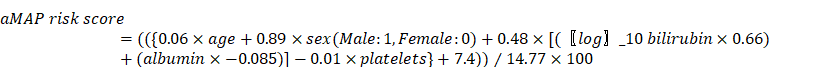Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(8):1272-1281. doi:10.7150/jca.79377 This issue Cite
Research Paper
The aMAP Score is an Independent Risk Factor for Intermediate-stage Hepatocellular Carcinoma: A Large Retrospective Cohort Study
1. Department of Oncology, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, Fujian, China.
2. Department of Oncology, The 900th Hospital of the People's Liberation Army Joint Service Support Force, Fuzong Clinical Medical College of Fujian Medical University, Fuzhou, Fujian, China.
3. Department of Gastroenterology, Xiamen Humanity Hospital, Xiamen, China.
4. Department of Gastroenterology, General Hospital of Southern Theater Command, People's Liberation Army of China, Guangzhou, Fujian, China.
5. Fujian Key Laboratory of Drug Target Discovery and Structural and Functional Research, Fuzhou, China.
6. Fujian Center for Safety Evaluation of New Drug, Fujian Medical University, Fuzhou, China.
* These authors contribute equally to the work.
Received 2022-9-29; Accepted 2023-4-11; Published 2023-5-8
Abstract
Background: A less effective nomogram for patients with intermediate-stage hepatocellular carcinoma (HCC) to predict overall survival (OS) is available. This study aimed to investigate the role of age-male-albumin-bilirubin-platelet (aMAP) scores in the prognosis of patients with intermediate-stage HCC and develop an aMAP score-based nomogram to predict OS.
Methods: Data on newly diagnosed intermediate-stage patients with HCC at Sun Yat-sen University Cancer Center between January 2007 and May 2012 were retrospectively collected. Independent risk factors affecting prognosis were selected by multivariate analyses. The optimal cut-off value for the aMAP score was determined using X-tile. The survival prognostic models were presented by the nomogram.
Results: For the 875 patients with intermediate-stage HCC included, the median OS was 22.2 months (95% CI 19.6-25.1). Patients were classified into three groups by X-tile plots (aMAP score < 49.42; 49.42 ≤ aMAP score < 56; aMAP score ≥ 56). Alpha-fetoprotein, lactate dehydrogenase, aMAP score, diameter of main tumor, number of intrahepatic lesions, and treatment regimen were independent risk factors for prognosis. A predicted model was constructed with a C-index of 0.70 (95% CI: 0.68-0.72) in the training goup, and its 1-, 3-, and 5-year area under the receiver operating curve were: 0.75, 0.73, and 0.72. The validation group of the C-index is 0.82. Calibration graphs showed good consistency between the actual and predicted survival rates. The decision curve analysis suggested the clinical utility of the model, which may help clinicians guide clinical decision-making.
Conclusion: The aMAP score was an independent risk factor for intermediate-stage HCC. The aMAP score-based nomogram has good discrimination, calibration, and clinical utility.
Keywords: hepatocellular carcinoma, intermediate-stage HCC, BCLC stage B, overall survival, aMAP score, predicted model, nomogram, TACE
Introduction
Liver cancer is the sixth most common tumor worldwide. Hepatocellular carcinoma (HCC) accounts for 75%-85% of liver cancer diagnoses [1]. The prognosis of HCC is not only affected by the size of the tumor burden but also by the residual liver function. In China, most patients have associated hepatitis B-related disease or concomitant Aspergillus flavus infection [2].
Intermediate-stage HCC, also known as Barcelona Clinic Liver Cancer (BCLC) stage B, is the most extensive stage of BCLC [3]. This group of patients may vary in terms of liver function and tumor burden, with a high Child-Pugh score of 5-9 points, significant differences in tumor size, varying tumor number, and distribution in one or two lobes, resulting in significant differences in the survival prognosis of patients [4]. The clinical benefit assessed by traditional means often differs somewhat from the actual benefit. Patients with better liver function and smaller tumor burden have a more optimistic prognosis. Studies have shown that for patients with fair liver function and small tumor burden, the 2-year overall survival rate may reach up to 63% [5].
Risk prediction models have been widely used in lung cancer, gastrointestinal tumors, and prostate cancer, among others. [6, 7]. A variety of models that predict the survival or recurrence rate of liver cancer have been established [8, 9]. Factors such as albumin, bilirubin, platelets, alpha-fetoprotein (AFP), tumor location, tumor number, and microvascular invasion can affect the prognosis of HCC [10, 11]. Thus, subsequent studies have adjusted for these risk factors to construct different models. The most widely known staging systems include the Okuda staging system, Child-Pugh liver function grading criteria, TNM staging system, Italian Liver Cancer Program (CLIP), and BCLC staging systems. However, the performance of these staging systems is unsatisfactory because of their low applicability in distinguishing heterogeneous cohorts. Therefore, many newly developed scoring systems, such as albumin-bilirubin (ALBI), platelet-albumin-bilirubin (PALBI), ALBI-T, and modified ALBI-T, have been developed [12, 13]. These scoring systems mainly use laboratory indicators such as albumin, bilirubin, platelets, and AFP, show superior predictive ability to more specifically predict the prognosis of individual patients, and affirm the irreplaceable role of clinical laboratory indicators in prognosis prediction. However, none of these models are suitable for patients with intermediate-stage HCC. There is still a lack of survival prognostic models for patients with intermediate-stage HCC.
Recently, Hou [14] developed an age-male-ALBI-platelet (aMAP) scoring system - the world's first diagnostic prediction model for predicting the 5-year risk of HCC across diseases and ethnic groups. They verified that five parameters, including age, sex, platelets, albumin, and bilirubin, were independent risk factors for the development of HCC. The patients were divided into three groups by aMAP scores: high risk, middle risk, and low risk groups (high risk group: aMAP score > 60; middle risk group: 50 ≤ aMAP score ≤ 60; low risk group: aMAP score < 50).
There are several prediction models based on aMAP scores, which are mostly used for predicting the likelihood of liver disease patients with different baseline characteristics developing HCC [15-17]. In addition, aMAP score as the primary variable was constructed to predict recurrence of HCC after radiofrequency ablation in a Chinese population prediction model by two other studies [18, 19]. Studies have shown that there is a certain relationship between the chronic development of liver cancer and the survival prognosis of patients [20].
The importance of the aMAP score in assessing the OS of intermediate-stage HCC has not been determined. Therefore, we retrospectively collected data from HCC patients to investigate the risk factors affecting OS and constructed a new model based on the aMAP score specialized for intermediate-stage HCC patients.
Materials and Methods
Data sources and patient selection
The clinical data of this study were mainly derived from a large retrospective multicenter study with a detailed introduction [21-24]. In this study, we included the baseline clinical data of intermediate-stage HCC patients in the derivation cohort at Sun Yat-sen University Cancer Center from January 2007 to May 2012 who received TACE or hepatectomy as first-line treatment.Validation cohort was established by IM-HCC patients from multiple centre between December 2018 to May 2022.Patients were followed up monthly during the period of initial treatment for the first 2 years while decreasing to every 3-6 months after 2 years of remission.
Patients who satisfied the inclusion criteria were enrolled and excluded if they met any of the exclusion criteria. The inclusion criteria were as follows: clinical diagnosis of stage B HCC; complete data any of the following at initial diagnosis (computerized tomography (CT) or magnetic resonance imaging of the abdominal region, radiography or CT of the chest, routine bloodwork test, biochemical routine test, serum AFP level, and coagulation indices); chronic hepatitis B virus-associated hepatocellular carcinoma; no history of other malignancies. The exclusion criteria were as follows: absence of laboratory parameters such as albumin (ALB), platelet count, and TBIL at initial diagnosis.
The Clinical Research Department of the Sun Yat-sen University Cancer Center approved the study protocol (2017-FXY-129). Since this study was a secondary analysis study and the patient data were anonymized, the need for informed consent was waived. Patients or the public were not involved in our study's design, conduct, reporting, or dissemination plan.
Definitions of variables and outcomes
Only baseline data of serum tumor markers, medical imaging, and biochemical and hematological parameters were included in the analysis. The primary site of the tumor in both the left and right lobes was defined as both lesions. aMAP score was calculated using sex, age, total bilirubin (μmol/L), albumin (g/L), and platelets (103/mm3). The formula [14] is as follows:
The primary study endpoint of this study was overall survival (OS).
Model construction, performance evaluation, and statistical analysis
The optimal cut-off value of the aMAP score and the model risk score were determined using X-tile software [25]. Categorical variables are presented as numbers and proportions. In this study, the Kaplan-Meier method was used to calculate the OS, and the log-rank test was used to compare the differences. Factors affecting OS were identified using the Cox proportional hazards model. Variables with p < 0.05, as determined by multivariate Cox regression analysis, were included in the final nomogram.
Internal validation was tested by performing 500 bootstrap resampling tests. External validation was performed by a completed independence group collected from multiple centre. Model discrimination was mainly evaluated using the C-index and area under the receiver operating characteristic curve (AUC). Calibration graphs were drawn to determine the degree of agreement between the predicted probability of a model and the observed probability. Decision curve analysis (DCA) is often used to evaluate model clinical utility.
Statistical analyses were mainly performed using X-tile software (Yale University School of Medicine, New Haven, CT, USA) and Empower (R) (www.empowerstats.com X&Y solutions, Inc. Boston, MA, USA), and R language software (version 4.2.2; Vienna, Austria; Fig. www.r-project.org).
This study was reported according to the requirements of the Transparent Reporting of a Multivariate Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement [26].
Results
Baseline characteristics and survival prognosis of HCC patients
Data on 5005 pathologically confirmed cases of HCC from January 2007 to May 2012 at Sun Yat-sen University Cancer Center were collected into the training group; 882 patients met the inclusion criteria. Among them, the data of baseline figures such as PTL, albumin, TBIL, of seven patients were lost. Finally, 875 patients with intermediate-stage HCC who met the inclusion criteria were included in the training group (Figure S1). 41 patients from multiple centre were finally met the inclusion criteria and included in the validation group. All patients had hepatitis B cirrhosis-associated liver cancer. Patient baseline characteristic are shown in Table 1.
The median OS of the training patients was 22.2 months, and the 1-, 3-, and 5-year OS rates were 0.67 (95% CI 0.63-0.70), 0.38 (95% CI 0.35-0.42), and 0.29 (95% CI 0.25-0.33).
The best cut-off value of aMAP score
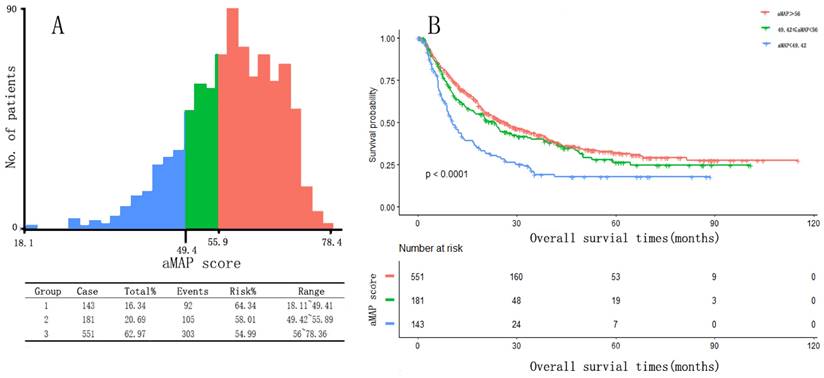
Two optimal cut-off points for the aMAP score were determined using X-tile: 49.42 and 56. Patients were divided into three groups with aMAP score < 49.42, 49.42 ≤ aMAP< 56, and aMAP score ≥ 56, accounting for 16.3%, 20.7%, and 63.0% of the total population. The median OS of the three groups was 10.4, 23.3 and 26.2 months. The Kaplan-Meier curve suggested that the aMAP score had a significant effect on OS (p < 0.0001) (Figure 1).
Univariate and multivariate analyses of risk factors and OS
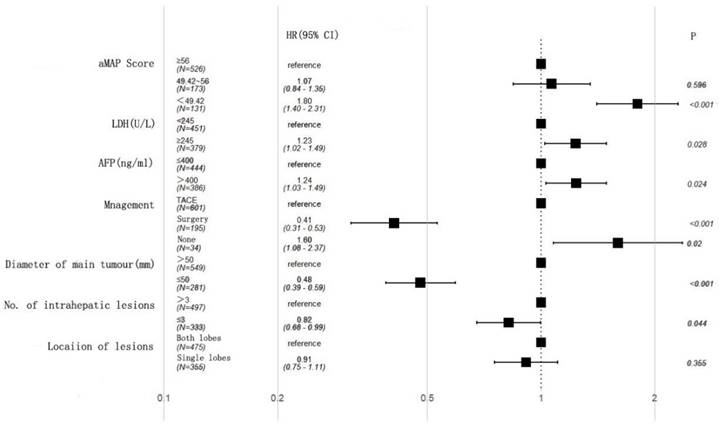
A univariate analysis showed that AFP, lactate dehydrogenase (LDH), aMAP score, diameter of main tumor, lesion location, number of intrahepatic lesions, and treatment were associated with OS (Table 2). A multivariate analysis further demonstrated that AFP (HR: 1.24, 95% CI 1.03-1.49, p = 0.024), LDH (HR: 1.23, 95% CI 1.02-1.49, p = 0.028), diameter of main tumor (HR: 0.48, 95% CI 0.39-0.59, p < 0.001), treatment (Surgery: HR: 0.41, 95% CI 0.31-0.53, p < 0.001; None: HR: 1.60, 95% CI 1.08-2,37, p = 0.02), aMAP score (49.42-56; HR: 1.07, 95% CI 0.84-1.35, p = 0.596; <49.42; HR: 1.80, 95% CI 1.40-2.31, p < 0.001), and number of intrahepatic lesions (HR: 0.82, 95% CI 0.68-0.99, p = 0.044) were independent factors (Figure 2).
aMAP score(age-male-ALBI-platelet) cut-off values and Kaplan-Meier curve. (a) X-tile plots used to generate optimal cut-off values of aMAP score; (B) Kaplan-Meier curve for different group (aMAP ≥ 56; 49.42 ≤ aMAP < 56; aMAP < 49.42)
Multivariate cox regression analysis of overall survival (OS) in the training cohort.
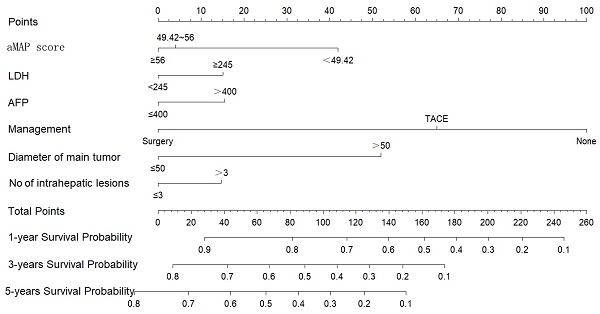
Nomogram of OS
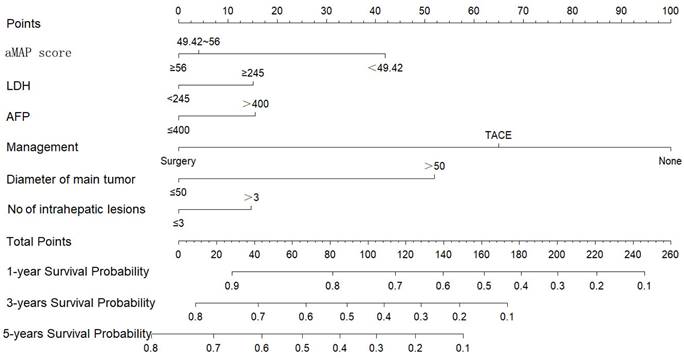
We constructed a nomogram to predict the 1-, 3-, and 5-year survival rates of intermediate-HCC patients (Figure 3). The scores for each factor were summed to provide an overall score and obtain the OS probability. For example, a patient with HCC without distant metastasis had a tumor diameter of 40 mm (0 points) at initial diagnosis, 2 intrahepatic lesions (0 points), aMAP score of 60 points (0 points), AFP of 500 ng/ml (17 points), and LDH of 246 ng/ml (16 points), and planning to receive transcatheter arterial chemoembolization (TACE) (65 points), had a total score of 98 points. The expected 1-, 3-, and 5-year OS was 75%, 45%, and 33%. We also designed calculation formulas to predict survival rates (Formula S1).
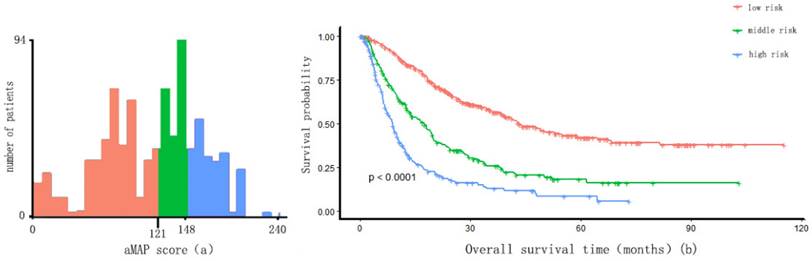
Individual patient scores were calculated according to the prognostic model, and patients were divided into a low-risk group (risk score ≤ 121 points), an intermediate-risk group (121 points < risk score ≤ 148 points), and a high-risk group (risk score > 148 points) using X-tile. Significant differences were observed in the overall survival time among the three groups (p < 0.01) (Figure 4). The survival time was 43.2, 17.5, and 8.9 months in the low-risk group, intermediate-risk group, and high-risk group, respectively.
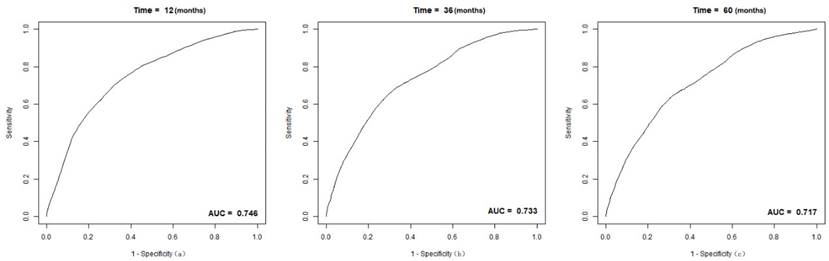
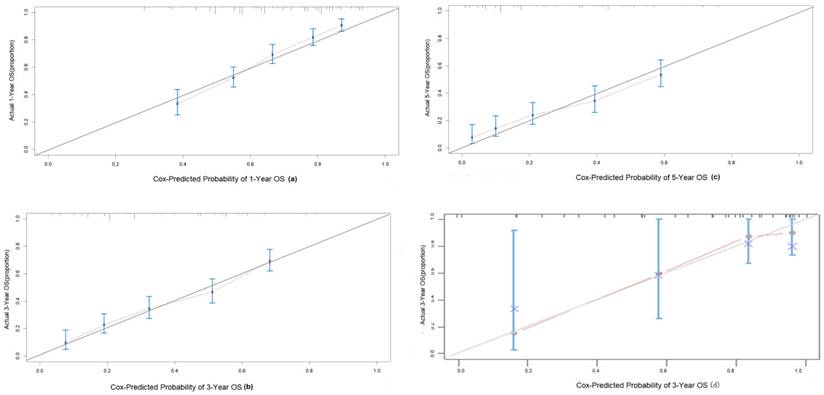
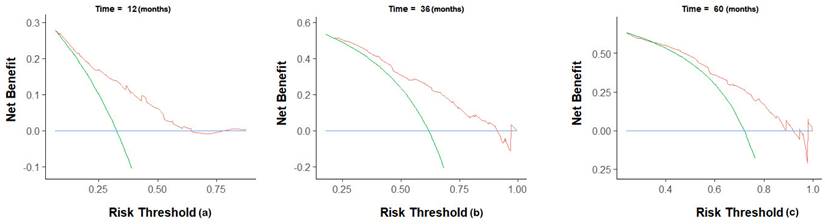
The C-index of the nomogram was 0.70(95% CI 0.68-0.72) in the training group, compared to a C-index of 0.82 in the validation group. The 1-, 3-, and 5-year AUC were 0.75, 0.73, 0.72 (Figure 5). Calibration curves showed good consistency between the actual and predicted survival rates for the prediction model (Figure 6). The clinical utility of the model was assessed using DCA (Figure 7). The intervention was supported when the predicted 1-, 3-, and 5-year overall survival rates of patients were between 0.2-0.6, 0.25-0.9, and 0.4-0.9.
Nomogram for predicting 1, 3, and 5-year OS of intermediate-stage hepatology carcinoma (HCC) patients.
Risk score cut-off values of nomogram and Kaplan-Meier curve. (a) X-tile plots used to generate optimal cut-off values of risk score of the nomogram and divide IM-HCC into three groups (low risk: risk score ≤ 121; middle risk: 121 < risk score ≤ 148; high risk: risk score > 148); (b) Kaplan-Meier curve for different group.
Discussion
It was widely used in HCC patients with AJCC TMN staging system world broad. However, its clinically utility is less efficient because only tumor burden was considered There is a significant difference in OS due to the heterogeneity of intermediate-stage HCC. The prognosis of HCC patients is not only affected by the size and number of tumors but also by the liver's residual function. Several models based on the liver reserve function have also been developed [27]. BCLC staging is the most well-known method that provides an accurate treatment regimen and predicts a patient's prognosis. However, BCLC staging is based on the Western populations. HCC in Western populations is mostly caused by alcoholic cirrhosis, while in Asian populations, it is mostly related to viral infections, especially hepatitis B virus; thus, the performance of BCLC staging in Asian populations is not satisfactory [28]. For this reason, prediction models based on Asian populations have been constructed in different countries, such as Okuda, CLIP, MESIAH, ITA.LI.CA, and HKLC score [27]. These models all have a specific population and are not well applied to the whole population, especially in the interim HCC population. Based on the characteristics of interim HCC patients, we need more targeted models.
The Intermediate HCC Patients' Demographic
| Characteristics | Total Patients (%) | |
|---|---|---|
| Training group (N=875) | Validation group (N=41) | |
| Sex | ||
| Male | 795 (90.86%) | 40 (97.56%) |
| Female | 80 (9.14%) | 1 (2.44%) |
| Age (years) | ||
| <55 | 445 (50.86%) | 18 (43.90%) |
| ≥55 | 430 (49.14%) | 23 (56.10%) |
| Treatment | ||
| TACE | 632 (72.23%) | 31 (75.60%) |
| Surgery | 206 (23.54%) | 6 (14.64%) |
| None | 37 (4.23%) | 4 (9.76%) |
| AFP (ng/ml) | ||
| ≤400 | 445 (50.85%) | 26 (63.41%) |
| >400 | 386 (44.11%) | 15 (36.59%) |
| NA | 44 (5.02%) | 0 |
| Hgb (g/L) | ||
| ≥120 | 668 (76.34%) | 30 (73.17%) |
| <120 | 207 (23.66%) | 11 (26.83%) |
| PLT (*10^9/L) | ||
| ≥100 | 651 (74.40%) | 26 (63.41%) |
| <100 | 222 (25.37%) | 15 (36.59%) |
| NA | 2 (0.22%) | 0 |
| WBC (*10^9/L) | ||
| <11 | 723 (84.07%) | 38 (92.68%) |
| ≥11 | 137 (15.93%) | 3 (7.32%) |
| AST (U/L) | ||
| ≥40 | 661 (75.54%) | 25 (60.97%) |
| <40 | 214 (24.46%) | 16 (39.03%) |
| NA | 15 (1.71%) | 0 |
| LDH (U/L) | ||
| <245 | 470 (53.78%) | 29 (70.73%) |
| ≥245 | 404 (46.22%) | 12 (26.27%) |
| NA | 1 (0.11%) | 0 |
| ALB (g/L) | ||
| ≥35 | 652 (74.51%) | 30 (73.17%) |
| <35 | 223 (25.49%) | 11 (26.83%) |
| TBIL (umol/L) | ||
| ≥17.1 | 488 (55.77%) | 19 (46.34%) |
| <17.1 | 387 (44.23%) | 22 (53.66%) |
| CRP (mg/L) | ||
| ≥10 | 501 (58.05%) | NA |
| <10 | 362 (41.95%) | NA |
| PT (s) | ||
| <13 | 630 (73.34%) | 27 (65.85%) |
| ≥13 | 229 (26.66%) | 14 (34.15%) |
| NA | 12(1.37%) | 0 |
| Location of lesions | ||
| Both lobes | 504 (57.60%) | 13 (31.70%) |
| Single lobe | 371 (42.40%) | 28 (68.30%) |
| Diameter of main tumor (mm) | ||
| >50 | 585 (66.86%) | 22 (53.66%) |
| ≤50 | 290 (33.14%) | 19 (46.34%) |
| Number of intrahepatic lesions | ||
| >3 | 523 (59.77%) | 30 (73.17%) |
| ≤3 | 352 (40.23%) | 11 (26.83%) |
| aMAP Score | ||
| ≥56 | 551 (62.97%) | 17 (41.46%) |
| 49.42≤aMAP<56 | 181 (20.69%) | 11 (26.83%) |
| <49.42 | 143 (16.34%) | 13 (31.71%) |
Univariate Analysis for Risk Factors of OS in Intermediate HCC patients
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Sex | |||
| Male | 1 | ||
| Female | 1.18 | 0.87-1.60 | 0.2780 |
| AFP (ng/ml) | |||
| ≤400 | 1 | ||
| >400 | 1.43 | 1.20-1.71 | <0.0001 |
| Hgb(g/L) | |||
| ≥120 | 1 | ||
| <120 | 1 | 0.81-1.22 | 0.9765 |
| PLT(*10^9/L) | |||
| ≥100 | 1 | ||
| <100 | 0.85 | 0.69-1.04 | 0.1149 |
| WBC(*10^9/L) | |||
| <11 | 1 | ||
| ≥11 | 1 | 0.79-1.28 | 0.9787 |
| PT (s) | |||
| <13 | 1 | ||
| ≥13 | 1.13 | 0.92-1.38 | 0.2417 |
| LDH(U/L) | |||
| <245 | 1 | ||
| ≥245 | 1.56 | 1.31-1.86 | <0.0001 |
| Diameter of main tumor (mm) | |||
| >50 | 1 | ||
| ≤50 | 0.45 | 0.37-0.55 | <0.0001 |
| Age (years) | |||
| <55 | 1 | ||
| ≥55 | 0.93 | 0.78-1.11 | 0.4494 |
| AST (U/L) | |||
| ≥40 | 1 | ||
| <40 | 1 | 0.81-1.22 | 0.9633 |
| Location of lesions | |||
| Both lobes | 1 | ||
| Single lobe | 0.69 | 0.57-0.82 | <0.0001 |
| ALB (g/L) | |||
| ≥35 | 1 | ||
| <35 | 1.30 | 1.07-1.59 | 0.0088 |
| CRP (mg/L) | |||
| ≥10 | 1 | ||
| <10 | 0.86 | 0.72-1.02 | 0.0900 |
| aMAP Score | |||
| ≥56 | 1 | ||
| 49.42≤aMAP<56 | 1.15 | 0.92-1.44 | 0.2080 |
| <49.42 | 1.85 | 1.47-2.34 | <0.0001 |
| TBIL (umol/L) | |||
| ≥17.1 | 1 | ||
| <17.1 | 1.04 | 0.87-1.24 | 0.6625 |
| Number of intrahepatic lesions | |||
| >3 | 1 | ||
| ≤3 | 0.64 | 0.53-0.76 | <0.0001 |
| Treatment | |||
| TACE | 1 | ||
| Surgery | 0.38 | 0.30-0.49 | <0.0001 |
| None | 1.53 | 1.06-2.22 | 0.0245 |
Besides the models mentioned above, researchers further established many models based on laboratory factors such as ALBI, PALBI and ALBI-T. Laboratory factors play an irreplaceable role in predicting prognosis because they show more objective and individual results. Although many different prediction models have been constructed to predict the prognosis and recurrence of HCC patients, there is still a lack of prediction models for intermediate-stage-HCC patients. Based on the characteristics of these patients, we need a more targeted model to predict survival outcomes in these patients.
The receiver operating (ROC) curves and area under ROC characteristic curve (AUC) values for intermediate-stage HCC patient cohort: (a) 1-year OS; (b) 3-year OS; (c) 5-year OS.
Calibration curves to predict OS in intermediate HCC patient cohort. (a) 1-year OS; (b) 3-year OS; (c) 5-year OS.
Decision curve analysis for prediction nomogram. (a) 1-year OS; (b) 3-year OS; (c) 5-year OS.
Our study retrospectively analyzed data and screened six risk factors, including AFP, LDH, aMAP score, diameter of main tumor, number of intrahepatic lesions, and treatment, that affect prognosis.
AFP is a glycoprotein (approximately 70 kDa) produced by the fetal liver and yolk sac and plays an important role in the occurrence and development of liver cancer [29]. Studies have found that high AFP expression in the serum or tumor cells indicates poor prognosis and is associated with vascular invasion, high tumor grade, and bulky liver cancer. High AFP concentration in serum is an independent prognostic parameter and is a more reliable prognostic predictor than AFP immunostaining of core biopsies [30]. It is well known that there is a significant correlation between tumor burden and prognosis. The larger the diameter and the greater the number of tumors, the worse the prognosis. Studies have found that the incidence of microvascular invasion and intrahepatic tumor metastasis is closely correlated with tumor diameter [31]. This is consistent with the conclusions of the present study.
Under anaerobic conditions, LDH can convert pyruvate into lactate for energy. A major feature of malignant tumors is hypoxia, with the tumor microenvironment being hypoxic; this affects the extracellular matrix composition, regulation of tumor immune response, and acceleration of tumor angiogenesis [32]. Under anaerobic conditions, the main energy supply for tumors is lactic acid, and it has been found that tumor cells preferentially convert glucose into lactic acid for energy supply, even under aerobic conditions [33]. This shift in metabolic mode favors the adaptation of tumor cells to an anaerobic environment. There is increasing evidence that poor prognosis and poor treatment response are associated with elevated LDH levels [34]. Zhang et al. also found a negative correlation between the OS and LDH levels [35].
TACE is used as a routine treatment for BCLC B stage patients. However, with the advancements in medical technology, hepatectomy leads to better results in some patients. On propensity matching score analysis of 257 patients who received surgical treatment and 135 patients who received TACE for BCLC B stage, a significant difference in the OS (p < 0.001) was observed [36]. The model constructed in this study also suggested that treatment was an independent risk factor for prognosis. Patients treated surgically have lower scores and better prognoses.
aMAP is a diagnostic prediction model for predicting the occurrence of liver cancer in patients with chronic hepatitis [14]. The indicators included in the model mainly included age, sex, ALBI, and platelet. Age is a well-known indicator of prognosis: the prognosis of older patients is significantly worse than that of younger patients [37]. ALBI is a simple, objective, evidence-based validated model for evaluating liver function and has been used internationally to predict the prognosis of patients with HCC. Platelets play a key role in the proliferation of HCC, and some studies suggest that their adhesion protein receptors GPIIb/IIIa and GPIb-IX-V may play a role in the distant metastasis of HCC [38]. In addition, antiplatelet therapy was found to significantly reduce the risk of death after resection in a large retrospective study, suggesting that elevated platelets are a poor prognostic factor [39]. Therefore, we conducted this study and showed that aMAP score could not only distinguish the prognosis of patients but also be used as an independent risk factor to predict the prognosis of patients.
In this study, the above factors were included in the model, and 500 internal bootstrap validations were performed to obtain a nomogram with a C-index of 0.70 (95% CI: 0.68, 0.72), in which the AUC for 1-, 3-, and 5-year OS were 0.75, 0.73, and 0.72 in the training group and a C-index of 0.82 in the validation group, which shows a good predictive ability. Meanwhile, the calibration chart of the 3-year prediction rate of the model coincides with the standard line, indicating that the OS predicted by the model is consistent with the actual. Our nomogram can accurately reflect the actual event occurrence. Moreover, our study provides a DCA curve to make a model utility evaluation index.
Our study has several strengths. First, this was a large retrospective cohort study with clinical data of 875 patients. As real-world data were used, the included population was more in line with that seen in real-world practice. Second, the nomogram we established was well performed when applied in the external group. Third, the study included the baseline characteristics of individual patients, and the differences in individual patients were fully considered. The targeted prognosis prediction for individual patients was in line with the concept of modern precision medicine. Moreover, the model was presented in the form of a nomogram, which is more convenient, intuitive, and operable in clinical settings. Patients were divided into three groups by risk score. A calculation formula based on this model was developed and designed to provide another way to predict prognosis.
However, there were some limitations. As a secondary analytical study, this study inevitably inherits the limitations of original data, including missing partial data and failure to collect more indicators that may affect the study results. At the same time, the data of this study came from a single center, which may have caused some selection bias. The selection bias may cause underestimation or overestimation of the prognosis when the model is applied to other populations.
In conclusion, aMAP score was an independent risk factor for predicting prognosis. Based on the aMAP score, we constructed a nomogram that can more objectively and accurately predict the 1-, 3-, and 5-year survival rates of individual patients with intermediate-stage HCC. Further DCA analysis indicates that our model has clinical applicability and can be useful in clinical decision-making.
Abbreviations
AFP: alpha-fetoprotein; ALBI: albumin-bilirubin; aMAP: age-male-albumin-bilirubin-platelet; AUC: area under the receiver operating characteristic curve; BCLC: Barcelona Clinic Liver Cancer; CLIP: Italian Liver Cancer Program; CT: computerized tomography; DCA: decision curve analysis; HCC: hepatocellular carcinoma; LDH: lactate dehydrogenase; OS: overall survival; PALBI: platelet-albumin-bilirubin; TACE: transcatheter arterial chemoembolization; TBIL: total bilirubin.
Supplementary Material
Supplementary figure and table, formula.
Acknowledgements
We are gratefully thankful for the statistical support from Empower U team of the Department of Epidemiology and Biostatistics, X&Y Solutions in Boston, and R language software version 4.1.1. We also would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81302067 and 81502360), Natural Science Foundation of Fujian Province (No. 2016J01576 and 2020J011147), and the Science and Technology Innovation Joint Foundation of Fujian Province (No. 2017Y9125).
Author Contributions
Conception and design: YC, WZ, LL, and YL. Collection and assembly of data: YS, SW, RW and QL. Data analysis and interpretation: YC, PZ, and XC. Manuscript writing: YC and XC. Final approval of the manuscript: All authors.
Data availability
Data are available in a public open access repository. The raw data were freely obtained from the Dryad Digital Repository database (www. Datadryad. org; Dryad data package; Shen, Lujun et al. (2019), Data from: Dynamically prognosticating patients with hepatocellular carcinoma through survival path mapping based on time-series data, Dryad, Dataset, https:// doi. org/ 10. 5061/ dryad. pd44k8r).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Chimed T, Sandagdorj T, Znaor A, Laversanne M, Tseveen B, Genden P. et al. Cancer incidence and cancer control in Mongolia: Results from the National Cancer Registry 2008-12. Int J Cancer. 2017;140:302-9
3. Llovet J, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2004;10:S115-20
4. Piscaglia F, Ogasawara S. Patient Selection for Transarterial Chemoembolization in Hepatocellular Carcinoma: Importance of Benefit/Risk Assessment. Liver Cancer. 2018;7:104-19
5. Golfieri R, Bargellini I, Spreafico C, Trevisani F. Patients with Barcelona Clinic Liver Cancer Stages B and C Hepatocellular Carcinoma: Time for a Subclassification. Liver Cancer. 2019;8:78-91
6. Lee CK, Goldstein D, Gibbs E, Joensuu H, Zalcberg J, Verweij J. et al. Development and validation of prognostic nomograms for metastatic gastrointestinal stromal tumour treated with imatinib. Eur J Cancer. 2015;51:852-60
7. Gravis G, Boher JM, Fizazi K, Joly F, Priou F, Marino P. et al. Prognostic Factors for Survival in Noncastrate Metastatic Prostate Cancer: Validation of the Glass Model and Development of a Novel Simplified Prognostic Model. Eur Urol. 2015;68:196-204
8. Kao WY, Su CW, Chiou YY, Chiu NC, Liu CA, Fang KC. et al. Hepatocellular Carcinoma: Nomograms Based on the Albumin-Bilirubin Grade to Assess the Outcomes of Radiofrequency Ablation. Radiology. 2017;285:670-80
9. He W, Peng B, Tang Y, Yang J, Zheng Y, Qiu J. et al. Nomogram to Predict Survival of Patients With Recurrence of Hepatocellular Carcinoma After Surgery. Clin Gastroenterol Hepatol. 2018;16:756-64 e10
10. Xing H, Zhang WG, Cescon M, Liang L, Li C, Wang MD. et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford). 2020;22:677-89
11. Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH. et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-17
12. Waked I, Berhane S, Toyoda H, Chan SL, Stern N, Palmer D. et al. Transarterial chemo-embolisation of hepatocellular carcinoma: impact of liver function and vascular invasion. Br J Cancer. 2017;116:448-54
13. Campani C, Vitale A, Dragoni G, Arena U, Laffi G, Cillo U. et al. Time-Varying mHAP-III Is the Most Accurate Predictor of Survival in Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Liver Cancer. 2021;10:126-36
14. Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q. et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73:1368-78
15. Sun Y, Li Z, Liao G, Xia M, Xu X, Cai S. et al. aMAP Score as a Predictor for Long-Term Outcomes in Patients with HBV-Related Acute-on-Chronic Liver Failure. Int J Gen Med. 2022;15:407-15
16. Qian Y, Li L, Ma L, Ji R, Ying S, Zhou J. et al. Validation of the hepatocellular carcinoma early detection screening algorithm Doylestown and aMAP in a cohort of Chinese with cirrhosis. Journal of clinical laboratory analysis. 2022;36:e24296
17. Caviglia G, Troshina G, Santaniello U, Rosati G, Bombaci F, Birolo G. et al. Long-Term Hepatocellular Carcinoma Development and Predictive Ability of Non-Invasive Scoring Systems in Patients with HCV-Related Cirrhosis Treated with Direct-Acting Antivirals. Cancers. 2022;14(3):828
18. Yang Y, Zhou Y, Zhang X, Xin Y, Chen Y, Fan Q. et al. Using the aMAP Risk Score to Predict Late Recurrence Following Radiofrequency Ablation for Hepatocellular Carcinoma in Chinese Population: A Multicenter Study. J Hepatocell Carcinoma. 2021;8:837-50
19. Xin Y, Zhang X, Yang Y, Chen Y, Wang Y, Zhou X. et al. Prediction of late recurrence after radiofrequency ablation of HBV-related hepatocellular carcinoma with the age-male-albumin-bilirubin-platelets (aMAP) risk score: a multicenter study. J Gastrointest Oncol. 2021;12:2930-42
20. Ghouri Y, Mian I, Rowe J. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. Journal of carcinogenesis. 2017;16:1
21. Shen L, Zeng Q, Guo P, Huang J, Li C, Pan T. et al. Dynamically prognosticating patients with hepatocellular carcinoma through survival paths mapping based on time-series data. Nature communications. 2018;9:2230
22. Lu L, Shen L, Wu Z, Shi Y, Hou P, Xue Z. et al. Trajectories of serum α-fetoprotein and intermediate-stage hepatocellular carcinoma outcomes after transarterial chemoembolization: A longitudinal, retrospective, multicentre, cohort study. EClinicalMedicine. 2022;47:101391
23. Lu L, Zheng P, Wu Z, Chen X. VersusHepatic Resection Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma: A Cohort Study. Frontiers in oncology. 2021;11:618937
24. Lu L, Su Z, Zheng P, Wu Z, Zhang Y, He H. et al. Association between platelet count and hepatocellular carcinoma overall survival: a large retrospective cohort study. BMJ open. 2020;10:e038172
25. Camp R, Dolled-Filhart M, Rimm D. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:7252-9
26. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): the TRIPOD Statement. Br J Surg. 2015;102:148-58
27. Tellapuri S, Sutphin PD, Beg MS, Singal AG, Kalva SP. Staging systems of hepatocellular carcinoma: A review. Indian J Gastroenterol. 2018;37:481-91
28. Han KH, Kudo M, Ye SL, Choi JY, Poon RT, Seong J. et al. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2011;81(Suppl 1):158-64
29. Sauzay C, Petit A, Bourgeois AM, Barbare JC, Chauffert B, Galmiche A. et al. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clin Chim Acta. 2016;463:39-44
30. Ridder DA, Weinmann A, Schindeldecker M, Urbansky LL, Berndt K, Gerber TS. et al. Comprehensive clinicopathologic study of alpha fetoprotein-expression in a large cohort of patients with hepatocellular carcinoma. Int J Cancer. 2022;150:1053-1066
31. Yang JD, Kim WR, Park KW, Chaiteerakij R, Kim B, Sanderson SO. et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56:614-21
32. LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18:356-65
33. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-33
34. Zhang JP, Wang HB, Lin YH, Xu J, Wang J, Wang K. et al. Lactate Dehydrogenase Is an Important Prognostic Indicator for Hepatocellular Carcinoma after Partial Hepatectomy. Transl Oncol. 2015;8:497-503
35. Zhang Q, Jiao X. LDH and GGT/ALT Ratio as Novel Prognostic Biomarkers in Hepatocellular Carcinoma Patients after Liver Transplantation. Comput Math Methods Med. 2021;2021:9809990
36. Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L. et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193
37. Kao W, Chiou Y, Hung H, Su C, Chou Y, Huo T. et al. Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofrequency ablation therapy. Journal of clinical gastroenterology. 2012;46:62-70
38. Lavergne M, Janus-Bell E, Schaff M, Gachet C, Mangin PH. Platelet Integrins in Tumor Metastasis: Do They Represent a Therapeutic Target? Cancers (Basel). 2017;9(10):133
39. Lee PC, Yeh CM, Hu YW, Chen CC, Liu CJ, Su CW. et al. Antiplatelet Therapy is Associated with a Better Prognosis for Patients with Hepatitis B Virus-Related Hepatocellular Carcinoma after Liver Resection. Ann Surg Oncol. 2016;23:874-83
Author contact
![]() Corresponding author: Xiong Chen, MD, Department of Oncology, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, Fujian, China. Tel: 86-0591-22859444; Fax: 86-0591-22859444; Email: cxiongzpcedu.cn
Corresponding author: Xiong Chen, MD, Department of Oncology, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, Fujian, China. Tel: 86-0591-22859444; Fax: 86-0591-22859444; Email: cxiongzpcedu.cn

 Global reach, higher impact
Global reach, higher impact