3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(9):1571-1578. doi:10.7150/jca.82736 This issue Cite
Research Paper
Pharmacokinetics and Safety of Multiple-Dose Alpelisib in Participants with Moderate or Severe Hepatic Impairment: A Phase 1, Open-Label, Parallel Group Study
1. Orlando Clinical Research Center, Orlando, USA.
2. Novartis Pharmaceuticals Corporation, East Hanover, USA.
3. Novartis Institutes for BioMedical Research, Basel, Switzerland.
4. Novartis Pharma S.A.S, Paris, France.
5. Novartis Healthcare Pvt. Ltd, Hyderabad, India.
6. Novartis Pharma AG, Basel, Switzerland.
7. Clinical Pharmacology Research Unit, Division of Clinical Pharmacology Department of Medicine, Miller School of Medicine, University of Miami, Miami, USA.
8. Katz Family Drug Discovery Center, University of Miami, Miami, USA.
Received 2023-1-17; Accepted 2023-4-2; Published 2023-5-21
Abstract
The pharmacokinetics (PK) and safety of single-dose alpelisib (300 mg) were assessed in participants with moderate to severe hepatic impairment (n = 6 each) compared with their matching healthy controls (n = 11). Blood samples were collected upto 144 hours post-dose and evaluated by liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay. The primary PK parameters (maximum plasma concentration [Cmax], area under the curve [AUC]inf and AUClast) and secondary PK parameters (AUC0-t, apparent total body clearance [CL/F], apparent volume of distribution [Vz/F], time of maximum observed concentration [Tmax], and half-life [T1/2]) of oral alpelisib 300 mg were determined from individual plasma concentration-time profiles using non‑compartmental analysis. Cmax of alpelisib decreased by approximately 17% in the moderate hepatic impairment group vs. the healthy control group (geometric mean ratio; GMR [90% confidence interval; CI], 0.833 [0.530, 1.31]). Cmax in the severe hepatic impairment group was comparable to that of the healthy control group (GMR [90% CI], 1.00 [0.636, 1.58]). AUClast for alpelisib decreased by approximately 27% in the moderate hepatic impairment group vs. the healthy control group (GMR [90% CI], 0.726 [0.487, 1.08]). AUClast was 26% higher in the severe hepatic impairment group compared with the healthy control group (GMR [90% CI], 1.26 [0.845, 1.87]). Overall, 3 participants (13.0%) experienced at least 1 adverse event which were either grade 1 or 2. Adverse events did not lead to study drug discontinuation. No grade 3 or 4 adverse events, serious adverse events or deaths were reported. The results indicate that a single dose of alpelisib was well tolerated in this study population. There was no significant impact of moderate or severe hepatic impairment on the exposure of alpelisib.
Keywords: Alpelisib, BYL719, Hepatic impairment, Pharmacokinetics
Introduction
The phosphatidylinositol 3-kinase (PI3K) signaling pathway contributes to several processes that are critical in mediating many aspects of cellular function, including nutrient uptake, anabolic reactions, cell growth, and survival [1]. The PI3K pathway is activated by multiple factors, including diverse oncogenic genomic alterations in PIK3CA, PIK3R1, PTEN, and other critical genes, which can serve as targets for anticancer therapy [1-3].
Alpelisib (BYL719; an oral, class I α-specific PI3K inhibitor belonging to the 2-aminothiazole class of compounds) strongly inhibits the PI3Kα isoform (both wildtype and mutant) over the β, δ, and γ isoforms, and is inactive against most other kinases [4]. Antitumor activity of alpelisib has been demonstrated in preclinical and early phases of clinical studies as a single agent and in combination with endocrine therapy [5, 6]. In the phase 3, SOLAR-1 study (NCT02437318), alpelisib plus fulvestrant significantly prolonged median progression-free survival versus placebo plus fulvestrant in patients with PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer (11.0 vs 5.7 months; P<0.001) [7].
Alpelisib is currently being investigated in combination with other drugs in various oncology indications and is anticipated to be used in participants with co-existing morbidities, including hepatic impairment. Hepatic dysfunction results in pathophysiological changes that lead to variable and difficult-to-predict effects on drug pharmacokinetics (PK) [8]. It is therefore important to determine the impact of hepatic impairment on the PK of alpelisib.
The first step in the metabolism of alpelisib in vivo is amide hydrolysis to BZG791, which is the major circulating metabolite in plasma. The metabolism of alpelisib to BZG791 is unlikely to be governed only by the liver, since it is primarily metabolized through both chemical and multi‑enzymatic amide hydrolysis, with only limited contribution from CYP3A4. As unchanged alpelisib can be eliminated by hepatobiliary export and/or intestinal secretion, liver metabolism and transport may account for an estimated fraction greater than 20% of the eliminated alpelisib [9]. In order to allow for safe and efficacious use of alpelisib in participants with impaired liver function, the impact of liver impairment on the PK of alpelisib and its primary metabolite BZG791 (although pharmacologically inactive) needs to be characterized.
The likelihood of an effect of mild hepatic impairment on alpelisib PK may be very low because of the large contribution of non-hepatic metabolic pathways, biliary excretion, and active intestinal secretion. Therefore, the study was conducted in participants with moderate and severe hepatic impairment. The objective of this phase 1, open-label, multicenter, parallel-group study (NCT02624557) was to characterize the PK and safety profile of alpelisib in participants with moderate and severe hepatic impairment (by Child-Pugh classification) and to develop dosing recommendations based on the findings. Here, we report the results from the final analysis of this study.
Materials and Methods
Study Design
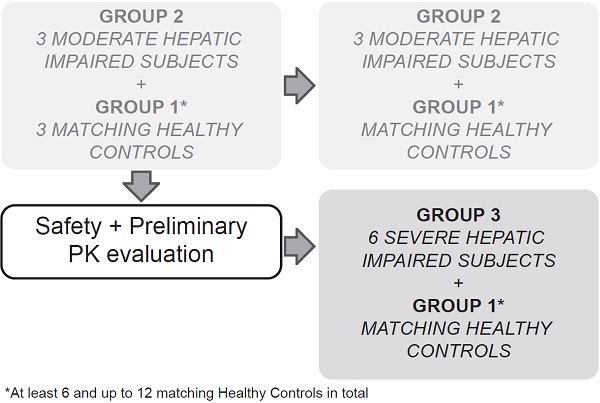
This multicenter, open-label, parallel-group study sequentially enrolled participants with moderate (Child-Pugh B or Group 2, score 7-9) and severe (Child-Pugh C or Group 3, score 10-15) hepatic impairment to assess the PK of alpelisib in participants with impaired hepatic function compared with healthy participants (Group 1, with apparent normal liver function) after a single dose of 300 mg alpelisib under fasted conditions (Figure 1). Enrollment commenced with three participants from the moderate hepatic impairment group. The enrollment of healthy matching controls did not start before his/her matching hepatic impaired participant had completed the end of study (EOS) visit. While enrollment in the moderate hepatic impairment group continued, an evaluation for safety and preliminary PK was made after the first 3 moderate impaired participants and their 3 matching healthy controls completed the study evaluations up to and including the EOS visit before starting enrollment into the severe hepatic impairment group.
Study design
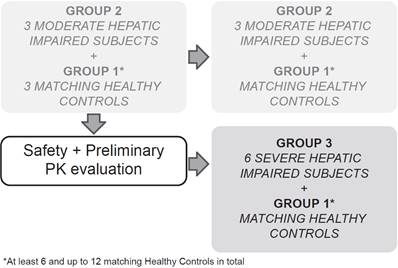
The total study duration for each participant was approximately 7 weeks, which comprised a 21‑day screening period (Day -21 to Day -1), an 8-day confinement period (Day -1 to Day 7), and a follow-up safety period ending at least 30 days (+5 days) after alpelisib administration to follow up on ongoing adverse events (AEs), concomitant medication, and the occurrence of serious adverse events (SAEs).
This study was conducted in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki. An independent ethics committee and institutional review boards approved the study protocol and all amendments at each participating center. Written informed consent was obtained from all participants.
Study Population
The study population consisted of participants (age, 18-75 years; weight, 50-120 kg; body mass index (BMI), 18.0-36.0 kg/m2) with moderate hepatic impairment (n=6) defined by Child-Pugh category B (7 to 9 total points) or severe hepatic impairment (n = 6) defined by Child-Pugh category C (10 to 15 total points; Table 1), who were otherwise healthy (exhibited physical signs consistent with stable hepatic impairment and were free of significant medical disorders unrelated to their hepatic disorder), and matched healthy control participants (matched by sex, race, age [±10 years], and body weight [±10%]; n = 12).
Child-Pugh classification and liver parameters at screening by hepatic function group (Full analysis set)
| Hepatic impairment classification | Moderate(total score 7-9; n = 6), n (%) | Severe(total score 10-15; n = 6), n (%) |
|---|---|---|
| Encephalopathy | ||
| Grade 1-2 | 6 (100) | 6 (100) |
| Ascites | ||
| Slight | 6 (100) | 0 |
| Moderate | 0 | 6 (100) |
| Total bilirubin (mg/dL) | ||
| <2 | 6 (100) | 0 |
| 2-3 | 0 | 2 (33.3) |
| >3 | 0 | 4 (66.7) |
| Serum albumin (g/dL) | ||
| >3.5 | 6 (100) | 1 (16.7) |
| 2.8-3.5 | 0 | 3 (50.0) |
| <2.8 | 0 | 2 (33.3) |
| International normalized ratio | ||
| <1.7 | 5 (83.3) | 3 (50.0) |
| Prothrombin time (seconds over control) | ||
| < 4 | 1 (16.7) | 2 (33.3) |
| 4 to 6 | 0 | 1 (16.7) |
| Score | ||
| 7 | 6 (100) | 0 |
| 10 | 0 | 3 (50.0) |
| 11 | 0 | 1 (16.7) |
| 12 | 0 | 1 (16.7) |
| 13 | 0 | 1 (16.7) |
Moderate = Child-Pugh class B; Severe = Child-Pugh class C.
Key exclusion criteria included any surgical or medical condition or medical history that could affect the PK of alpelisib, use of any medication or food supplement 14 days prior to dosing or during the study that could affect the PK of alpelisib, medical history of liver transplant or immunosuppressant therapy, diabetes mellitus or fasting plasma glucose (FPG) levels > 160 mg/dL or > 8.8 mmol/L, donation or loss of ≥ 400 mL blood or plasma < 8 weeks prior to dosing, and history of psychiatric illness within the past 2 years. Detailed eligibility criteria can be found in Table S1.
Study Assessments
Pharmacokinetic sample collection and analysis
Blood samples for assessing plasma concentration-time profiles of alpelisib were collected in K3-EDTA from all participants at pre-dose and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 48, 72, 96, and 144 hours post-dose. Plasma samples were stored at -70°C.
Plasma concentrations of alpelisib and BZG791 were determined by a previously validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay. The lower limits of quantitation (LLOQ) are currently 5.0 ng/mL for alpelisib and 1.0 ng/mL for BZG791. Values below the assay LLOQ were reported as 0 ng/mL.
Plasma protein binding sample collection and analysis
For plasma protein binding (PPB) analysis, blood samples were collected in K3-EDTA at two time points, pre-dose prior to alpelisib administration for the PPB of BZG791 and 3 hours after administration for the PPB of 14C-alpelisib. Plasma samples were stored at -20°C until analyzed.
Protein binding was determined by ultrafiltration method. Plasma, spiked with the intended compound (14C-alpelisib or BZG791), was introduced into ultrafiltration devices and the ultrafiltrate was obtained. Plasma protein binding was calculated based on compound concentration determined in the ultrafiltrate and in the spiked plasma sample before ultrafiltration. Stability was assessed in previous assay; though nonspecific adsorption was not investigated for this assay, neither analytes is known for unspecific binding from the in vitro assays performed with alpelisib or BZG791.
Safety Assessments
The safety of single-dose oral alpelisib 300 mg was assessed throughout the study by recording AEs, clinical laboratory parameters, electrocardiograms (ECGs), and physical examinations; event severity (according to National Cancer Institute Common Terminology Criteria for Adverse Events [NCI‑CTCAE] version 4.03) and relationship to study drug were also recorded.
Statistical Analysis
Population Size
The sample size (six participants per hepatic impairment group with a within-study control population) was based on practical considerations and guidance from the United States Food and Drug Administration and European Medicines Agency [10, 11].
Pharmacokinetic Analyses
The primary PK parameters (maximum concentration [Cmax], area under the curve from time zero to infinity [AUCinf] or AUClast) and secondary PK parameters (AUC0-t, apparent total body clearance [CL/F], apparent volume of distribution [Vz/F], time of maximum observed concentration [Tmax], and half‑life [T1/2]) of oral alpelisib 300 mg were determined from individual plasma concentration-time profiles using non-compartmental analysis (Phoenix WinNonlin Version 6.4 - Pharsight, Mountain View, CA).
Log-transformed parameters (Cmax, AUClast and AUCinf) for both total and unbound alpelisib were analyzed by means of an analysis of covariance (ANCOVA) model, with hepatic function group as the fixed effect; supportive analyses were performed using the same model, with age, weight, sex, and race as covariates. The differences between the control group and each one of the hepatic function groups and the two-sided 90% confidence intervals (CIs) were derived from the model. These were back‑transformed to obtain the point estimates and the 90% CIs for the ratios of the geometric means on the original scale.
Results
Of the 23 enrolled participants, 11 were in the healthy control group, six were in the moderate hepatic impairment group, and six were in the severe hepatic impairment group. All six participants in each impairment group were individually matched with a participant from the healthy control group. One participant from the healthy control group served as a matching control for two participants (one participant in the moderate hepatic impairment group and one participant in the severe hepatic impairment group). All participants completed the study and were included in the safety and PK analyses sets. Most participants were male (56.5%) and Caucasian (91.3%); age, gender, body weight, and other baseline characteristics were similar across treatment groups. Median creatinine clearance for all participants was 127.28 mL/min (normal); all participants had normal creatinine clearance except in one participant in Group 3 who had a mild renal impairment (56.81 mL/min) (Table 2).
Pharmacokinetics
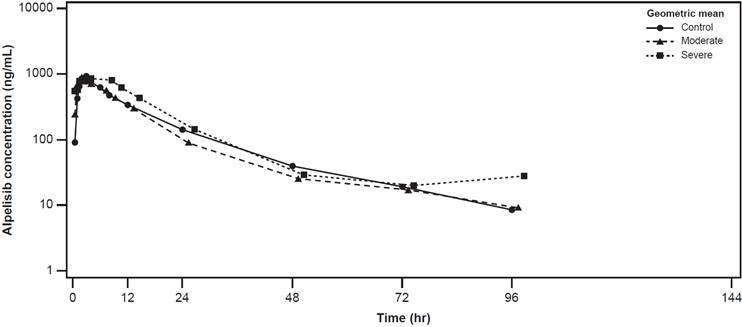
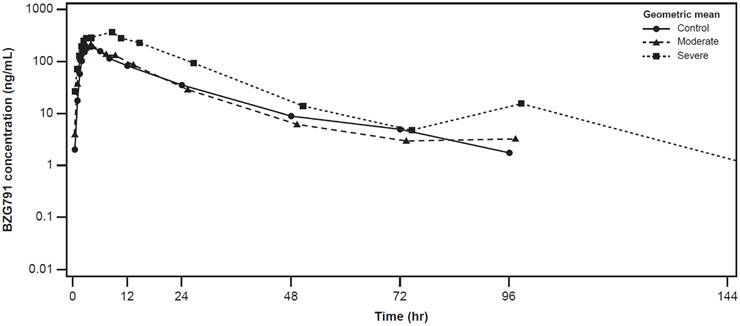
The plasma concentration-time profiles of alpelisib were largely similar across the healthy control, moderate hepatic impairment, and severe hepatic impairment groups (Figure 2). The plasma concentration-time profiles of BZG791 were largely similar in the healthy control and moderate hepatic impairment groups. Plasma concentrations were consistently higher in the severe hepatic impairment group from around 12 hours to 144 hours post-dose (Figure 3).
Participant demographics by hepatic function group
| Characteristic | Control (N = 11) | Moderate (N = 6) | Severe (N = 6) | All Participants (N = 23) |
|---|---|---|---|---|
| Age, median (range), years | 57.0 (47.0 - 68.0) | 57.0 (53.0 - 62.0) | 62.5 (48.0 - 66.0) | 58.0 (47.0 - 68.0) |
| Sex, n (%) | ||||
| Male | 6 (54.5) | 3 (50.0) | 4 (66.7) | 13 (56.5) |
| Female | 5 (45.5) | 3 (50.0) | 2 (33.3) | 10 (43.5) |
| Race, n (%) | ||||
| Caucasian | 10 (90.9) | 5 (83.3) | 6 (100) | 21 (91.3) |
| Black | 1 (9.1) | 1 (16.7) | 0 | 2 (8.7) |
| Ethnicity, n (%) | ||||
| Hispanic/Latino | 5 (45.5) | 4 (66.7) | 3 (50.0) | 12 (52.2) |
| Mixed Ethnicity | 2 (18.2) | 0 | 0 | 2 (8.7) |
| Other | 4 (36.4) | 2 (33.3) | 3 (50.0) | 9 (39.1) |
| Weight, median (range), Kgs | 82.60 (55.1 - 112.9) | 74.70 (52 - 103.1) | 82.75 (68.7 - 91.5) | 81.50 (52 - 112.9) |
| Height, median (range), cms | 166.5 (150 - 180) | 164.1 (159 - 174) | 169.5 (158 - 185) | 166.3 (150 - 185) |
| Body mass index, median (range), Kg/m2 | 28.14 (24.24 - 34.85) | 28.295 (19.33 - 36.17) | 28.43 (21.99 - 33.8) | 28.14 (19.33 - 36.17) |
| Creatinine clearance, median (range), mL/min | 112.29 (81.03 - 201.61) | 131.16 (111.27 - 140.97) | 137.725 (56.81 - 231.53) | 127.28 (56.81 - 231.53) |
Cmax of alpelisib decreased by approximately 17% in the moderate hepatic impairment compared with the healthy control group (geometric mean ratio; GMR [90% CI], 0.833 [0.530, 1.31]). Cmax in the severe hepatic impairment group was comparable to that of the healthy control group (GMR [90% CI], 1.00 [0.636, 1.58]) (Table 3).
AUClast for alpelisib decreased by approximately 27% in the moderate hepatic impairment group compared with the healthy control group (GMR [90% CI], 0.726 [0.487, 1.08]). AUClast was 26% higher in the severe hepatic impairment group compared with the healthy control group (GMR [90% CI], 1.26 [0.845, 1.87]). Values of AUCinf were similar to those of AUClast across the hepatic impairment groups (Table 3).
Cmax of BZG791 was comparable between the moderate hepatic impairment and healthy control groups (GMR [90% CI], 0.942 [0.639, 1.39]). In the severe hepatic impairment group, Cmax increased by 74% (GMR [90% CI], 1.74 [1.18, 2.56]) (Table 4).
AUClast for BZG791 decreased by approximately 12% in the moderate hepatic impairment group compared with the healthy control group (GMR [90% CI], 0.883 [0.598, 1.30]). It increased by 145% in the severe hepatic impairment group vs. the healthy control group (GMR [90% CI], 2.45 [1.65, 3.62]). Values of AUCinf were similar to those of AUClast across the hepatic impairment groups (Table 4). The metabolic ratio of BZG791 also increased (geometric mean [range], 0.472 [0.323 to 0.580]) in participants in the severe hepatic impairment group compared with participants in the healthy control and moderate hepatic impairment groups.
Geometric mean (SD) concentration-time profiles for plasma alpelisib by hepatic function group (Pharmacokinetic analysis set)
Geometric mean (SD) concentration-time profiles for plasma BZG791 by hepatic function group (Pharmacokinetic analysis set)
Summary of statistical analysis of primary PK parameters for alpelisib by hepatic function group (Pharmacokinetic analysis set)
| PK parameter (unit) | Group (Control, N=11; Moderate, N=6; Severe, N=6) | Adjusted geo-mean | Comparison(s) | Group comparison | ||
|---|---|---|---|---|---|---|
| Geo-mean ratio | 90% CI | |||||
| Lower | Upper | |||||
| Cmax (ng/mL) | Control | 1180 | - | - | - | - |
| Moderate | 986 | Moderate/Control | 0.833 | 0.530 | 1.31 | |
| Severe | 1190 | Severe/Control | 1.00 | 0.636 | 1.58 | |
| AUClast (ng*hr/mL) | Control | 13300 | - | - | - | - |
| Moderate | 9630 | Moderate/Control | 0.726 | 0.487 | 1.08 | |
| Severe | 16700 | Severe/Control | 1.26 | 0.845 | 1.87 | |
| AUCinf (ng*hr/mL) | Control | 13700 | - | - | - | - |
| Moderate | 9990 | Moderate/Control | 0.730 | 0.499 | 1.07 | |
| Severe | 17100 | Severe/Control | 1.25 | 0.859 | 1.83 | |
| Tmax (hr) | Control | 2.02 | - | - | - | - |
| Moderate | 1.75 | Moderate/Control | -0.267 | - | - | |
| Severe | 2.75 | Severe/Control | 0.733 | - | - | |
AUCinf, area under the curve from time zero to infinity; CI, confidence interval; Cmax, maximum blood concentration; Tmax, time at which Cmax is reached.
For Tmax, median is presented under 'Adjusted geo-mean', difference of medians under 'Geo-mean ratio'.
Geometric mean ratio of the healthy control participant group and each one of the hepatic function groups was estimated using an ANCOVA model of the log-transformed PK parameters. Included in the model were hepatic function group as a fixed effect and sex as covariate for Cmax, hepatic function group, weight and sex as covariates for AUClast and AUCinf. The results were back transformed to get adjusted geometric mean, geometric mean ratio, and 90% CI.
Summary of statistical analysis of primary PK parameters for BZG791 by hepatic function group (Pharmacokinetic analysis set)
| PK parameter (unit) | Group (Control, N=11; Moderate, N=6; Severe, N=6) | Adjusted geo-mean | Comparison(s) | Group comparison | ||
|---|---|---|---|---|---|---|
| Geo-mean ratio | 90% CI | |||||
| Lower | Upper | |||||
| Cmax (ng/mL) | Control | 242 | - | - | - | - |
| Moderate | 228 | Moderate/Control | 0.942 | 0.639 | 1.39 | |
| Severe | 421 | Severe/Control | 1.74 | 1.18 | 2.56 | |
| AUClast (ng*hr/mL) | Control | 3110 | - | - | - | - |
| Moderate | 2750 | Moderate/Control | 0.883 | 0.598 | 1.30 | |
| Severe | 7620 | Severe/Control | 2.45 | 1.65 | 3.62 | |
| AUCinf (ng*hr/mL) | Control | 3200 | - | - | - | - |
| Moderate | 2840 | Moderate/Control | 0.887 | 0.608 | 1.29 | |
| Severe | 7640 | Severe/Control | 2.39 | 1.64 | 3.49 | |
| Tmax (hr) | Control | 4.00 | - | - | - | - |
| Moderate | 4.00 | Moderate/Control | 0 | - | - | |
| Severe | 6.00 | Severe/Control | 2.00 | - | - | |
AUCinf, area under the curve from time zero to infinity; CI, confidence interval; Cmax, maximum blood concentration; Tmax, time at which Cmax is reached.
For Tmax, median is presented under 'Adjusted geo-mean', difference of medians under 'Geo-mean ratio'.
Geometric mean ratio of the healthy control participant group and each one of the hepatic function groups was estimated using an ANCOVA model of the log-transformed PK parameters. Included in the model were hepatic function group as a fixed effect and sex as covariate for Cmax, hepatic function group, weight and sex as covariates for AUClast and AUCinf. The results were back transformed to get adjusted geometric mean, geometric mean ratio, and 90% CI.
Adverse events, regardless of study drug relationship, by hepatic function group, preferred term, and maximum grade (Safety set)
| Primary system organ class Preferred term, n (%)a | Control (N = 11) | Moderate (N=6) | Severe (N=6) | All Participants (N=23) |
|---|---|---|---|---|
| Any Grade | 1 (9.1) | 2 (33.3) | 0 | 3 (13.0) |
| Grade 1 | 1 (9.1) | 1 (16.7) | 0 | 2 (8.7) |
| Grade 2 | 0 | 1 (16.7) | 0 | 1 (4.3) |
| Nausea | 0 | 2 (33.3) | 0 | 2 (8.7) |
| Blood pressure increased | 0 | 1 (16.7) | 0 | 1 (4.3) |
| Dizziness | 1 (9.1) | 0 | 0 | 1 (4.3) |
aA participant with multiple occurrences of an adverse event under a hepatic function group is counted only once in the adverse event category for that group. A participant with multiple adverse events within a primary system organ class is counted only once in the total row at maximum severity grade.
The average protein binding values of both alpelisib and BZG791 by group were also similar, indicating no significant effect of hepatic impairment on protein binding (Supplementary Table S2, Table S3, and Table S4). The PK parameter statistics for unbound alpelisib and BZG791 were similar to those of total alpelisib and total BZG791 in the healthy control and moderate hepatic impairment groups but were increased in the severe hepatic impairment group (the values of which also exhibited greater variability), which may be because of a slightly increased levels of fraction unbound in the severe hepatic impairment group.
Safety
Of the 23 participants treated, three (13.0%) experienced at least one AE. All AEs were of either grade 1 (nausea and dizziness) or grade 2 (increased blood pressure) severity; there were no grade 3/4 AEs. No AEs were reported in the severe hepatic impairment group (Table 5). No clinically significant changes from baseline in vital signs or hematology and biochemistry laboratory values were reported in the study. No deaths, SAEs, or other significant AEs were reported in the study.
Discussion
The objective of this study was to characterize the PK and safety profile of a single, oral 300 mg dose of alpelisib, administered in the fasted state, in participants with moderate and severe hepatic impairment (by Child-Pugh classification). The healthy control participants in this study were enrolled based on matched demographics with hepatic impaired participants with respect to age, race, sex, and body weight.
Compared with the healthy control group, Cmax of alpelisib was around 17% lower in the moderate hepatic impairment group. Drug exposures as assessed by AUClast and AUCinf were both around 27% lower in the moderate hepatic impaired group compared with the healthy control group. Cmax of alpelisib in the severe hepatic impairment group was comparable to that of the healthy control group. Drug exposure as assessed by AUCinf provided similar results as drug exposure assessed by AUClast.
It is likely that the differences in alpelisib maximum concentration and exposure across the hepatic impairment groups are an effect of variability rather than a true effect of hepatic impairment, especially with the effect showing an opposite trend in the moderate hepatic impairment group compared with the severe hepatic impairment group. Some variability is generally expected with small sample sizes (six participants in each of the hepatic impairment groups), and a prior food effect study with alpelisib had shown that alpelisib PK variability is higher in the fasted state compared with the fed state.
No apparent relationship was found between any PK parameter of alpelisib and a hepatic laboratory parameter.
The PK parameters of the metabolite BZG791, Cmax, AUClast, and AUCinf, were comparable in the moderate hepatic impairment group and the healthy control group, but were significantly increased (Cmax by 74%, AUClast by 145%, and AUCinf by 139%) in the severe hepatic impairment group because of higher exposure in the severe hepatic impairment group compared with the healthy control group. The metabolic ratio of BZG791 was also significantly increased in the severe hepatic impairment group compared with the healthy control group.
The current disposition model of alpelisib based on the results of the human ADME study postulates that approximately 73% of alpelisib is hydrolyzed to BZG791 systemically (i.e., extrahepatically), with 22% contribution of CYP3A4-mediated metabolism and a minor (4%) contribution of renal excretion. While alpelisib and BZG791 are substrates for BCRP, export of BCRP is not expected a large role in the disposition of alpelisib (and thus sensitive to any polymorphisms related to transporter expression) but may play a role in the disposition of BZG791 [9].
Hepatic impairment should result in a loss or reduction of metabolic activity and transporter activity in the liver. BZG791 exposure was also changed in the mild to moderate impairment group, suggesting that the hydrolysis pathway outside of the liver is not affected by the loss of oxidative metabolism or export in the liver, which is supportive of the current model. In severe hepatic impaired participants, the exposure of BZG791 was found to be significantly increased. This observed increase in BZG791 PK parameters and metabolic ratio without the exposure of alpelisib being significantly impacted in the severe impairment group compared with the control group may be explained either by an inhibition of BZG791 disposition pathways (e.g. via BCRP as it is not considered to be metabolized further) or by a compensatory mechanism. A confounding impact on the results by genetic polymorphisms of BCRP or concurrent impaired renal function (observed in one participant in the severe hepatic impairment group) can be considered unlikely due to the low contribution to the disposition of alpelisib itself.
Protein binding values in the healthy control, moderate hepatic impairment, and severe hepatic impairment groups were similar for both alpelisib and BZG791 and was in line with previously reported in vitro protein binding values. The GMRs of unbound alpelisib and unbound BZG791 were similar to that of total alpelisib and total BZG791, respectively. However, the values of the PK variability for unbound alpelisib and BZG791 were found to be increased across all treatment groups compared with that of total bound alpelisib and BZG791.
Collectively, these results indicate that moderate or severe hepatic impairment has no impact on the clearance, elimination, or distribution of alpelisib. Therefore, it is reasonable to conclude that mild hepatic impairment also has no impact. The increase in BZG791 exposure in the severe impairment group is, however, indicative of a shift in the metabolic pathways. The data from this study has led to the alpelisib label recommendations for patients with hepatic impairment.
Very few AEs were reported in this study, mainly of grade 1 or grade 2 severity. No clinically significant changes from baseline in vital signs or hematology and biochemistry laboratory values were reported in the study. No deaths, SAEs, or other significant AEs were reported in the study.
A single dose of alpelisib was well tolerated in this study population. No significant impact of moderate or severe hepatic impairment was observed on the clearance, elimination, or distribution of alpelisib. In conclusion, this analysis supports that no dose adjustment of alpelisib is required in participants with mild, moderate, or severe hepatic impairment.
Abbreviations
ADME: absorption, distribution, metabolism and excretion; AE: adverse event; ANCOVA: analysis of covariance; AUC: area under the curve; AUC0-t: area under the concentration-time curve from time zero to time t; AUCinf: area under the concentration-time curve (AUC) from time zero to infinity; AUClast: area under the concentration-time curve (AUC) from time zero to the last measurable concentration sampling time (Tlast); BCRP: breast cancer resistance protein; BMI: body mass index; Bpm: beats per minute; CI: confidence interval; CL/F: total body clearance of drug from the plasma; Cmax: maximum plasma concentration; CTCAE: Common Terminology Criteria for Adverse Events; ECG: electrocardiogram; EDTA: ethylene diamine tetraacetic acid; EOS: end of study; FPG: fasting plasma glucose; GMR: geometric mean ratio; LLOQ: lower limits of quantitation; LCMS/MS: liquid chromatography-tandem mass spectrometry; NCI: National Cancer Institute; PI3K: phosphatidylinositol 3'-kinase; PK: pharmacokinetics; PPB: plasma protein binding; SAE: serious adverse event; SD: standard deviation; Tmax: time of maximum observed concentration; T1/2: half-life; ULN: upper limit of normal; Vz/F: volume of distribution during terminal elimination phase.
Supplementary Material
Supplementary tables.
Acknowledgements
We thank the participants who participated in this trial and their families, as well as the staff members at individual trial centers who provided support; Olivier Heudi (Novartis Institutes for BioMedical Research) who took care of the bioanalytics, Leonel Reis da Silva (Novartis Institutes for Biomed. Research) who conducted the protein binding experiments, and Avinash Yerramsetti (Novartis Healthcare Pvt Ltd) for providing medical editorial assistance with this manuscript.
Funding
The study was initiated, funded, and sponsored by Novartis Pharmaceuticals Corporation. The study was designed by the investigators and the sponsor. The sponsor in collaboration with the investigators undertook design and conduct of the study. The study investigators and their respective research teams collected the data; Novartis Pharmaceuticals Corporation compiled the data for summation and analysis. All authors were responsible for data interpretation. Dr. Marbury prepared the article in conjunction with all the authors, including employees of the sponsor. The corresponding author had final responsibility for the decision to submit the manuscript for publication.
Competing Interests
Dr. Marbury is an employee and equity owner of Orlando Clinical Research Center.
Dr. El-Hashimy is an employee of Novartis Pharmaceutical Corporation and holds Novartis stock options.
Dr. Blumenstein is an employee of Novartis Institutes for Biomed. Research and holds Novartis stock options.
Letellier is an employee of Novartis Pharma S.A.S and holds Novartis stock options.
Dr. Sengupta is an employee of Novartis Healthcare Pvt. Ltd.
Lorenzo is an employee of Novartis Pharma AG and holds Novartis shares
Dr. Preston reports grants from Novartis during the conduct of the study.
References
1. Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nature Reviews Clinical Oncology. 2018;15:273
2. Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nature reviews Cancer. 2015;15:7-24
3. Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nature Reviews Molecular Cell Biology. 2010;11:329
4. Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M. et al. Characterization of the Novel and Specific PI3Kα Inhibitor NVP-BYL719 and Development of the Patient Stratification Strategy for Clinical Trials. Molecular Cancer Therapeutics. 2014;13:1117-29
5. Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A. et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7:283ra51
6. Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM. et al. Phosphatidylinositol 3-Kinase alpha-Selective Inhibition With Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study. J Clin Oncol. 2018;36:1291-9
7. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS. et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. New England Journal of Medicine. 2019;380:1929-40
8. Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. European Journal of Clinical Pharmacology. 2008;64:1147
9. James A, Blumenstein L, Glaenzel U, Jin Y, Demailly A, Jakab A. et al. Absorption, distribution, metabolism, and excretion of [(14)C]BYL719 (alpelisib) in healthy male volunteers. Cancer Chemother Pharmacol. 2015;76:751-60
10. Food and Drug Administration. Guidance for industry. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072123.pdf. Published May 2003. Accessed March 28. 2019
11. Committee for Medicinal Products for Human Use (CHMP). Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003122.pdf. Published February 2005. Accessed March 28. 2019
Author contact
![]() Corresponding author: Dr. Thomas Marbury, President of Orlando Research Center, Orlando, Florida 32809, USA. Tel: +1 407-240-7878; Fax: +1 407-240-9846; E-mail: tmarburynet.
Corresponding author: Dr. Thomas Marbury, President of Orlando Research Center, Orlando, Florida 32809, USA. Tel: +1 407-240-7878; Fax: +1 407-240-9846; E-mail: tmarburynet.

 Global reach, higher impact
Global reach, higher impact