable selection bias cannot be completely ruled out because of the retrospective and non-randomized design. For instance, other lymphocyte-related biomarkers, including platelet-to-lymphocyte ratio and monocyte-to-lymphocyte ratio [17, 18] and inflammation-related hematological factors such as C-reactive protein [29] and erythrocyte sedimentation rate [14], which were assessed as significant prognostic factors for UTUC in previous studies, were not included in the present study. Second, because the surgical procedures included in this study were performed by multiple surgeons, there might be heterogeneity in the surgical process and expertise. In addition, there might be variations among surgeons with regard to the criteria for determining the implementation of ACH, type of ACH regimen, and postoperative follow-up method. These factors may have affected the postoperative survival outcomes. LN dissection, which is considered an important part of the current surgical treatment for UTUC, was not performed in a significant number (approximately 80%) of patients. Therefore, the accurate LN status may not have been evaluated. The possibility that this had an effect on the survival outcomes could not be excluded. Last, since our study data were obtained from a single institution, a validation process through a multicenter study involving a large number of patients is needed in the future to generalize the results of this study.
In conclusion, our PS-matching study results indicate that the postoperative NLR can be predictive of survival outcomes after RNU for UTUC. If the predictive role of postoperative NLR is validated through additional multicenter and prospective studies in the future, it is expected to serve as a useful tool to determine whether to follow-up and provide adjuvant treatment for patients with UTUC.
Ethical approval of studies
The study design and use of patient information stored in the hospital database were approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (approval number: H-2303-130-1414). The requirement for informed consent was waived by the IRB because this was a retrospective study with complete removal of personal identifiers, and data were analyzed anonymously. This study was conducted according to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Author Contributions
Conception and design: Hyung Suk Kim, Ja Hyeon Ku; Data acquisition: Hyung Suk Kim, Ja Hyeon Ku; Data analysis and interpretation: Hyung Suk Kim, Ja Hyeon Ku; Drafting the manuscript: Hyung Suk Kim; Critical revision of the manuscript for scientific and factual content: Hyung Suk Kim and Ja Hyeon Ku; Statistical analysis: Hyung Suk Kim, Hui Seung Lee; Supervision: Ja Hyeon Ku.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ristau BT, Tomaszewski JJ, Ost MC. Upper tract urothelial carcinoma: current treatment and outcomes. Urology. 2012;79:749-56
2. Rouprêt M, Babjuk M, Compérat E. et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059-71
3. Rouprêt M, Babjuk M, Burger M. et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. 2021;79:62-79
4. Lucca I, Leow JJ, Shariat SF, Chang SL. Diagnosis and management of upper tract urothelial carcinoma. Hematol Oncol Clin North Am. 2015;29:271-88
5. Chromecki TF, Bensalah K, Remzi M. et al. Prognostic factors for upper urinary tract urothelial carcinoma. Nat Rev Urol. 2011;8:440-7
6. Verhoest G, Shariat S, Chromecki T. et al. Predictive factors of recurrence and survival of upper tract urothelial carcinomas. World J Urol. 2011;29:495-501
7. Lughezzani G, Burger M, Margulis V. et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62:100-14
8. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223-6
9. Kim M, Moon KC, Choi WS. et al. Prognostic value of systemic inflammatory responses in patients with upper urinary tract urothelial carcinoma. World J Urol. 2015;33:1439-57
10. Kim HS, Ku JH. Systemic inflammatory response based on neutrophil-to-lymphocyte ratio as a prognostic marker in bladder cancer. Disease Markers. 2016;2016:12
11. Luo H-L, Chen Y-T, Chuang Y-C. et al. Subclassification of upper urinary tract urothelial carcinoma by the neutrophil-to-lymphocyte ratio (NLR) improves prediction of oncological outcome. BJU Int. 2014;113:E144-E9
12. Tanaka N, Kikuchi E, Kanao K. et al. A multi-institutional validation of the prognostic value of the neutrophil-to-lymphocyte ratio for upper tract urothelial carcinoma treated with radical nephroureterectomy. Ann Surg Oncol. 2014;21:4041-8
13. Kohada Y, Hayashi T, Goto K. et al. Preoperative risk classification using neutrophil-lymphocyte ratio and hydronephrosis for upper tract urothelial carcinoma. Jpn J Clin Oncol. 2018;48:841-50
14. Sung HH, Jeon HG, Jeong BC. et al. Clinical significance of prognosis using the neutrophil-lymphocyte ratio and erythrocyte sedimentation rate in patients undergoing radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU Int. 2015;115:587-94
15. Kishimoto N, Takao T, Kuribayashi S. et al. The neutrophil-to-lymphocyte ratio as a predictor of intravesical recurrence in patients with upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. Int J Clin Oncol. 2017;22:153-8
16. Antonucci M, Defidio L, De Dominicis M. et al. Utility of preoperative neutrophil/lymphocyte ratio as a new objective prognostic tool in endoscopically treated upper tract urothelial carcinoma: a retrospective evaluation. J Endourol. 2020;34:993-1000
17. Shao Y, Li W, Wang D, Wu B. Prognostic value of preoperative lymphocyte-related systemic inflammatory biomarkers in upper tract urothelial carcinoma patients treated with radical nephroureterectomy: a systematic review and meta-analysis. World J Surg Oncol. 2020;18:273
18. Chien TM, Li CC, Lu YM, Chou YH, Chang HW, Wu WJ. The predictive value of systemic immune-inflammation index on bladder recurrence on upper tract urothelial carcinoma outcomes after radical nephroureterectomy. J Clin Med. 2021 10
19. Nishihara K, Suekane S, Ueda K, Nakiri M, Matsuo M, Igawa T. High postoperative neutrophil-to-lymphocyte ratio as a poor prognostic marker in patients with upper tract urothelial carcinoma. Oncol Lett. 2019;17:5241-50
20. Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. Can body mass index predict survival outcomes in patients treated with radical nephroureterectomy for upper-tract urothelial carcinoma? Int Urol Nephrol. 2015;47:1311-20
21. Kim HS, Ku JH, Jeong CW, Kwak C, Kim HH. Laparoscopic radical nephroureterectomy is associated with worse survival outcomes than open radical nephroureterectomy in patients with locally advanced upper tract urothelial carcinoma. World J Urol. 2016;34:859-69
22. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-45
23. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883-99
24. Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218-30
25. Templeton AJ, McNamara MG, Šeruga B. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124
26. Wei Y, Jiang Y-Z, Qian W-H. Prognostic role of NLR in urinary cancers: a meta-analysis. PloS one. 2014;9:e92079
27. Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. The prognostic significance of the early postoperative neutrophil-to-lymphocyte ratio in patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Ann Surg Oncol. 2016;23:335-42
28. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265-81
29. Tanaka N, Kikuchi E, Shirotake S. et al. The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: a multi-institutional study. Eur Urol. 2014;65:227-34
Author contact
![]() Corresponding author: Ja Hyeon Ku, M.D. PhD., Department of Urology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea; Tel: 82-2-2072-0361; Fax: 82-2-742-4665; E-mail: kuuro70ac.kr
Corresponding author: Ja Hyeon Ku, M.D. PhD., Department of Urology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea; Tel: 82-2-2072-0361; Fax: 82-2-742-4665; E-mail: kuuro70ac.kr

 Global reach, higher impact
Global reach, higher impact