Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(9):1541-1552. doi:10.7150/jca.84600 This issue Cite
Research Paper
Differences between Advanced Large Cell Neuroendocrine Carcinoma and Advanced Small Cell Lung Cancer: A Propensity Score Matching Analysis
Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, Jiangxi, P.R. China.
Received 2023-3-23; Accepted 2023-5-13; Published 2023-5-21
Abstract
Background: Nowadays, the characteristics and treatment of advanced pulmonary large cell neuroendocrine carcinoma (LCNEC) remain controversial. This study aimed to analyze the similarity of clinical characteristics, survival outcomes and treatment modalities between advanced LCNEC and advanced small cell lung cancer (SCLC) to provide more evidence for the study of advanced LCNEC.
Methods: All SCLC and LCNEC patient data were obtained from the SEER database (2010-2019). Pearson's χ2 test was used to compare the differences in clinical characteristics. Propensity score matching (PSM) was utilized to balance the bias of the variables between patients. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify prognostic factors. KM analysis was used to calculate survival.
Results: A total of 1094 patients with IV LCNEC and 20939 patients with IV SCLC were included in this study. The demographic characteristics and tumor characteristics of IV LCNEC and IV SCLC were different (p < 0.05). After PSM, the overall survival (OS) for IV LCNEC and IV SCLC was 6.0 months, the cancer-specific survival (CSS) was 7.0 months, and there was no significant difference in OS or CSS between the two groups. Risk/protective factors for OS and CSS were similar for IV LCNEC and IV SCLC patients. Survival outcomes were similar in patients with IV LCNEC and IV SCLC with different treatment modalities; chemoradiotherapy significantly improved OS and CSS in patients with IV LCNEC (9.0 months) and SCLC (10.0 months), however, radiotherapy alone did not improve survival in patients with IV LCNEC.
Conclusions: These results confirmed that the prognosis and treatment modalities are similar and that advanced LCNEC could be treated as advanced SCLC, which provide new evidence for the treatment of advanced LCNEC patients.
Keywords: large cell neuroendocrine carcinoma, small cell lung cancer, advanced, propensity score matching analysis, SEER database.
Introduction
Lung cancer is one of the most common tumors worldwide, increasing the global medical and economic burden [1]. Pulmonary large cell neuroendocrine carcinoma (LCNEC) is a rare tissue type of lung cancer, accounting for approximately 3% of all types of lung cancer, and its characteristics have not received sufficient attention due to its rarity [2]. LCNEC exhibits neuroendocrine characteristics, immunohistochemistry (IHC) features, biological markers and morphological characteristics, which are similar to small cell lung cancer (SCLC); therefore, the WHO classified LCNEC and SCLC as pulmonary high-grade neuroendocrine carcinoma (HGNEC) in 2015 [3]. Of note, due to the highly metastatic characteristics of SCLC and LCNEC, patients are often in an advanced stage of the tumor at the time of diagnosis, which increases the difficulty of treatment [4, 5].
The WHO reclassified LCNEC as pulmonary HGNEC, however, LCNEC is not fully equivalent to SCLC [6]. Previous studies found that LCNEC has both SCLC and non-small cell lung cancer (NSCLC) genetic profiles [7]. Based on molecular studies, the expression of some genes in LCNEC (STK11, KEAP1) was similar to that in NSCLC; therefore, LCNEC was more likely to be a hybrid subtype of SCLC and NSCLC [8]. Controversially, the characteristics of early stage LCNEC appear to be consistent with NSCLC when analyzed from clinical features, whereas in stage IV LCNEC the metastatic pattern resembles that of SCLC [9]. Advanced SCLC and advanced LCNEC are often indicative of tumors with organ metastases, their prognosis is extremely poor and their treatment has always been challenging. The median OS (mOS) for stage IV SCLC was reported to be 7.0 months, with a 3-year survival rate of only 7.2% [10], and the mOS for stage IV LCNEC was 4.0 (3.5-4.6) months [9], which is lower than that for other types of lung cancer. In addition, the treatments for advanced LCENC have been contradictory. Some studies suggest that advanced LCNEC should be treated with the SCLC-type modality because the SCLC-type modality shows better OS and progression-free survival (PFS) [11, 12], yet others oppose it [13].
Advanced LCNEC is a rare and aggressive type of cancer. Whether patients with stage IV LCNEC should be treated as NSCLC or SCLC remains controversial [14]. The Surveillance, Epidemiology, and End Results (SEER) database is unique in the number of cases, especially for LCNEC patients. In this study, we obtained stage IV SCLC and IV NSCLC data from SEER database and performed 1:1 propensity score matching (PSM) analysis to compare the clinical characteristics, prognostic factors and treatment modalities of advanced LCNEC and SCLC.
Materials and Methods
Data collection
SEER is a United States cancer patient-based database that collects data on approximately 30% of all cancer patients with the goal of reducing the burden of cancer (https://seer.cancer.gov/). LCNEC and SCLC data were downloaded from Incident SEER Research Plus Data, 17 Registries, Nov 2021 Sub (2000-2015). Inclusion criteria: (1) all subjects were diagnosed in 2010-2019; (2) age > 18; (3) primary site (ICD-O-3 /WHO 2008): lung and bronchus; (4) histology code (ICD-O-3 Hist/behav): 8013/3, 8002/3, 8041/3, 8042/3, 8043/3 and 8044/3. (5) The tumor stage was IV. Exclusion criteria: (1) Follow-up data unknown and missing; (2) Incomplete clinical data and other relevant information.
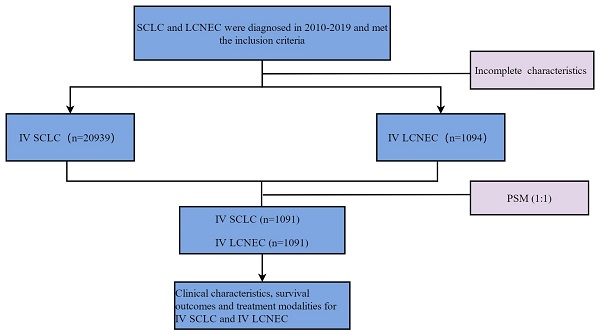
The variables collected included demographic characteristics of patients: age, gender, race, marital status. Tumor characteristics: laterality, T stage, N stage, brain metastasis, bone metastasis, liver metastasis, lung metastasis, primary site. Treatment: Radiation, chemotherapy. Survival data: Survival months, overall survival (OS) and cancer-specific survival (CSS). To facilitate statistical analysis, we reclassified some variables: age (≥ 65,< 65), marital status (married, unmarried), T stage (T0,T1,T2,T3,T4), N stage (N0,N1,N2,N3), laterality (left, right, others), radiotherapy (Yes, No), primary site (main bronchus, upper lobe, middle lobe, lower lobe, others). OS and CSS were the primary endpoints in this study. Patients diagnosed in 2016-2017 were reclassified to T stage and N stage according to the "2016 SEER Manual Section V: Stage of Disease at Diagnosis" document. Definition of Treatment options: (1) Radiotherapy: Yes: patients were treated with radiotherapy as first course of treatment. No: patients were not treated with radiotherapy as first course of treatment. (2) Chemotherapy: Yes: patients were treated with chemotherapy. No: patients were not treated with chemotherapy. (3) Chemoradiotherapy: Yes: patients were both treated with radiotherapy and chemotherapy. No: patients were treated with radiotherapy/chemotherapy alone or were given neither chemotherapy nor radiotherapy. The flow chart of patient screening is shown in Figure 1.
Propensity score matching
To reduce the effect of selection bias, PSM was applied to SCLC and LCNEC groups in this study. The matching ratio for stage IV SCLC and LCNEC groups was 1:1 and the caliper value was set to 0.03 through the “nearest” method (Figure 2). The variables used for matching were as follows: age, gender, race, marital status, T stage, N stage, laterality, primary site, brain metastasis, bone metastasis, liver metastasis, lung metastasis, radiotherapy, chemotherapy.
Statistical Methods
All statistical analyses were performed with SPSS 23.0(SPSS Inc., Chicago, IL, USA) and R version 4.2.1. P-value < 0.05 was considered statistically significant. The "MatchIt" package of R was used to perform PSM analyses. Kaplan-Meier (KM) analysis was used to compare the prognosis of different treatment modalities. Pearson's χ2 test was utilized to compare the baseline characteristics of the stage IV SCLC and LCNEC groups. Univariable and multivariate Cox proportional hazard models were used to identify risk factors for OS and CSS in the stage IV LCNEC and SCLC groups.
Flow chart for screening patients. LCNEC: large cell neuroendocrine carcinoma; SCLC: small cell lung cancer.
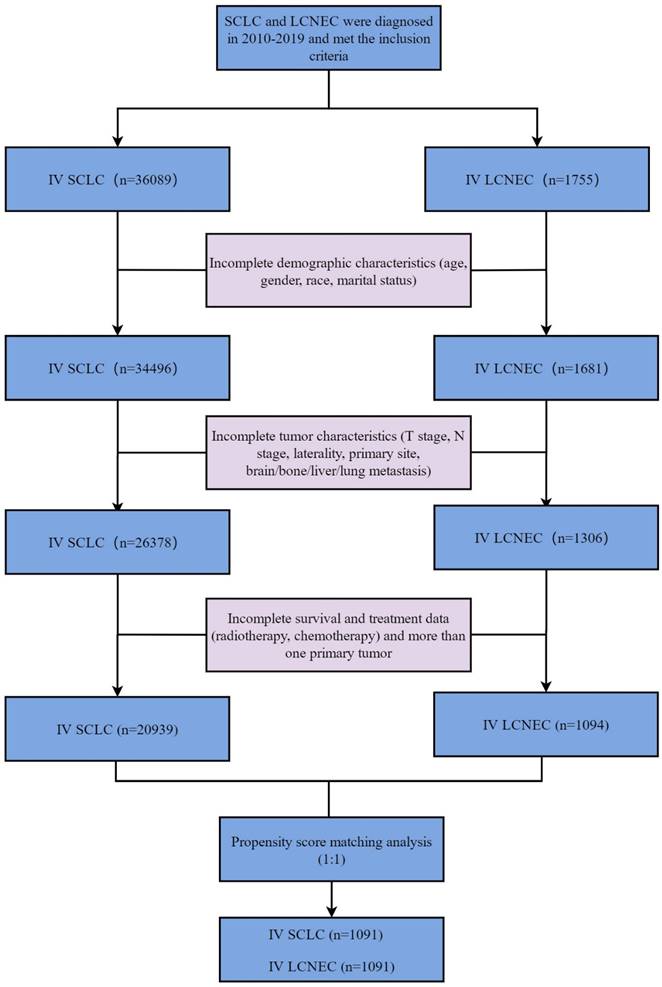
Standardized mean differences before and after PSM. PSM: propensity score matching.
Patient characteristics before PSM
| Variable | SCLC | LCNEC | p-value |
|---|---|---|---|
| All participants | 20939 (100.0%) | 1094 (100.0%) | |
| Age(years) | 0.005 | ||
| ≥65 | 12664 (60.5%) | 614 (56.1%) | |
| <65 | 8275 (39.5%) | 480 (43.9%) | |
| Gender | <0.001 | ||
| Male | 10699 (51.1%) | 619 (56.6%) | |
| Female | 10240 (48.9%) | 475 (43.4%) | |
| Race | <0.001 | ||
| Black | 1860 (8.9%) | 136 (12.4%) | |
| White | 18180 (86.8%) | 909 (83.1%) | |
| Others | 899 (4.3%) | 49 (4.5%) | |
| Marital status | 0.031 | ||
| Married | 10772 (51.4%) | 600 (54.8%) | |
| Unmarried | 10167 (48.6%) | 494 (45.2%) | |
| T stage | <0.001 | ||
| T0 | 288 (1.4%) | 19 (1.7%) | |
| T1 | 2268 (10.8%) | 176 (16.1%) | |
| T2 | 4904 (23.4%) | 270 (24.7%) | |
| T3 | 4599 (22%) | 269 (24.6%) | |
| T4 | 8880 (42.4%) | 360 (32.9%) | |
| N stage | <0.001 | ||
| N0 | 2609 (12.5%) | 253 (23.1%) | |
| N1 | 1404 (6.7%) | 107 (9.8%) | |
| N2 | 11248 (53.7%) | 481 (44%) | |
| N3 | 5678 (27.1%) | 253 (23.1%) | |
| Laterality | 0.381 | ||
| Left | 8677 (41.4%) | 431 (39.4%) | |
| Right | 11440 (54.6%) | 621 (56.8%) | |
| Others | 822 (3.9%) | 42 (3.8%) | |
| Primary site | <0.001 | ||
| Main bronchus | 2533 (12.1%) | 86 (7.9%) | |
| Upper lobe | 10059 (48%) | 544 (49.7%) | |
| Middle lobe | 794 (3.8%) | 48 (4.4%) | |
| Lower lobe | 4449 (21.2%) | 278 (25.4%) | |
| Others | 3104 (14.8%) | 138 (12.6%) | |
| Brain Metastasis | <0.001 | ||
| Yes | 5539 (26.5%) | 420 (38.4%) | |
| No | 15400 (73.5%) | 674 (61.6%) | |
| Bone Metastasis | 0.179 | ||
| Yes | 7646 (36.5%) | 377 (34.5%) | |
| No | 13293 (63.5%) | 717 (65.5%) | |
| Liver Metastasis | <0.001 | ||
| Yes | 8795 (42%) | 353 (32.3%) | |
| No | 12144 (58%) | 741 (67.7%) | |
| Lung Metastasis | 0.147 | ||
| Yes | 4335 (20.7%) | 247 (22.6%) | |
| No | 16604 (79.3%) | 847 (77.4%) | |
| Radiotherapy | <0.001 | ||
| Yes | 9718 (46.4%) | 597 (54.6%) | |
| No | 11221 (53.6%) | 497 (45.4%) | |
| Chemotherapy | <0.001 | ||
| Yes | 16669 (79.6%) | 745 (68.1%) | |
| No | 4270 (20.4%) | 349 (31.9%) |
Abbreviations: PSM: propensity score matching, SCLC: small cell lung cancer, LCNEC: large cell neuroendocrine carcinoma.
Results
Basic characteristics of patients
In this study, a total of 22033 patients were diagnosed from 2010 to 2019, including stage IV SCLC (n=20939) and stage IV LCNEC (n=1094) groups (Table 1). The basic characteristics of the patients are shown in Table 1. Compared with the stage IV SCLC group, age, gender, race, marital status, T stage, N stage, primary site, brain metastasis, liver metastasis, radiotherapy and chemotherapy were significantly different in stage IV LCNEC group before PSM (p < 0.05). T4 (42.4%), N2 (53.7%) and N3 (27.1%) were common in IV SCLC patients. Brain metastases (38.4% vs 26.5%) were more common and liver metastases (32.3% vs 42.0%) were less common in IV LCNEC than in SCLC. More IV LCNEC patients chose radiotherapy (54.6% vs 46.4%) and fewer patients chose chemotherapy (68.1% vs 79.6%) than IV SCLC patients.
Patient characteristics after PSM
| Variable | SCLC | LCNEC | p-value |
|---|---|---|---|
| All participants | 1091(100.0%) | 1091(100.0%) | |
| Age(years) | 1.000 | ||
| ≥65 | 613 (56.2%) | 612 (56.1%) | |
| <65 | 478 (43.8%) | 479 (43.9%) | |
| Gender | 0.518 | ||
| Male | 600 (55%) | 616 (56.5%) | |
| Female | 491 (45%) | 475 (43.5%) | |
| Race | 0.963 | ||
| Black | 138 (12.6% | 135 (12.4%) | |
| White | 907 (83.1%)) | 908 (83.2%) | |
| Others | 46 (4.2%) | 48 (4.4%) | |
| Marital status | 0.390 | ||
| Married | 577 (52.9%) | 598 (54.8%) | |
| Unmarried | 514 (47.1%) | 493 (45.2%) | |
| T stage | 0.380 | ||
| T0 | 17 (1.6%) | 19 (1.7%) | |
| T1 | 160 (14.7%) | 173 (15.9%) | |
| T2 | 308 (28.2%) | 270 (24.7%) | |
| T3 | 273 (25%) | 269 (24.7%) | |
| T4 | 333 (30.5%) | 360 (33%) | |
| N stage | 0.386 | ||
| N0 | 272 (24.9%) | 250 (22.9%) | |
| N1 | 95 (8.7%) | 107 (9.8%) | |
| N2 | 495 (45.4%) | 481 (44.1%) | |
| N3 | 229 (21%) | 253 (23.2%) | |
| Laterality | 0.772 | ||
| Left | 428 (39.2%) | 430 (39.4%) | |
| Right | 627 (57.5%) | 619 (56.7%) | |
| Others | 36 (3.3%) | 42 (3.8%) | |
| Primary site | 0.224 | ||
| Main bronchus | 68 (6.2%) | 86 (7.9%) | |
| Upper lobe | 587 (53.8%) | 543 (49.8%) | |
| Middle lobe | 55 (5%) | 48 (4.4%) | |
| Lower lobe | 252 (23.1%) | 276 (25.3%) | |
| Others | 129 (11.8%) | 138 (12.6%) | |
| Brain Metastasis | 0.539 | ||
| Yes | 432 (39.6%) | 417 (38.2%) | |
| No | 659 (60.4%) | 674 (61.8%) | |
| Bone Metastasis | 0.083 | ||
| Yes | 338 (31%) | 377 (34.6%) | |
| No | 753 (69%) | 714 (65.4%) | |
| Liver Metastasis | 0.550 | ||
| Yes | 339 (31.1%) | 353 (32.4%) | |
| No | 752 (68.9%) | 738 (67.6%) | |
| Lung Metastasis | 0.758 | ||
| Yes | 240 (22%) | 247 (22.6%) | |
| No | 851 (78%) | 844 (77.4%) | |
| Radiotherapy | 0.577 | ||
| Yes | 580 (53.2%) | 594 (54.4%) | |
| No | 511 (46.8%) | 497 (45.6%) | |
| Chemotherapy | 0.378 | ||
| Yes | 765 (70.1%) | 745 (68.3%) | |
| No | 326 (29.9%) | 346 (31.7%) |
Abbreviations: PSM: propensity score matching, SCLC: small cell lung cancer, LCNEC: large cell neuroendocrine carcinoma.
The stage IV SCLC group (n=1091) and stage IV LCNEC group (n=1091) were selected for further analysis after 1:1 PSM, and the baseline features were well-balanced between the IV SCLC and IV LCNEC groups (Table 2).
KM analysis for IV LCNEC and IV SCLC
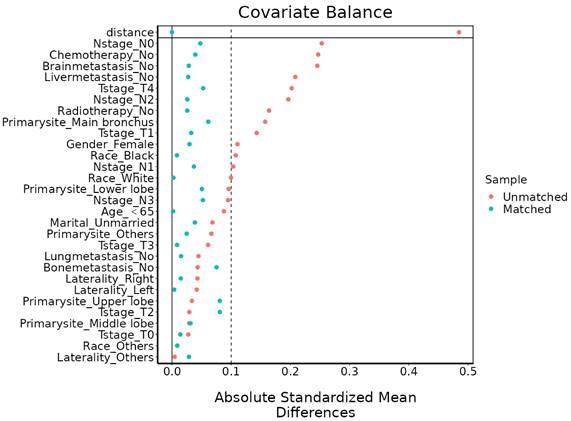
KM analysis was used to compare OS or CSS in the stage IV LCNEC and stage IV SCLC groups (Figure 3). The mOS of IV LCNEC was 6.0(95%CL: 5.44-6.56 months), IV SCLC was 7.0 (95%CL: 6.89-7.11 months); median CSS (mCSS) of IV LCNEC was 7.0 (95%CL: 6.34-7.66 months), IV SCLC was 8.0 (95%CL: 7.89-8.11 months). There was no statistically significant difference in OS (p = 0.19) or CSS (p = 0.19) between stage IV LCNEC and stage IV SCLC groups before PSM. After PSM, the mOS of IV LCNEC was 6.0 (95%CL: 5.44-6.56 months), IV SCLC was 6.0 (95%CL: 5.45-6.55 months); the mCSS of IV LCNEC was 7.0 (95%CL: 6.34-7.66 months), IV SCLC was 7.0 (95%CL: 6.40-7.61 months). There was no statistically significant difference in OS (p = 0.25) and CSS (p = 0.15) between the stage IV LCNEC and stage IV SCLC groups after PSM. The 1-year, 2-year and 3-year survival rates are shown in Table 3.
The survival probability of OS and CSS in IV LCNEC and IV SCLC
| Before PSM | OS | CSS | ||
|---|---|---|---|---|
| LCNEC | SCLC | LCNEC | SCLC | |
| 1-year survival probability | 25.29% | 24.15% | 27.46% | 26.30% |
| 2-year survival probability | 11.07% | 7.34% | 12.58% | 8.46% |
| 3-year survival probability | 6.12% | 4.21% | 7.47% | 5.04% |
| After PSM | ||||
| 1-year survival probability | 25.22% | 24.64% | 27.39% | 26.82% |
| 2-year survival probability | 11.01% | 8.12% | 12.52% | 9.09% |
| 3-year survival probability | 6.09% | 5.35% | 7.44% | 6.25% |
Abbreviations: OS: overall survival, CSS: cancer-specific survival, PSM: propensity score matching, SCLC: small cell lung cancer, LCNEC: large cell neuroendocrine carcinoma.
Univariable Cox analysis for IV LCNEC and IV SCLC after PSM
Univariate Cox analysis was performed with OS and CSS in stage IV LCNEC and stage IV SCLC patients after PSM. The results of univariate Cox analysis showed that age, N stage, marital status, primary site, radiotherapy, chemotherapy, brain metastasis, liver metastasis was significantly associated with OS in stage IV SCLC and stage IV LCNEC patients (Table 4); Besides, T stage was significantly associated with OS in stage IV SCLC patients, bone metastasis was significantly associated with OS in stage IV LCNEC patients. Age, N stage, primary site, brain metastasis, liver metastasis, radiotherapy, and chemotherapy were correlated with CSS of IV LCNEC and IV SCLC after PSM (Table 5). Besides, T stage was correlated with CSS of SCLC; bone metastasis was correlated with CSS of LCNEC.
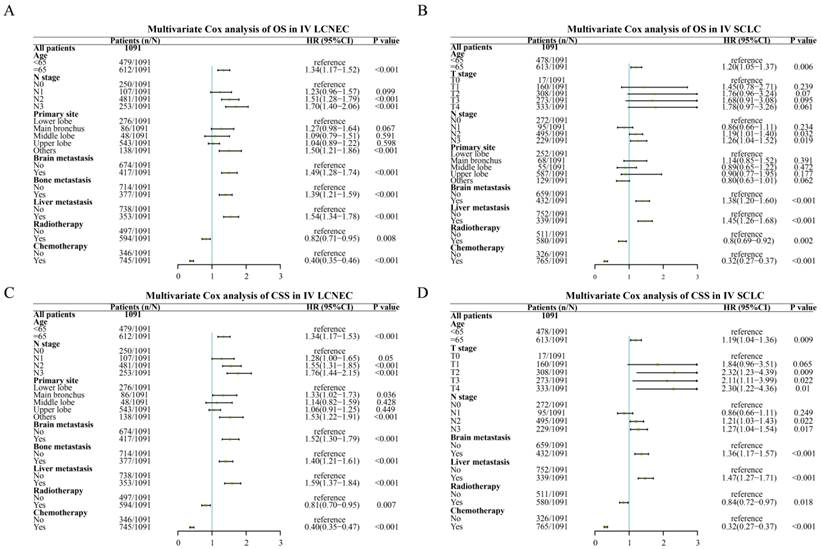
Multivariate Cox analysis for IV LCNEC and IV SCLC after PSM
In the multivariate Cox analysis with stage IV LCNEC and IV SCLC patients, age, N stage, brain metastasis, liver metastasis were independent risk factors for OS while radiotherapy, chemotherapy were common independent protective factors for OS in stage IV SCLC and stage IV LCNEC patients (Figure 4). Besides, liver metastasis was only an independent risk factor for IV LCNEC patients. In addition, age, N stage, primary site, brain metastasis, liver metastasis were common independent risk/protective factors for CSS in IV SCLC and IV LCNEC patients. T stage was an independent risk factor for CSS in SCLC and bone metastasis was an independent risk factor for CSS in LCNEC.
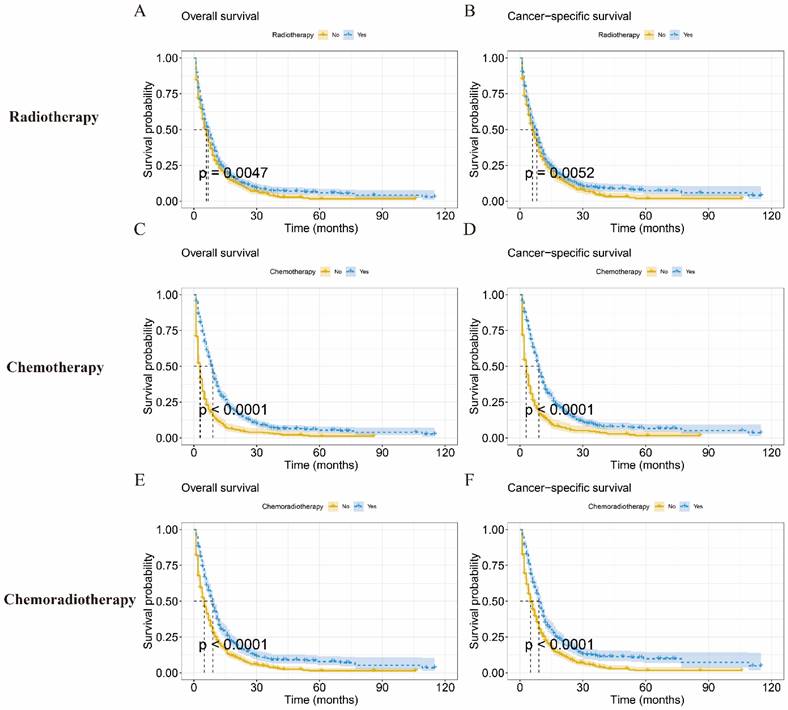
Prognosis of each treatment modality in IV LCNEC patients
To assess the prognostic impact of each treatment modality on patients with IV LCNEC, we compared treatment outcomes with radiotherapy, chemotherapy, and chemoradiotherapy, respectively. The mOS of radiotherapy was 7.0 months (95%CI: 6.08-7.91months) (Figure 5A), chemotherapy was 9.0 months (95%CI: 8.31-9.69months) (Figure 5B), chemoradiotherapy was 9.0 months (95%CI: 8.01-9.98 months) (Figure 5C).The KM analysis showed that radiotherapy, chemotherapy and chemoradiotherapy could improve the survival probability of IV LCNEC patients (p < 0.05). The mCSS of radiotherapy, chemotherapy and chemoradiotherapy were 8.0 months (95%CI: 7.13-8.88 months), 9.0 months (95%CI: 8.32-9.69 months) and 9.0 months (95%CI: 8.09-9.92 months) respectively (Figure 5D-F).
Evaluation of different treatment modalities of IV SCLC and IV LCNEC
To identify the effect of treatment modalities on OS and CSS for stage IV SCLC and stage IV LCNEC, patients were divided into four groups according to treatment modalities before PSM: Control: patients were not treated with radiotherapy or chemotherapy since being diagnosed. Radiotherapy: patients were treated with radiotherapy alone. Chemotherapy: patients were treated with chemotherapy alone. Chemoradiotherapy: patients were both treated with radiotherapy and chemotherapy. The baseline characteristics of IV SCLC and IV LCNEC were shown in Table S1 and Table S2.
KM curves in IV LCNEC and IV SCLC patients before and after PSM. A: The KM curve of OS before PSM (p=0.19). B: The KM curve of CSS before PSM (p=0.19). C: The KM curve of OS after PSM (p=0.25). D: The KM curve of CSS after PSM (p=0.15). LCNEC: large cell neuroendocrine carcinoma; SCLC: small cell lung cancer; OS: overall survival; CSS: cancer-specific survival; PSM: propensity score matching; KM: Kaplan-Meier.
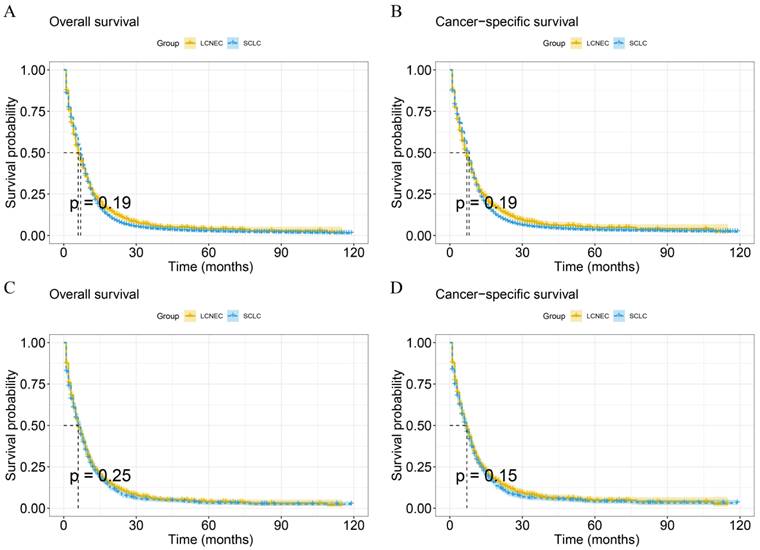
The multivariate Cox analysis of IV LCNEC and IV SCLC after PSM. A: The multivariate Cox analysis of OS in IV LCNEC patients; B: The multivariate Cox analysis of OS in IV SCLC patients; C: The multivariate Cox analysis of CSS in IV LCNEC patients; D: The multivariate Cox analysis of CSS in IV SCLC patients. LCNEC: large cell neuroendocrine carcinoma; SCLC: small cell lung cancer; OS: overall survival; CSS: cancer-specific survival; PSM: propensity score matching.
Univariable Cox analysis of OS in IV SCLC and IV LCNEC after PSM
| Variable | SCLC | LCNEC | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | ||||
| <65 | ||||
| ≥65 | 1.30 (1.15-1.48) | <0.001 | 1.36 (1.20-1.55) | <0.001 |
| Gender | ||||
| Female | ||||
| Male | 1.10 (0.97-1.24) | 0.146 | 1.10 (0.97-1.25) | 0.139 |
| Race | ||||
| Black | ||||
| White | 1.10 (0.91-1.33) | 0.311 | 1.16 (0.96-1.41) | 0.129 |
| Others | 1.40 (0.99-1.99) | 0.060 | 0.93 (0.65-1.32) | 0.679 |
| Marital status | ||||
| Unmarried | ||||
| Married | 0.91 (0.80-1.03) | 0.123 | 0.92 (0.81-1.04) | 0.179 |
| T stage | ||||
| T0 | ||||
| T1 | 1.79 (0.99-3.22) | 0.054 | 1.15 (0.69-1.90) | 0.595 |
| T2 | 1.95 (1.09-3.47) | 0.024 | 1.27 (0.78-2.08) | 0.341 |
| T3 | 1.88 (1.05-3.36) | 0.033 | 1.53 (0.94-2.51) | 0.090 |
| T4 | 1.76 (0.99-3.14) | 0.054 | 1.54 (0.94-2.51) | 0.086 |
| N stage | ||||
| N0 | ||||
| N1 | 0.87 (0.68-1.12) | 0.296 | 1.18 (0.93-1.50) | 0.176 |
| N2 | 1.19 (1.02-1.39) | 0.029 | 1.38 (1.17-1.62) | <0.001 |
| N3 | 1.13 (0.94-1.36) | 0.193 | 1.40 (1.16-1.69) | <0.001 |
| Laterality | ||||
| Left | ||||
| Right | 0.99 (0.87-1.12) | 0.833 | 1.05 (0.92-1.20) | 0.443 |
| Others | 0.73 (0.50-1.07) | 0.103 | 1.05 (0.76-1.45) | 0.779 |
| Primary site | ||||
| Lower lobe | ||||
| Main bronchus | 0.96 (0.72-1.27) | 0.770 | 1.11 (0.86-1.43) | 0.434 |
| Middle lobe | 0.90 (0.66-1.22) | 0.485 | 0.98 (0.71-1.35) | 0.888 |
| Upper lobe | 0.84 (0.72-0.99) | 0.032 | 0.93 (0.80-1.09) | 0.379 |
| Others | 0.87 (0.70-1.09) | 0.238 | 1.29 (1.05-1.60) | 0.018 |
| Brain Metastasis | ||||
| No | ||||
| Yes | 1.16 (1.03-1.32) | 0.019 | 1.17 (1.03-1.33) | 0.017 |
| Bone Metastasis | ||||
| No | ||||
| Yes | 1.12 (0.97-1.28) | 0.112 | 1.39 (1.22-1.59) | <0.001 |
| Liver Metastasis | ||||
| No | ||||
| Yes | 1.44 (1.26-1.65) | <0.001 | 1.47 (1.28-1.68) | <0.001 |
| Lung Metastasis | ||||
| No | ||||
| Yes | 1.02 (0.88-1.19) | 0.779 | 1.09 (0.94-1.27) | 0.235 |
| Radiotherapy | ||||
| No | ||||
| Yes | 0.70 (0.61-0.79) | <0.001 | 0.84 (0.74-0.95) | 0.005 |
| Chemotherapy | ||||
| No | ||||
| Yes | 0.32 (0.28-0.37) | <0.001 | 0.43 (0.37-0.49) | <0.001 |
Abbreviations: OS: overall survival, PSM: propensity score matching, SCLC: small cell lung cancer, LCNEC: large cell neuroendocrine carcinoma.
Univariable Cox analysis of CSS in IV SCLC and IV LCNEC after PSM
| Variable | SCLC | LCNEC | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | ||||
| <65 | ||||
| ≥65 | 1.28 (1.13-1.46) | <0.001 | 1.36 (1.19-1.55) | <0.001 |
| Gender | ||||
| Female | ||||
| Male | 1.11 (0.97-1.26) | 0.121 | 1.11 (0.98-1.27) | 0.113 |
| Race | ||||
| Black | ||||
| White | 1.08 (0.89-1.31) | 0.430 | 1.19 (0.98-1.46) | 0.085 |
| Others | 1.34 (0.93-1.92) | 0.116 | 0.97 (0.67-1.39) | 0.859 |
| Marital status | ||||
| Unmarried | ||||
| Married | 0.92 (0.81-1.05) | 0.221 | 0.93 (0.82-1.06) | 0.273 |
| T stage | ||||
| T0 | ||||
| T1 | 1.99 (1.05-3.79) | 0.036 | 1.12 (0.66-1.88) | 0.679 |
| T2 | 2.23 (1.19-4.20) | 0.013 | 1.24 (0.74-2.06) | 0.414 |
| T3 | 2.10 (1.12-3.96) | 0.022 | 1.55 (0.93-2.58) | 0.090 |
| T4 | 2.03 (1.08-3.82) | 0.028 | 1.52 (0.92-2.52) | 0.104 |
| N stage | ||||
| N0 | ||||
| N1 | 0.87 (0.67-1.13) | 0.285 | 1.24 (0.97-1.58) | 0.091 |
| N2 | 1.20 (1.03-1.41) | 0.023 | 1.42 (1.20-1.68) | <0.001 |
| N3 | 1.15 (0.95-1.39) | 0.155 | 1.45 (1.20-1.77) | <0.001 |
| Laterality | ||||
| Left | ||||
| Right | 0.99 (0.86-1.12) | 0.832 | 1.04 (0.91-1.19) | 0.558 |
| Others | 0.66 (0.44-0.99) | 0.045 | 1.00 (0.71-1.41) | 0.991 |
| Primary site | ||||
| Lower lobe | ||||
| Main bronchus | 0.98 (0.74-1.31) | 0.911 | 1.15 (0.89-1.50) | 0.283 |
| Middle lobe | 0.92 (0.67-1.26) | 0.588 | 1.01 (0.73-1.41) | 0.936 |
| Upper lobe | 0.86 (0.73-1.01) | 0.064 | 0.95 (0.81-1.11) | 0.527 |
| Others | 0.85 (0.67-1.07) | 0.162 | 1.31 (1.05-1.64) | 0.015 |
| Brain Metastasis | ||||
| No | ||||
| Yes | 1.16 (1.02-1.32) | 0.024 | 1.18 (1.03-1.35) | 0.015 |
| Bone Metastasis | ||||
| No | ||||
| Yes | 1.14 (0.99-1.31) | 0.061 | 1.40 (1.23-1.61) | <0.001 |
| Liver Metastasis | ||||
| No | ||||
| Yes | 1.45 (1.26-1.66) | <0.001 | 1.51 (1.32-1.74) | <0.001 |
| Lung Metastasis | ||||
| No | ||||
| Yes | 1.02 (0.88-1.19) | 0.794 | 1.11 (0.95-1.29) | 0.188 |
| Radiotherapy | ||||
| No | ||||
| Yes | 0.71(0.63-0.81) | <0.001 | 0.83 (0.73-0.95) | 0.006 |
| Chemotherapy | ||||
| No | ||||
| Yes | 0.32 (0.27-0.37) | <0.001 | 0.43 (0.38-0.50) | <0.001 |
Abbreviations: CSS: cancer-specific survival, PSM: propensity score matching, SCLC: small cell lung cancer, LCNEC: large cell neuroendocrine carcinoma.
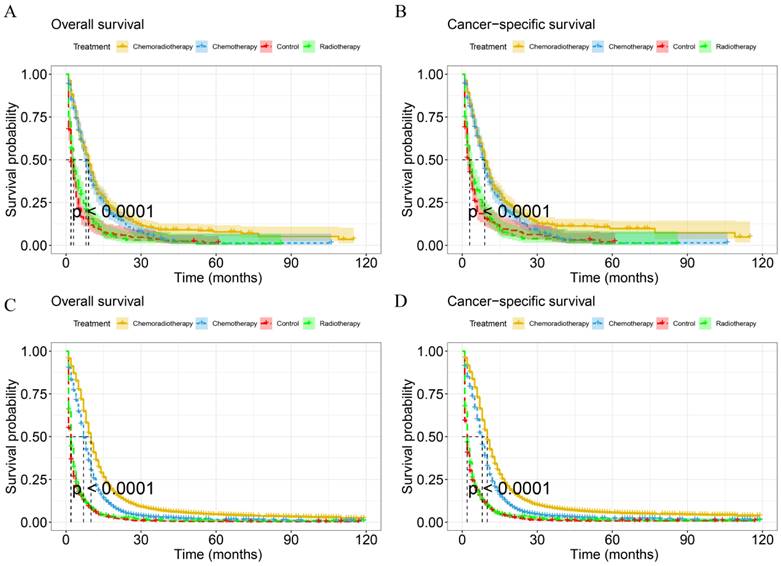
KM analysis was used to compare the difference in survival probability between patients with different treatment modalities (Figure 6). The mOS and mCSS of chemoradiotherapy group were 10.0 months (95%CI: 9.81-10.19 months) and 10.0 months (9.81-10.19 months) respectively, with a better survival probability than other groups in IV SCLC (p < 0.001). Besides, chemotherapy and radiotherapy alone had better OS and CSS than the control group in IV SCLC (p<0.05). For IV LCNEC patients, the mOS and mCSS in the chemoradiotherapy group were 9.0 months (95%CI: 8.02-9.99 months) and 9.0 months (95%CI: 8.09-9.92 months) respectively, which had a higher probability of survival than the other treatment groups (p < 0.001). In addition, the OS and CSS of the chemotherapy group were better than those of the control group (p < 0.001). However, the OS and CSS of radiotherapy alone were not significantly different compared to the control group in IV LCNEC patients (p = 0.228, p = 0.391).
Discussion
Similar histological features of LCNEC and SCLC were demonstrated in some studies [15], and further exploration of the differences in clinical features, prognostic factors, and treatment modalities between LCNEC and SCLC is warranted, especially for advanced LCNEC patients. Our study confirmed significant differences in the clinical characteristics of advanced LCNEC and SCLC, as reflected by demographic characteristics and tumor characteristics. Furthermore, after PSM, there was no significant difference in OS and CSS between IV LCNEC and IV SCLC, and their risk/protective factors were broadly similar based on the results of univariable and multivariable proportional hazards regression analysis, except for T stage and bone metastasis. In terms of treatment modality, chemoradiotherapy was the optimal treatment modality for advanced LCNEC and advanced SCLC patients, with better OS and CSS than other treatment modalities. However, radiotherapy alone could benefit advanced SCLC patients but did not seem to benefit advanced LCNEC patients.
Varlotto conducted a large retrospective study that included patients with LCNEC, SCLC and NSCLC from 2001-2007 and they concluded that the clinical features of LCNEC were more inclined to NSCLC than to SCLC [13]. Wang identified significant differences in both demographic and clinical characteristics of LCNEC from SCLC, except for marital status [16]. To our knowledge, our study was the first to confirm that there are significant differences in demographics and treatment modalities between LCNEC and SCLC at an advanced stage of the disease. However, Isaka suggested that the clinical characteristics of LCNEC and SCLC were similar [17], his study included only 10 patients with LCNEC, which may be biased by the small sample size. Derks found a similar pattern of organ metastases in advanced LCNEC as in SCLC, but liver metastases were less common in SCLC [9]. Our study found that brain metastases were common in advanced LCNEC patients compared with advanced SCLC patients, although the brain was reported to be the most common organ of metastasis in SCLC [18], which suggests that brain metastases may be more common in advanced LCNEC patients. In addition, Derks' study reported a higher probability of brain metastasis in advanced LCNEC/SCLC than in NSCLC [9]. Radiotherapy was more common in advanced LCNEC than SCLC, while previous studies have shown that SCLC appears to be more sensitive to radiotherapy [19, 20]. This may because LCENC with organ metastases is often recommended as the primary treatment with postsurgical radiotherapy [21], while chemotherapy combined with immunotherapy is preferred for advanced SCLC [22], which also increases the proportion of patients with IV LCNEC treated with radiotherapy. In the future, with the improvement of pathological detection techniques, LCNEC patients continue to be identified, and the differences with SCLC may change as the number of patient increases.
KM curves of IV LCNEC patients with each treatment modality. Kaplan-Meier curve of OS in radiotherapy (A), chemotherapy (C), and chemoradiotherapy (E) for IV LCNEC patients. Kaplan-Meier curve of CSS in radiotherapy (B), chemotherapy (D), and chemoradiotherapy (F) for IV LCNEC patients. OS: overall survival; CSS: cancer-specific survival; LCNEC: large cell neuroendocrine carcinoma; KM: Kaplan-Meier.
SCLC is considered to have the worst prognosis and highest malignancy of all lung cancer tissue types, especially in the extensive stage [23]; nevertheless, our study suggested that advanced LCNEC may also be highly malignant. The prognosis of SCLC and LCNEC remains controversial, Tomonari found no significant difference in OS and PFS between SCLC and LCNEC patients after surgery [24]; however, Varlotto found that the 1-, 2-, and 4-year OS probabilities of LCNEC (76%, 56%, 41%) were significantly higher than those of SCLC (69%, 49%, 32%) in patients undergoing surgery without radiotherapy, which was similar to the prognosis of NSCLC [13]. Isaka also reported a better prognosis for stage IA LCNEC than for SCLC patients with small-sized tumors [17]. Nevertheless, few studies have compared the prognosis of LCNEC and SCLC with different stages. Derks reported that LCNEC presented a better OS than SCLC in the early-stage disease but presented a similar OS to SCLC in the advanced stage [9]. Our study favors Derks' finding that OS and CSS were not significantly different between patients with advanced LCENC and SCLC, and similar results were reported after PSM analysis. Although the prognostic differences between studies on LCNEC and SCLC were inconsistent, our study and most studies confirmed that the prognosis of LCNEC and SCLC is similar, especially in the advanced stages of the disease.
KM curves for IV LCNEC and IV SCLC in different treatment modalities. KM curves of OS (A) and CSS (B) for IV LCNEC in different treatment modalities. KM curves of OS(C) and CSS (D) for IV SCLC in different treatment modalities. LCNEC: large cell neuroendocrine carcinoma; SCLC: small cell lung cancer; OS: overall survival; CSS: cancer-specific survival; KM: Kaplan-Meier.
We confirmed that OS and CSS prognostic factors for IV LCNEC and IV SCLC were similar after PSM, ranging from demographic characteristics to tumor characteristics. Risk factors for SCLC have been widely reported in other studies [18, 25]. The prognostic factors for LCNEC have received increasing attention in recent years, and age, gender, insurance, marital status, and tumor size have been confirmed as risk factors for LCNEC [26-28]. Unlike other studies, gender, race, and marriage were not reported to correlate with the prognosis of IV LCNEC, nor was IV SCLC in this study. In addition, chemotherapy and radiotherapy prolonged OS and CSS and improved prognosis in IV LCNEC and IV SCLC, which is consistent with IV NSCLC [9].
At present, the standard treatment of advanced LCNEC remains controversial, and also underappreciated due to its low morbidity [16]. Varlotto compared the characteristics of patients with LCNEC, SCLC, and NSCLC and confirmed that the characteristics and prognosis of LCNEC were more similar to those of NSCLC; therefore, he concluded that treatment of LCNEC should continue with NSCLC regimens [13]. Unfortunately, he did not control for sample selection bias, which may eventually lead to biased results; secondly, he did not compare the characteristics and prognosis of advanced LCNEC. Sun conducted a study, in which patients with LCNEC were treated with SCLC regimens and NSCLC regimens, and the results showed a better prognosis in the SCLC regimens group; thus, he concluded that LCNEC should be treated with the SCLC regimens [12]. Derks suggested that early stage LCNEC treatment strategy should refer to the treatment of NSCLC regimens, while advanced stage LCNEC should be treated with SCLC regimens [9]. Previous studies have explored the treatment of stage IV LCNEC, but due to sample size limitations, there are still no consistent conclusions [2]. Our results confirmed that advanced LCNEC and SCLC benefit to a similar extent in each treatment modality based on a large sample of IV LCNEC patients, suggesting that the treatment of stage IV LCNEC patients might favor SCLC regimens.
There are still several limitations in our study. First, some patient information such as smoking, specific chemotherapy and radiation regimens, immunotherapy and targeted therapies were not provided in the SEER database, which may have had an impact on our results. Second, the information bias introduced by retrospective studies may cause errors in our results. Third, we failed to present information on stage IV LCNEC patients from our own database due to the limitations of diagnostic techniques and the low morbidity and high mortality of LCNEC. More prospective studies are needed to further explore the similarity between SCLC and LCNEC in the future.
Conclusion
The clinical features of advanced LCNEC differ from those of advanced SCLC, while the survival outcomes and treatment modalities are similar. In summary, advanced LCNEC is similar to advanced SCLC, and advanced LCNEC could be treated with advanced SCLC regimens, which provide new evidence for the treatment of advanced LCNEC patients.
Supplementary Material
Supplementary tables.
Acknowledgements
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81660493), the National Clinical Research Center for Geriatrics-Jiangxi Branch Center. 2021ZDG02001 and the Natural Science Foundation of Jiangxi Province (Grant No.20202ACBL206019).
Author contributions
Weichang Yang: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing-original draft, Writing-review & editing. Xiaoqun Ye: Data curation, Investigation, Methodology, Validation, Writing - review & editing. Wenjun Wang:Data curation, Investigation. Zhouhua Li: Data curation, Investigation, Methodology, Software. Juan Wu: Data curation. Xiaofeng Xu: Data curation, Investigation, Methodology. Chong Chen: Conceptualization, Project administration, Resources, Supervision.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet (London, England). 2021;398:535-54
2. Corbett V, Arnold S, Anthony L, Chauhan A. Management of Large Cell Neuroendocrine Carcinoma. Frontiers in oncology. 2021;11:653162
3. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB. et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10:1243-60
4. Ko J, Winslow MM, Sage J. Mechanisms of small cell lung cancer metastasis. EMBO molecular medicine. 2021;13:e13122
5. Rossi G, Bisagni A, Cavazza A. High-grade neuroendocrine carcinoma. Current opinion in pulmonary medicine. 2014;20:332-9
6. Marx A, Chan JK, Coindre JM, Detterbeck F, Girard N, Harris NL. et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10:1383-95
7. Baine MK, Rekhtman N. Multiple faces of pulmonary large cell neuroendocrine carcinoma: update with a focus on practical approach to diagnosis. Translational lung cancer research. 2020;9:860-78
8. Rekhtman N. Lung neuroendocrine neoplasms: recent progress and persistent challenges. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2022;35:36-50
9. Derks JL, Hendriks LE, Buikhuisen WA, Groen HJ, Thunnissen E, van Suylen RJ. et al. Clinical features of large cell neuroendocrine carcinoma: a population-based overview. The European respiratory journal. 2016;47:615-24
10. Xu L, Zhang G, Song S, Zheng Z. Surgery for small cell lung cancer: A Surveillance, Epidemiology, and End Results (SEER) Survey from 2010 to 2015. Medicine. 2019;98:e17214
11. Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM. et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35:3484-515
12. Sun JM, Ahn MJ, Ahn JS, Um SW, Kim H, Kim HK. et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung cancer (Amsterdam, Netherlands). 2012;77:365-70
13. Varlotto JM, Medford-Davis LN, Recht A, Flickinger JC, Schaefer E, Zander DS. et al. Should large cell neuroendocrine lung carcinoma be classified and treated as a small cell lung cancer or with other large cell carcinomas? Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6:1050-8
14. Atieh T, Huang CH. Treatment of Advanced-Stage Large Cell Neuroendocrine Cancer (LCNEC) of the Lung: A Tale of Two Diseases. Frontiers in oncology. 2021;11:667468
15. Derks JL, Rijnsburger N, Hermans BCM, Moonen L, Hillen LM, von der Thüsen JH. et al. Clinical-Pathologic Challenges in the Classification of Pulmonary Neuroendocrine Neoplasms and Targets on the Horizon for Future Clinical Practice. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2021;16:1632-46
16. Wang J, Ye L, Cai H, Jin M. Comparative study of large cell neuroendocrine carcinoma and small cell lung carcinoma in high-grade neuroendocrine tumors of the lung: a large population-based study. Journal of Cancer. 2019;10:4226-36
17. Isaka M, Nakagawa K, Ohde Y, Okumura T, Watanabe R, Ito I. et al. A clinicopathological study of peripheral, small-sized high-grade neuroendocrine tumours of the lung: differences between small-cell lung carcinoma and large-cell neuroendocrine carcinoma. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2012;41:841-6
18. Wang Q, Gümüş ZH, Colarossi C, Memeo L, Wang X, Kong CY. et al. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2023;18:31-46
19. Crockett C, Belderbos J, Levy A, McDonald F, Le Péchoux C, Faivre-Finn C. Prophylactic cranial irradiation (PCI), hippocampal avoidance (HA) whole brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS) in small cell lung cancer (SCLC): Where do we stand? Lung cancer (Amsterdam, Netherlands). 2021;162:96-105
20. Zugazagoitia J, Paz-Ares L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2022;40:671-80
21. Collen C, Christian N, Schallier D, Meysman M, Duchateau M, Storme G. et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Annals of oncology: official journal of the European Society for Medical Oncology. 2014;25:1954-9
22. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ. et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. The New England journal of medicine. 2018;379:2220-9
23. Wang Q, Gümüş ZH, Colarossi C, Memeo L, Wang X, Kong CY. et al. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2022
24. Kinoshita T, Yoshida J, Ishii G, Aokage K, Hishida T, Nagai K. The differences of biological behavior based on the clinicopathological data between resectable large-cell neuroendocrine carcinoma and small-cell lung carcinoma. Clinical lung cancer. 2013;14:535-40
25. Moser SS, Bar J, Kan I, Ofek K, Cohen R, Khandelwal N. et al. Real World Analysis of Small Cell Lung Cancer Patients: Prognostic Factors and Treatment Outcomes. Current oncology (Toronto, Ont). 2021;28:317-31
26. Gu J, Gong D, Wang Y, Chi B, Zhang J, Hu S. et al. The demographic and treatment options for patients with large cell neuroendocrine carcinoma of the lung. Cancer medicine. 2019;8:2979-93
27. Lowczak A, Kolasinska-Cwikla A, Osowiecka K, Glinka L, Palucki J, Rzepko R. et al. Outcomes of Patients with Pulmonary Large Cell Neuroendocrine Carcinoma in I-IV Stage. Medicina (Kaunas, Lithuania). 2021;57(2):118
28. Shah S, Gosain R, Groman A, Gosain R, Dasari A, Halfdanarson TR. et al. Incidence and Survival Outcomes in Patients with Lung Neuroendocrine Neoplasms in the United States. Cancers. 2021;13(8):1753
Author contact
![]() Corresponding author: Xiaoqun Ye, Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, Jiangxi, P.R. China; Email: 511201663com.
Corresponding author: Xiaoqun Ye, Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, Jiangxi, P.R. China; Email: 511201663com.

 Global reach, higher impact
Global reach, higher impact