Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(11):2093-2108. doi:10.7150/jca.86495 This issue Cite
Research Paper
The Valuable Prognostic Impact of Regional Lymph Node Removed on Outcomes for IIIA N0 NSCLC Patients
1. Department of Respiratory Medicine, Shanghai Tenth People's Hospital, Tongji University; Shanghai 200040, China.
2. Tongji University School of Medicine; Shanghai 200072, China.
3. Department of Respiratory Medicine, ChongMing Branch of Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai 202150, China.
#These authors have contributed equally to this work.
Received 2023-5-25; Accepted 2023-6-29; Published 2023-7-9
Abstract
Background: Regional lymph nodes (RLNs) removed combined with surgery is a standard option for patients at stage I to IIIA NSCLC. The objective of the study is to clarify the effect of removing different number of RLNs on survival outcomes for patients at stage IIIA N0 NSCLC.
Methods: Patients at stage IIIA N0 NSCLC from 2004 to 2015 were identified from Surveillance, Epidemiology, and End Results (SEER) database. Prior propensity score method (PSM), survival time was compared among different number (0, 1-3 and ≥4) of RLNs removed groups. After PSM, lung cancer-specific survival (LCSS) and overall survival (OS) were compared. Kaplan-Meier analysis and Cox regression analyses were used to clarify the impact of the factors on the prognosis with hazard ratio (HR) and 95% confidence interval (CI).
Results: A total of 11,583 patients at stage IIIA N0 NSCLC were included. Prior PSM, survival indicators including 1-year mortality rate, 5-year mortality rate, median survival time (MDST) and mean survival time (MST) from good to bad were all: ≥4, 1-3 and none RLNs removed group. After PSM, Kaplan-Meier survival analyses and univariate Cox regression analyses on OS and LCSS revealed a statistically significance on survival curve (P<0.001) between each two of the three groups (none, 1-3 and ≥4 RLNs removed group). Multivariable Cox regression analyses on OS and LCSS showed an independent association of RLNs removed with higher OS (HR, 0.275; 95% CI, 0.259-0.291; P<0.001) and LCSS (HR, 0.239; 95% CI, 0.224-0.256; P<0.001) compared with none RLN removed and no statistical difference with OS (HR, 1.118; 95% CI, 0.983-1.271; P=0.088) and LCSS (HR, 1.107; 95% CI, 0.954-1.284; P=0.179) between 1-3 RLNs removed and ≥4 RLNs removed.
Conclusions: Removing RLNs was beneficial to survival outcomes of patients at stage IIIA N0 NSCLC. Compared with 1-3 RLNs removed, ≥4 RLNs removed could bring a better survival time but not an independent prognostic factor (P>0.05).
Keywords: regional lymph nodes, non-small cell lung cancer, lung cancer-specific survival, overall survival
1. Introduction
Lung cancer (LC) is the leading cause of cancer-related death [1]. In the latest global cancer statistics, LC is with high incidence and high mortality characteristics which is 11.4% and 18.0% of all cancers, ranking the first and second, respectively [2]. 1,796,144 people in 185 countries died of LC in 2020, indicating that the research about LC is of great significance. NSCLC is associated with the characteristics of high morbidity and mortality in all lung malignancy subtypes. Approximately, 85% of lung cancer are NSCLC and the 5-year survival rate for NSCLC patients is about 15-20% [3, 4]. However, only about one-third of NSCLC patients who were diagnosed early may be cured by resection of tumor [5]. As the epidemic of NSCLC, it is concerned by people and has become a serious global public health problem.
According to National Comprehensive Cancer Network Guidelines (Version 1.2020, 2019) in the US, suitable patients at stage I to IIIA NSCLC may be recommended to have a surgery to cure which may be the best way to cure NSCLC. During the surgery, RLNs is a standard treatment for patients with NSCLC [6]. However, the optimal number of RLNs during surgery is still controversial for the IIIA NSCLC patients. The ACOSOG Z0030 trial suggested the optimal number of resection lymph node (LN) should be 10 [7]. The American College of Surgeons' Commission on Cancer considered a removal of at least 10 regional LNs might be adequate [8]. Currently, the Union for International Cancer Control and American Joint Committee on Cancer both endorsed 6 LNs for resection [9].
At present, there are few clinic studies on the impact of the RLNs removed on the survival of NSCLC patients, especially for IIIA stage. Our study is aimed to clarify the optimal number of RLNs removed for IIIA N0 NSCLC, and finger out the prognostic factors for them.
2. Materials and Methods
2.1. Data source
We conducted this retrospective study to clarify the impact of the number of RLNs removed on patients at stage IIIA N0 NSCLC. The data were retrieved from SEER database, which was established by the National Cancer Institute in the United States (US) in 1973. The SEER database keeps providing incidence, survival, and mortality data for histopathologic cancer subtypes and data by molecular subtyping, covering approximately 30% population of the US [10].
2.2. Study population
Eligible LC patients were identified initially from SEER database between 2004 and 2015. We limited the cohort to NSCLC patients diagnosed with adenocarcinoma (pathological codes 8140/3), squamous cell carcinoma (pathological codes 8070/3), adenosquamous carcinoma (pathological codes 8560/3), large cell carcinoma (pathological codes 8012/3) and other types of NSCLC. The patients with not applicable LCSS were excluded. The number of RLNs removed was divided into three categories: none RLN removed group, 1-3 RLNs removed group and ≥4 RLNs removed group. Detailed criterion is shown in Figure 1. “Excluded LCSS N/A not first” meant the research has ruled out that the tumors studied were not the first tumors to occur.
2.3. Covariates
Baseline clinical characteristics including age, survival time, size of tumor, gender, race, region, year of diagnosis, primary site, grade, laterality, pathology of tumor, stage, radiation record (RT), chemotherapy record (CT), radiation sequence with surgery, insurance, high school education (%), marital status, median household income (US dollars, tens) and number of RLNs removed were collected.
2.4. Statistical analyses
Potential deviation between none RLN removed group and RLNs group, 1-3 RLNs removed and ≥4 RLNs removed group were controlled by PSM analysis (1:3). The survival curves were created by using Kaplan-Meier analysis with log-rank test to compare OS and LCSS for the various RLNs removed categories, RT record and CT record among the cohort after PSM. Univariate and multivariate Cox regression analyses were used to clarify the impact and independence of predictors to survival outcomes: OS and LCSS. Predictors (P<0.05) identified in Kaplan-Meier analyses or univariable analyses were included into multivariable analysis.
Continuous variables were compared by using t-test, and categorical variables were compared by using chi-square. Data were analyzed using IBM SPSS version 21.0 (IBM Corp, Armonk, NY, USA). The forest plots were generated by GraphPad Prism (version 8.0, GraphPad Software Inc, San Diego, CA, USA). Statistical significance was set at a two-tailed P<0.05.
3. Results
3.1. Study cohort characteristics
Figure 1 shows a flow diagram of the study for the detailed criterion about patients from the SEER database. A total of 11,583 patients at stage IIIA T4, M0, N0 NSCLC were included among the cohort, with the histological type of squamous cell carcinoma (n=3730), adenocarcinoma (n=4720), adenosquamous carcinoma (n=148), large cell carcinoma (n=335) and other types of NSCLC (n=2650). These 11,583 patients were divided into three categories: 8751 patients without any RLN removed group (75.5%), 601 patients with 1-3 RLNs removed group (5.2%) and 2231 patients with ≥4 RLNs removed group (19.3%).
Study flow diagram. SEER, Surveillance, Epidemiology and End Results; LC, lung cancer; NSCLC, non-small cell lung cancer; N/A, not applicable; OS, overall survival; LCSS, lung cancer-specific survival; NOS, not otherwise specified; RLN, regional lymph node.
We compared the clinical, histological, social, demographic and therapeutic characteristics between none RLN removed group and RLNs removed group, 1-3 RLNs removed and ≥4 RLNs removed group both prior and after PSM (Table 1,2). Prior PSM, compared with none RLN removed group, the proportions from not first to first were age between 65 to 74 (38.1%), diagnosis year between 2012-2015 (34.0%) and poorly differentiated (36.3%) in RLNs removed group. Besides, female (47.8%), white race (84.7%), tumor size ≤1 cm (96.4%), tumor on upper lobe (62.9%), laterality right-origin of primary (57.9%), adenocarcinoma (43.3%), no CT record (59.6%), insured (53.5%) married (56.2%) and income >5000, ≤7000 (50.7%) were more common in RLNs removed group. Compared with 1-3 RLNs removed group, the proportions from not first to first were diagnosis year between 2012-2015 (35.5%) and pacific coast region (43.2%) in ≥4 RLNs removed group. Besides, age between 55 to 64 (26.7), male (52.8%), tumor size ≤1 cm (97.0%), tumor on upper lobe (63.3%), poorly differentiated (36.8%), laterality right-origin of primary (58.0%), adenocarcinoma (44.1%), no CT record (59.8%), insured (55.2%) married (57.2%) and income >5000, ≤7000 (51.5%) were more common in ≥4 RLNs removed group. The largest to small proportion of applications of RT were: none RLNs removed group, RLNs removed group and 1-3 RLNs removed group and ≥4 RLNs removed group.
After PSM, there were 8496 patients without any RLN removed and 2832 patients with RLNs removed. Among the cohort of RLNs removed, there were 601 patients with 1-3 RLNs removed and 1803 patients with ≥4 RLNs removed.
3.2. Survival outcomes
The 1-year mortality rate was 51.8% (5998 of 11583), including 5543 deaths (63.3%), 123 deaths (20.5%) and 332 deaths (14.8%) in the none RLN removed group, 1-3 RLNs removed group and ≥4 RLNs removed group (P<0.05). 5-year survival rate, MDST and MST prior PSM were shown in Table 3. All survival indicators from good to bad were: ≥4 RLNs removed group, 1-3 RLNs removed group and none RLN removed group.
Baseline characteristics of patients at stage IIIA N0 NSCLC with and without RLNs removed prior and after PSM
| Variable | Full cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| None RLN removed | With RLNs removed | P value | None RLN removed | With RLNs removed | P value | ||
| Age | <0.001 | <0.001 | |||||
| ≤45 | 132 (1.5) | 52 (1.8) | 130 (1.5) | 52 (1.8) | |||
| 45-54 | 644 (7.4) | 270 (9.5) | 625 (7.4) | 270 (9.5) | |||
| 55-64 | 1641 (18.8) | 736 (26.0) | 1585 (18.7) | 736 (26.0) | |||
| 65-74 | 2668 (30.5) | 1079 (38.1) | 2573 (30.3) | 1079 (38.1) | |||
| ≥75 | 3666 (41.9) | 695 (24.5) | 3583 (42.4) | 695 (24.5) | |||
| Sex | 0.007 | 0.019 | |||||
| Female | 3929 (44.9) | 1354 (47.8) | 4650 (54.7) | 1478 (52.2) | |||
| Male | 4822 (55.1) | 1478 (52.2) | 3846 (45.3) | 1354 (47.8) | |||
| Race | <0.001 | <0.001 | |||||
| White | 6916 (79.0) | 2398 (84.7) | 6719 (79.1) | 2399 (84.7) | |||
| Black | 1182 (13.5) | 260 (9.2) | 1142 (13.4) | 260 (9.2) | |||
| Others | 647 (7.4) | 168 (5.9) | 629 (7.4) | 168 (5.9) | |||
| Unknown | 6 (0.1) | 6 (0.2) | 6 (0.1) | 6 (0.2) | |||
| Tumor Size | <0.001 | <0.001 | |||||
| ≤1 cm | 5986 (68.4) | 2731 (96.4) | 5967 (70.2) | 2731 (96.4) | |||
| >1, ≤2 cm | 3 (0.0) | 2 (0.1) | 3 (0.0) | 2 (0.1) | |||
| >2, ≤3 cm | 7 (0.1) | 2 (0.1) | 7 (0.1) | 2 (0.1) | |||
| >3, ≤4 cm | 7 (0.1) | 3 (0.1) | 7 (0.1) | 3 (0.1) | |||
| >4 cm | 2 (0.0) | 0 (0) | 2 (0.0) | 0 (0.0) | |||
| Unknown | 2746 (31.4) | 94 (3.3) | 2510 (29.5) | 94 (3.3) | |||
| Diagnosis year | <0.001 | <0.001 | |||||
| 2004-2007 | 4232 (48.4) | 931 (32.2) | 4061 (47.8) | 913 (32.2) | |||
| 2008-2011 | 2916 (33.3) | 955 (33.7) | 2849 (33.5) | 955 (33.7) | |||
| 2012-2015 | 1603 (18.3) | 964 (34.0) | 1586 (18.7) | 964 (34.0) | |||
| Tumor location | <0.001 | <0.001 | |||||
| Main bronchus | 558 (6.4) | 53 (1.9) | 484 (5.7) | 42 (1.5) | |||
| Upper lobe | 4401 (50.3) | 1782 (62.9) | 4333 (51.0) | 1782 (62.9) | |||
| Middle lobe | 288 (3.3) | 89 (3.1) | 280 (3.3) | 89 (3.1) | |||
| Lower lobe | 1944 (22.2) | 809 (28.6) | 1913 (22.5) | 809 (28.6) | |||
| Overlapping lesion | 110 (1.3) | 57 (2.0) | 110 (1.3) | 57 (2.0) | |||
| Not otherwise specified | 1450 (16.6) | 42 (1.5) | 1376 (16.2) | 53 (1.9) | |||
| Differentiation grade | <0.001 | <0.001 | |||||
| Well differentiated | 309 (3.5) | 387 (13.7) | 304 (3.6) | 387 (13.7) | |||
| Moderately differentiated | 1365 (15.6) | 1018 (35.9) | 1342 (15.8) | 1018 (35.9) | |||
| Poorly differentiated | 2391 (27.3) | 1028 (36.3) | 2364 (27.8) | 1028 (36.3) | |||
| Undifferentiated | 146 (1.7) | 51 (1.8) | 145 (1.7) | 51 (1.8) | |||
| Unknown | 4540 (51.9) | 348 (12.3) | 4341 (51.1) | 348 (12.3) | |||
| Laterality | <0.001 | <0.001 | |||||
| Left-origin of primary | 3882 (44.4) | 1189 (42.0) | 3759 (44.2) | 1189 (42.0) | |||
| Right-origin of primary | 4678 (53.5) | 1639 (57.9) | 4565 (53.7) | 1639 (57.9) | |||
| One side, unspecified | 32 (0.4) | 2 (0.1) | 3 (0.4) | 2 (0.1) | |||
| Paired site | 147 (1.7) | 2 (0.1) | 136 (1.6) | 2 (0.1) | |||
| Not a paired site | 12 (0.1) | 0 (0.0) | 6 (0.1) | 0 (0.0) | |||
| Histologic type | <0.001 | <0.001 | |||||
| Adenocarcinoma | 3490 (39.9) | 1230 (43.4) | 3422 (40.3) | 1230 (43.4) | |||
| Squamous cell carcinoma | 2908 (33.2) | 822 (29.0) | 2780 (32.7) | 822 (29.0) | |||
| Adenosquamous | 83 (0.9) | 65 (2.3) | 82 (1.0) | 65 (2.3) | |||
| Large cell carcinoma | 264 (3.0) | 71 (2.5) | 255 (3.0) | 71 (2.5) | |||
| Other types of NSCLC | 2006 (22.9) | 644 (22.7) | 1957 (23.0) | 644 (22.7) | |||
| Radiotherapy record | <0.001 | <0.001 | |||||
| Beam radiation | 3597 (41.1) | 620 (21.9) | 3354 (39.5) | 620 (21.9) | |||
| Beam with implants or isotopes | 21 (0.2) | 2 (0.1) | 13 (0.2) | 2 (0.1) | |||
| Radiation, but not specified | 75 (0.9) | 12 (0.4) | 73 (0.9) | 12 (0.4) | |||
| Radioactive implants | 24 (0.3) | 7 (0.2) | 22 (0.3) | 7 (0.2) | |||
| Recommended, unknown | 64 (0.7) | 21 (0.7) | 64 (0.8) | 21 (0.7) | |||
| Refused | 158 (1.8) | 7 (0.2) | 158 (1.9) | 7 (0.2) | |||
| None/Unknown | 4812 (55.0) | 2163 (76.4) | 4812 (56.6) | 2163 (76.4) | |||
| Radiation sequence | <0.001 | <0.001 | |||||
| Prior to surgery | 26 (0.3) | 185 (6.5) | 26 (0.3) | 185 (6.5) | |||
| After surgery | 124 (1.4) | 433 (15.3) | 124 (1.5) | 433 (15.3) | |||
| Before and after surgery | 7 (0.1) | 19 (0.7) | 7 (0.1) | 19 (0.7) | |||
| Intraoperative radiation | 5 (0.1) | 0 (0) | 0 (0.0) | 1 (0.0) | |||
| Unknown, but both given | 1 (0.0) | 3 (01) | 4 (0.0) | 0 (0.0) | |||
| No radiation and/or surgery | 8588 (98.1) | 2191 (77.4) | 8334 (98.1) | 2191 (77.4) | |||
| In and before/after surgery | 0 (0) | 1 (0.0) | 1 (0.0) | 3 (0.1) | |||
| Chemotherapy record | 0.001 | 0.006 | |||||
| Yes | 3835 (43.8) | 1143 (40.4) | 3680 (43.3) | 1143 (40.4) | |||
| No | 4916 (56.2) | 1689 (59.6) | 4816 (56.7) | 1689 (59.6) | |||
| Marital status | <0.001 | <0.001 | |||||
| Married | 4056 (46.3) | 1591 (56.2) | 3952 (46.5) | 1591 (56.2) | |||
| Separated | 94 (1.1) | 27 (1.0) | 91 (1.1) | 27 (1.0) | |||
| Single | 1210 (13.8) | 340 (12.0) | 1170 (13.8) | 340 (12.0) | |||
| Divorced | 1024 (11.7) | 366 (12.9) | 987 (11.6) | 366 (12.9) | |||
| Unmarried or domestic partner | 5 (0.1) | 2 (0.1) | 5 (0.1) | 2 (0.1) | |||
| Widowed | 2054 (23.5) | 410 (14.5) | 1991 (23.4) | 410 (14.5) | |||
| Unknown | 308 (3.5) | 96 (3.4) | 300 (3.5) | 96 (3.4) | |||
| Median family income (dollar, tens) | <0.001 | <0.001 | |||||
| ≤5000 | 2801 (32.0) | 724 (25.6) | 2273 (32.1) | 724 (25.6) | |||
| >5000, ≤7000 | 4105 (46.9) | 1435 (50.7) | 3965 (46.7) | 1435 (50.7) | |||
| >7000, ≤9000 | 1568 (17.9) | 579 (20.4) | 1537 (18.1) | 579 (20.4) | |||
| >9000 | 277 (3.2) | 94 (3.3) | 271 (3.2) | 94 (3.3) | |||
Abbreviations: NSCLC, non-small cell lung cancer; RLN, regional lymph node.
Baseline characteristics of patients at stage IIIA N0 NSCLC with and without RLNs removed prior and after PSM
| Variable | Full cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| 1-3 RLNs removed | ≥4 RLNs removed | P value | 1-3 RLNs removed | ≥4 RLNs removed | P value | ||
| Age | 0.591 | 0.782 | |||||
| ≤45 | 11 (1.8) | 41 (1.8) | 11 (1.8) | 32 (1.8) | |||
| 45-54 | 58 (9.7) | 212 (9.5) | 58 (9.7) | 163 (9.0) | |||
| 55-64 | 141 (23.5) | 594 (26.7) | 141 (23.5) | 471 (26.1) | |||
| 65-74 | 234 (38.9) | 845 (37.9) | 234 (38.9) | 676 (37.5) | |||
| ≥75 | 157 (26.1) | 538 (24.1) | 157 (26.1) | 461 (25.6) | |||
| Sex | 0.244 | 0.604 | |||||
| Female | 300 (49.9) | 1054 (47.2) | 301 (50.1) | 925 (51.3) | |||
| Male | 301 (50.1) | 1177 (52.8) | 300 (49.9) | 878 (48.7) | |||
| Race | 0.888 | 0.951 | |||||
| White | 511 (85.0) | 1887 (84.6) | 511 (85.0) | 1533 (85.0) | |||
| Black | 57 (9.5) | 203 (9.1) | 57 (9.5) | 162 (9.0) | |||
| Others | 32 (5.3) | 136 (6.1) | 32 (5.3) | 104 (5.8) | |||
| Unknown | 1 (0.2) | 5 (0.2) | 1 (0.2) | 4 (0.2) | |||
| Tumor Size | 0.009 | 0.059 | |||||
| ≤1 cm | 567 (94.3) | 2164 (97.0) | 567 (94.3) | 1740 (96.5) | |||
| >1, ≤2 cm | 0 (0.0) | 2 (0.1) | 0 (0.0) | 2 (0.1) | |||
| >2, ≤3 cm | 1 (0.2) | 1 (0.0) | 1 (0.2) | 1 (0.1) | |||
| >3, ≤4 cm | 0 (0.0) | 3 (0.1) | 0 (0.0) | 3 (0.2) | |||
| >4 cm | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Unknown | 33 (5.5) | 61 (2.7) | 33 (5.5) | 57 (3.2) | |||
| Diagnosis year | <0.001 | 0.123 | |||||
| 2004-2007 | 231 (38.4) | 682 (30.6) | 231 (38.4) | 614 (34.1) | |||
| 2008-2011 | 199 (33.1) | 756 (33.9) | 199 (33.1) | 617 (34.2) | |||
| 2012-2015 | 171 (28.5) | 793 (35.5) | 171 (28.5) | 572 (31.7) | |||
| Tumor location | 0.001 | 0.046 | |||||
| Main bronchus | 370 (61.6) | 1412 (63.3) | 13 (2.2) | 27 (1.5) | |||
| Upper lobe | 20 (3.3) | 69 (3.1) | 370 (61.6) | 1136 (63.0) | |||
| Middle lobe | 168 (28.0) | 641 (28.7) | 20 (28.0) | 61 (3.4) | |||
| Lower lobe | 23 (3.8) | 30 (1.3) | 168 (28.0) | 530 (29.4) | |||
| Overlapping lesion | 7 (1.2) | 50 (2.2) | 7 (1.2) | 19 (1.1) | |||
| Not otherwise specified | 13 (2.2) | 29 (1.3) | 23 (3.8) | 30 (1.7) | |||
| Differentiation grade | 0.005 | 0.161 | |||||
| Well differentiated | 80 (13.3) | 307 (13.8) | 80 (13.3) | 262 (14.5) | |||
| Moderately differentiated | 202 (33.6) | 816 (36.6) | 202 (33.6) | 638 (35.4) | |||
| Poorly differentiated | 206 (34.3) | 822 (36.8) | 206 (34.3) | 637 (35.3) | |||
| Undifferentiated | 12 (2.0) | 39 (1.7) | 12 (2.0) | 37 (2.1) | |||
| Unknown | 101 (16.8) | 247 (11.1) | 101 (16.8) | 229 (12.7) | |||
| Laterality | 0.046 | 0.048 | |||||
| Left-origin of primary | 346 (57.6) | 1293 (58.0) | 253 (42.1) | 750 (41.6) | |||
| Right-origin of primary | 253 (42.1) | 936 (42.0) | 346 (57.6) | 1053 (58.4) | |||
| One side, unspecified | 0 (0.0) | 2 (0.1) | 0 (0.0) | 0 (0.0) | |||
| Paired site | 2 (0.3) | 0 (0.0) | 2 (0.3) | 0 (0.0) | |||
| Not a paired site | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Histologic type | 0.031 | 0.555 | |||||
| Adenocarcinoma | 247 (41.1) | 983 (44.1) | 247 (41.1) | 799 (44.3) | |||
| Squamous cell carcinoma | 162 (27.0) | 660 (29.6) | 162 (27.0) | 479 (26.6) | |||
| Adenosquamous | 20 (3.3) | 45 (2.0) | 20 (3.3) | 45 (2.5) | |||
| Large cell carcinoma | 13 (2.2) | 58 (2.6) | 13 (2.2) | 40 (2.2) | |||
| Other types of NSCLC | 159 (26.5) | 485 (21.7) | 159 (26.5) | 440 (24.4) | |||
| Radiotherapy record | <0.001 | 0.016 | |||||
| Beam radiation | 177 (29.5) | 443 (19.9) | 177 (29.5) | 410 (22.7) | |||
| Beam with implants or isotopes | 0 (0.0) | 2 (0.1) | 3 (0.5) | 0 (0.0) | |||
| Radiation, but not specified | 3 (0.5) | 9 (0.4) | 0 (0.0) | 8 (0.4) | |||
| Radioactive implants | 3 (0.5) | 4 (0.2) | 3 (0.5) | 4 (0.2) | |||
| Recommended, unknown | 4 (0.7) | 17 (0.8) | 4 (0.7) | 13 (0.7) | |||
| Refused | 3 (0.5) | 4 (0.2) | 3 (0.5) | 4 (0.2) | |||
| None/Unknown | 411 (68.4) | 1752 (78.5) | 411 (68.4) | 1364 (75.7) | |||
| Radiation sequence | <0.001 | <0.001 | |||||
| Prior to surgery | 36 (6.0) | 149 (6.7) | 36 (6.0) | 120 (6.7) | |||
| After surgery | 140 (23.3) | 293 (13.1) | 140 (23.3) | 288 (16.0) | |||
| Before and after surgery | 4 (0.7) | 15 (0.7) | 4 (0.7) | 13 (0.7) | |||
| Intraoperative radiation | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | |||
| Unknown, but both given | 2 (0.3) | 1 (0.0) | 2 (0.3) | 1 (0.1) | |||
| No radiation and/or surgery | 418 (69.6) | 1773 (79.5) | 418 (69.6) | 1381 (76.6) | |||
| In and before/after surgery | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Chemotherapy record | 0.748 | 0.399 | |||||
| Yes | 246 (40.9) | 897 (40.2) | 246 (40.9) | 703 (39.0) | |||
| No | 355 (59.1) | 1334 (59.8) | 355 (59.1) | 1100 (61.0) | |||
| Marital status | 0.060 | 0.702 | |||||
| Married | 314 (52.2) | 1277 (57.2) | 314 (52.2) | 997 (55.3) | |||
| Separated | 8 (1.3) | 19 (0.9) | 8 (1.3) | 18 (1.0) | |||
| Single | 70 (11.6) | 270 (12.1) | 70 (11.6) | 207 (11.5) | |||
| Divorced | 83 (13.8) | 283 (12.7) | 83 (13.8) | 241 (13.4) | |||
| Unmarried or domestic partner | 0 (0.0) | 2 (0.1) | 0 (0.0) | 0 (0.0) | |||
| Widowed | 109 (18.1) | 301 (13.5) | 109 (18.1) | 284 (15.8) | |||
| Unknown | 17 (2.8) | 79 (3.5) | 17 (2.8) | 56 (3.1) | |||
| Median family income (dollar, tens) | 0.001 | 0.029 | |||||
| ≤5000 | 188 (31.3) | 536 (24.0) | 188 (31.3) | 474 (26.3) | |||
| >5000, ≤7000 | 287 (47.8) | 1148 (51.5) | 287 (47.8) | 933 (51.7) | |||
| >7000, ≤9000 | 101 (16.8) | 478 (21.4) | 101 (16.8) | 344 (19.1) | |||
| >9000 | 25 (4.2) | 69 (3.1) | 25 (4.2) | 52 (2.9) | |||
Abbreviations: NSCLC, non-small cell lung cancer; RLN, regional lymph node.
Overall survival, lung cancer-specific survival, median survival time and mean survival time in stage IIIA N0 NSCLC patients with different number of RLNs removed prior PSM
| Variable | Number | 5-year survival rate (%) | Median survival time (months) | Mean survival time (months) | |
|---|---|---|---|---|---|
| Overall survival | Lung cancer-specific survival | ||||
| Overall patients | 11,583 | 6.6 | 8.0 | 11.0 | 21.6 |
| Number of RLNs removed | |||||
| None | 8,751 | 2.9 | 3.5 | 8.0 | 15.5 |
| 1-3 | 601 | 17.0 | 20.8 | 27.0 | 39.1 |
| ≥4 | 2,231 | 18.6 | 22.1 | 31.0 | 40.9 |
Abbreviations: NSCLC, non-small cell lung cancer; RLNs, regional lymph nodes.
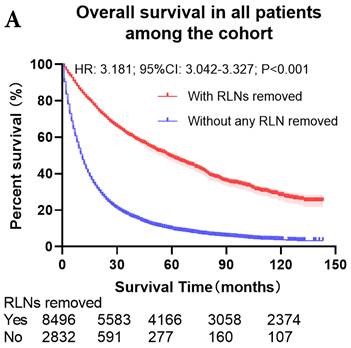
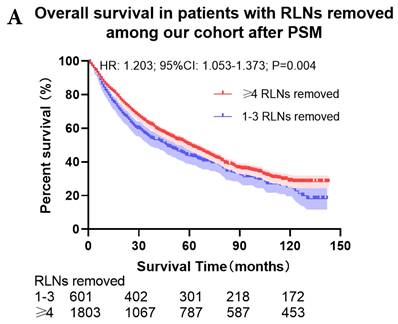
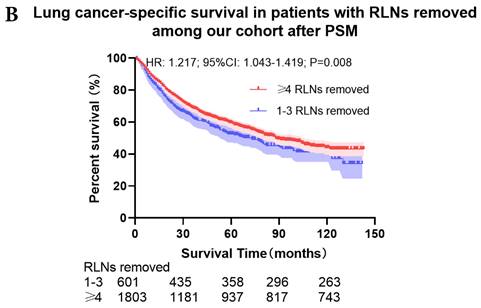
The survival curves showed that there were statistically significances both on OS and LCSS among the cohort after PSM with regard to number of RLNs removed (P<0.001 and P<0.001, respectively), CT (P<0.001 and P<0.001 with curves crossing, respectively) and RT (P<0.001 with curves crossing and P<0.001 with curves crossing, respectively) between without and with RLNs removed group by using Kaplan-Meier analysis with log-rank test (Figure 2, Figure S1). Between 1-3 RLNs removed group and ≥4 RLNs removed group, the survival curves showed that there were also statistically significances both on OS and LCSS among the cohort after PSM with regard to number of RLNs removed (P=0.002 and P=0.004, respectively) and RT (P<0.001 with curves crossing and P<0.001 with curves crossing, respectively) by using Kaplan-Meier analysis with log-rank test (Figure 3, Figure S2A, S2B). In terms of CT, there were statistically significant differences on LCSS among the cohort after PSM with regard to (P=0.008 with curves crossing) except OS (P=0.833) between 1-3 RLNs removed group and ≥4 RLNs removed group (Figure S2C, S2D).
Univariate and multivariable analysis on OS and LCSS between without and with RLNs removed group, 1-3 RLNs removed group and ≥4 RLNs removed group were shown in Table 4,5. Multivariable Cox regression analysis revealed independent associations of RLNs removed with higher OS (HR, 0.275; 95% CI, 0.259-0.291; P<0.001) and LCSS (HR, 0.239; 95% CI, 0.224-0.256; P<0.001) compared with none RLNs removed (Table 4). Furthermore, a smaller number of RLNs removed (1-3 RLNs) was found to be no statistical difference with OS (HR, 1.118; 95% CI, 0.983-1.271; P=0.088) and LCSS (HR, 1.107; 95% CI, 0.954-1.284; P=0.179) compared with a larger number of RLNs removed (≥4 RLNs) (Table 5). In addition, the forest plots of HRs for OS were generated to show the same multivariable Cox regression analysis outcomes of treatments which is also factors that can be changed even when the patient have been suffered from NSCLC. between without and with RLNs removed group, 1-3 RLNs removed group and ≥4 RLNs removed group more visually (Figure 4).
Comparison of survival curve: role of RLNs removed on survival outcome: (a) RLNs removed that can influence OS; (b) LNs removed that can influence LCSS. RLN, regional lymph node; HR, hazard ratio; CI, confidence interval.
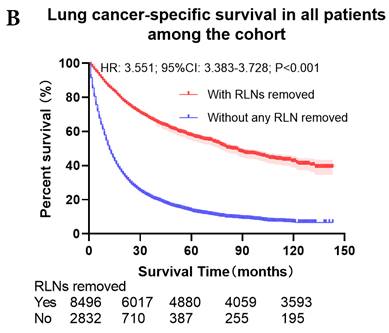
Comparison of survival curve: role of different number of RLNs removed on survival outcome: (a) Different number of RLNs removed that can influence OS; (b) Different number of RLNs removed that can influence LCSS. RLN, regional lymph node; HR, hazard ratio; CI, confidence interval.
Univariable and multivariable Cox analyses of OS and LCSS in all patients among our cohort after PSM
| Variables | Overall survival | Lung cancer-specific survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||
| Age | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| ≤45 | Reference | Reference | Reference | Reference | |||||||
| 45 to 54 | 1.112 (0.926-1.358) | 0.240 | 1.241 (1.024-1.504) | 0.028 | 1.086 (0.890-1.326) | 0.415 | 1.196 (0.979-1.461) | <0.001 | |||
| 55 to 64 | 1.112 (0.936-1.346) | 0.214 | 1.335 (1.111-1.603) | 0.002 | 1.037 (0.858-1.253) | 0.705 | 1.242 (1.026-1.503) | 0.079 | |||
| 65 to 74 | 1.316 (1.100-1.574) | 0.003 | 1.562 (1.302-1.873) | <0.001 | 1.177 (0.977-1.419) | 0.086 | 1.413 (1.169-1.708) | 0.026 | |||
| ≥75 | 1.922 (1.608-2.298) | <0.001 | 1.892 (1.576-2.271) | <0.001 | 1.705 (1.416-2.053) | <0.001 | 1.703 (1.408-2.059) | <0.001 | |||
| Sex | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Male | Reference | Reference | Reference | Reference | |||||||
| Female | 0.823 (0.789-0.859) | 0.785 (0.751-0.822) | 0.826(0.789-0.865) | 0.798 (0.760-0.838) | |||||||
| Race | 0.001 | 0.001 | <0.002 | 0.055 | |||||||
| White | Reference | Reference | Reference | Reference | |||||||
| Black | 1.110 (1.043-1.182) | 0.001 | 1.014 (0.950-1.083) | 0.674 | 1.132 (1.058-1.211) | <0.001 | 1.019 (0.950-1.094) | 0.600 | |||
| Other | 0.953 (0.878-1.036) | 0.259 | 0.851 (0.781-0.928) | <0.001 | 1.018 (0.933-1.111) | 0.688 | 0.897 (0.819-0.983) | 0.020 | |||
| Unknown | 0.390 (0.146-1.039) | 0.060 | 0.407 (0.152-1.090) | 0.074 | 0.469 (0.176-1.250) | <0.130 | 0.515 (0.192-1.380) | 0.187 | |||
| Tumor size | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| ≤1 cm | Reference | Reference | Reference | Reference | |||||||
| >1, ≤2 cm | 0.583 (0.146-2.332) | 0.446 | 0.719 (0.179-2.881) | 0.641 | 0.324 (0.048-2.428) | 0.283 | 0.453 (0.064-3.226) | 0.430 | |||
| >2, ≤3 cm | 0.650 (0.270-1.562) | 0.335 | 0.663 (0.275-1.596) | 0.359 | 0.635 (0.238-1.694) | 0.365 | 0.663 (0.248-1.770) | 0.412 | |||
| >3, ≤4 cm | 0.776 (0.349-1.728) | 0.535 | 0.926 (0.415-2.071) | 0.852 | 0.778 (0.324-1.871) | 0.576 | 0.943 (0.391-2.275) | 0.896 | |||
| >4 cm | 1.611 (0.403-6.442) | 0.500 | 1.317 (0.327-5.306) | 0.699 | 0.937 (0.132-6.653) | 0.948 | 0.718 (0.101-5.131) | 0.741 | |||
| Unknown | 2.226 (2.124-2.333) | <0.001 | 1.414 (1.336-1.495) | <0.001 | 2.335 (2.221-2.456) | <0.001 | 1.432 (1.348-1.521) | <0.001 | |||
| Diagnosis year | <.001 | <0.001 | <0.001 | <0.001 | |||||||
| 2004-2007 | Reference | Reference | Reference | Reference | |||||||
| 2008-2011 | 0.812 (0.775-0.850) | <.001 | 0.896 (0.838-0.959) | 0.002 | 0.793 (0.755-0.833) | <0.001 | 0.903 (0.839-0.972) | 0.007 | |||
| 2012-2015 | 0.503 (0.470-0.538) | <.001 | 0.698 (0.640-0.760) | <0.001 | 0.476 (0.443-0.513) | <0.001 | 0.693 (0.631-0.761) | <0.001 | |||
| Tumor location | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Main bronchus | Reference | Reference | Reference | Reference | |||||||
| Upper lobe | 0.586 (0.532-0.644) | <0.001 | 0.738 (0.669-0.814) | <0.001 | 0.556 (0.502-0.615) | <0.001 | 0.718 (0.647-0.797) | <0.001 | |||
| Middle lobe | 0.665 (0.575-0.770) | <0.001 | 0.756 (0.651-0.878) | <0.001 | 0.628 (0.537-0.736) | <0.001 | 0.735 (0.625-0.863) | <0.001 | |||
| Lower lobe | 0.648 (0.586-0.717) | <0.001 | 0.772 (0.696-0.856) | <0.001 | 0.602 (0.541-0.670) | <0.001 | 0.738 (0.661-0.824) | <0.001 | |||
| Overlapping lesion | 0.691 (0.571-0.836) | <0.001 | 0.881 (0.727-1.069) | 0.199 | 0.693 (0.566-0.848) | <0.001 | 0.904 (0.737-1.109) | 0.335 | |||
| NOS | 1.244 (1.120-1.382) | <0.001 | 0.894 (0.799-1.002) | 0.054 | 1.239 (1.108-1.385) | <0.001 | 0.890 (0.789-1.004) | 0.058 | |||
| Grade | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Well differentiated | Reference | Reference | Reference | Reference | |||||||
| Moderate differentiated | 0.345 (0.310-0.385) | <0.001 | 0.604 (0.540-0.676) | <0.001 | 1.638 (1.437-1.867) | <0.001 | 1.463 (1.281-1.671) | <0.001 | |||
| Poorly differentiated | 0.532 (0.502-0.564) | <0.001 | 0.843 (0.792-0.898) | <0.001 | 2.354 (2.076-2.670) | <0.001 | 1.863 (1.638-2.118) | <0.001 | |||
| Undifferentiated | 0.745 (0.709-0.782) | <0.001 | 1.069 (1.015-1.126) | 0.011 | 3.186 (2.608-3.892) | <0.001 | 2.344 (1.895-2.898) | <0.001 | |||
| Unknown | 0.978 (0.838-1.141) | 0.777 | 1.325 (1.123-1.565) | 0.001 | 3.174 (2.806-3.591) | <0.001 | 1.703 (1.500-1.933) | <0.001 | |||
| Laterality | <0.001 | 0.675 | <0.001 | 0.861 | |||||||
| Left-origin of primary | Reference | Reference | Reference | Reference | |||||||
| Right-origin of primary | 0.959 (0.920-1.001) | 0.056 | 0.998 (0.956-1.043) | 0.941 | 0.942 (0.900-0.986) | 0.011 | 0.981 (0.936-1.029) | 0.436 | |||
| Only one side, unspecified | 2.152 (1.493-3.102) | <0.001 | 1.262 (0.872-1.826) | 0.217 | 2.034 (1.361-3.039) | 0.001 | 1.143 (0.762-1.715) | 0.519 | |||
| Paired site | 2.233 (1.878-2.654) | <0.001 | 1.054 (0.880-1.262) | 0.570 | 2.192 (1.817-2.645) | <0.001 | 0.994 (0.818-1.209) | 0.954 | |||
| Not a paired site | 1.430 (0.595-3.439) | 0.424 | 0.727(0.299-1.765) | 0.480 | 1.678 (0.698-4.034) | 0.248 | 0.806 (0.331-1.960) | 0.634 | |||
| Histologic type | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Adenocarcinoma | Reference | Reference | Reference | Reference | |||||||
| Squamous cell carcinoma | 1.179 (1.122-1.238) | <0.001 | 1.192 (1.130-1.258) | <0.001 | 1.148 (1.088-1.212) | <0.001 | 1.178 (1.111-1.249) | <0.001 | |||
| Adenosquamous | 1.065 (0.885-1.281) | 0.507 | 1.355 (1.124-1.634) | 0.001 | 1.098 (0.901-1.338) | 0.353 | 1.420 (1.164-1.734) | 0.001 | |||
| Large cell carcinoma | 1.428 (1.265-1.612) | <0.001 | 1.283 (1.125-1.463) | <0.001 | 1.431 (1.255-1.632) | <0.001 | 1.265 (1.097-1.457) | 0.001 | |||
| Other types of NSCLC | 1.055 (0.999-1.114) | 0.053 | 1.019 (0.963-1.078) | 0.515 | 1.065 (1.004-1.130) | 0.035 | 1.036 (0.975-1.100) | 0.259 | |||
| RLNs removed | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| With RNLs removed | Reference | Reference | Reference | Reference | |||||||
| Without any RLN removed | 0.275 (0.259-0.291) | 0.284 (0.265-0.305) | 0.239 (0.224-0.256) | 0.252 (0.233-0.273) | |||||||
| Radiotherapy record | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Beam radiation | Reference | Reference | Reference | Reference | |||||||
| Beam with implants or isotopes | 1.401 (0.829-2.368) | 0.208 | 1.520 (0.888-2.602) | 0.127 | 1.545 (0.896-2.665) | 0.118 | 1.673 (0.956-2.926) | 0.071 | |||
| Radiation, but not specified | 1.004 (0.788-1.279) | 0.976 | 0.911 (0.714-1.163) | 0.455 | 1.004 (0.772-1.305) | 0.978 | 0.907 (0.696-1.181) | 0.469 | |||
| Radioactive implants | 0.865 (0.574-1.304) | 0.488 | 0.774 (0.502-1.193) | 0.246 | 0.661 (0.398-1.098) | 0.110 | 0.629 (0.374-1.059) | 0.081 | |||
| Recommended, unknown | 1.043 (0.804-1.352) | 0.752 | 1.081 (0.832-1.404) | 0.560 | 1.130 (0.863-1.480) | 0.374 | 1.211 (0.923-1.589) | 0.166 | |||
| Refused | 1.797 (1.524-2.120) | <0.001 | 1.154 (0.975-1.367) | 0.096 | 1.880 (1.581-2.237) | <0.001 | 1.246 (1.043-1.490) | 0.015 | |||
| None/Unknown | 1.095 (1.048-1.145) | <0.001 | 1.332 (1.260-1.409) | <0.001 | 1.085 (1.034-1.138) | 0.001 | 1.345 (1.267-1.428) | <0.001 | |||
| Radiation sequence | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Prior to surgery | Reference | Reference | Reference | Reference | |||||||
| After surgery | 1.385 (1.132-1.696) | 0.002 | 1.095 (0.893-1.343) | 0.384 | 1.444 (1.151-1.812) | 0.002 | 1.140 (0.907-1.434) | 0.262 | |||
| Before and after surgery | 1.006 (0.589-1.719) | 0.983 | 0.735 (0.427-1.267) | 0.268 | 1.194 (0.682-2.091) | 0.535 | 0.832 (0.471-1.471) | 0.527 | |||
| Intraoperative radiation | 3.062 (0.428-21.911) | 0.265 | 2.616(0.344-19.890) | 0.353 | 0.005 (0.000-1.558E+) | 0.843 | 0.103(0.000-4.858E+) | 0.814 | |||
| Unknown, but both given | 1.358 (0.432-4.268) | 0.601 | 0.413 (0.132-1.420) | 0.167 | 1.128 (0.278-4.575) | 0.866 | 0.388 (0.092-1.647) | 0.199 | |||
| No radiation and/or surgery | 1.883 (0.599-5.920) | 0.279 | 1.143 (0.363-3.604) | 0.819 | 2.362 (0.749-7.452) | 0.143 | 1.342 (0.424-4.249) | 0.617 | |||
| In and before/after surgery | 2.122 (1.777-2.534) | <0.001 | 0.693 (0.575-0.836) | <0.001 | 2.271 (1.859-2.775) | <0.001 | 0.720 (0.583-0.888) | 0.002 | |||
| Chemotherapy record | |||||||||||
| No | Reference | Reference | Reference | Reference | |||||||
| Yes | 0.714 (0.684-0.745) | <0.001 | 0.655 (0.625-0.686) | <0.001 | 0.778 (0.743-0.814) | <0.001 | 0.704 (0.670-0.741) | <0.001 | |||
| Marital status | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Married | Reference | Reference | Reference | Reference | |||||||
| Separated | 1.121 (0.917-1.371) | 0.266 | 1.123 (0.917-1.376) | 0.263 | 1.181 (0.956-1.459) | 0.123 | 1.175 (0.949-1.456) | 0.138 | |||
| Single | 1.198 (1.112-1.278) | <0.001 | 1.184 (1.106-1.267) | <0.001 | 1.165 (1.086-1.251) | <0.001 | 1.144 (1.062-1.232) | <0.001 | |||
| Divorced | 1.060 (0.991-1.135) | 0.091 | 1.126 (1.050-1.208) | 0.001 | 1.059 (0.984-1.140) | 0.125 | 1.124 (1.043-1.213) | 0.002 | |||
| Unmarried or domestic partner | 0.671 (0.216-2.082) | 0.490 | 0.559 (0.180-1.739) | 0.315 | 0.770 (0.248-2.388) | 0.651 | 0.691 (0.222-2.150) | 0.523 | |||
| Widowed | 1.387 (1.315-1.462) | <0.001 | 1.145 (1.080-1.215) | <0.001 | 1.345 (1.269-1.425) | <0.001 | 1.124 (1.054-1.198) | <0.001 | |||
| Unknown | 1.051 (0.933-1.183) | 0.414 | 1.029 (0.912-1.161) | 0.642 | 1.011 (0.887-1.151) | 0.875 | 0.987 (0.864-1.127) | 0.846 | |||
| Median family income (dollar, tens) | <0.001 | 0.002 | <0.001 | 0.548 | |||||||
| ≤5000 | Reference | Reference | Reference | Reference | |||||||
| >5000, ≤7000 | 0.857 (0.817-0.899) | <0.001 | 0.938 (0.891-0.986) | 0.013 | 0.852 (0.809-.0897) | <0.001 | 0.936 (0.886-0.989) | 0.019 | |||
| >7000, ≤9000 | 0.806 (0.758-0.857) | <0.001 | 0.891 (0,831-0.954) | 0.001 | 0.813 (0.761-0.869) | <0.001 | 0.896 (0.832-0.966) | 0.004 | |||
| >9000 | 0.806 (0.713-0.912) | 0.001 | 0.840 (0.739-0.956) | 0.008 | 0.792 (0.692-0.907) | 0.001 | 0.826 (0.718-0.951) | 0.008 | |||
Abbreviations: OS, overall survival; LCSS, lung cancer-specific survival; NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval; RLN, regional lymph node.
Univariable and multivariable Cox analyses of OS and LCSS in patients with RLNs removed after PSM
| Variables | Overall survival | Lung cancer-specific survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||
| Age | <0.001 | <0.001 | 0.001 | <0.001 | |||||||
| ≤45 | Reference | Reference | Reference | Reference | |||||||
| 45 to 54 | 1.525 (0.902-2.578) | 0.116 | 1.667 (0.981-2.832) | 0.059 | 1.214 (0.712-2.070) | 0.476 | 1.363 (0.795-2.338) | 0.260 | |||
| 55 to 64 | 1.347 (0.814-2.230) | 0.247 | 1.426 (0.857-2.373) | 0.172 | 0.990 (0.595-1.647) | 0.969 | 1.088 (0.650-1.821) | 0.748 | |||
| 65 to 74 | 1.674 (1.017-2.756) | 0.043 | 1.935 (1.167-3.208) | 0.010 | 1.261 (0.764-2.082) | 0.364 | 1.508 (0.907-2.510) | 0.114 | |||
| ≥75 | 2.233 (1.353-3.684) | 0.002 | 2.909 (1.750-4.838) | <0.001 | 1.476 (0.890-2.447) | 0.131 | 2.002 (1.196-3.353) | 0.008 | |||
| Sex | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Male | Reference | Reference | Reference | Reference | |||||||
| Female | 0.574 (0.512-0.644) | 0.618 (0.548-0.697) | 0.565 (0.495-0.646) | 0.627 (0.545-0.721) | |||||||
| Race | 0.480 | 0.860 | |||||||||
| White | Reference | Reference | |||||||||
| Black | 1.034 (0.853-1.254) | 0.734 | 1.087 (0.873-1.354) | 0.456 | |||||||
| Other | 0.836 (0.645-1.085) | 0.178 | 1.033 (0.784-1.362) | 0.816 | |||||||
| Unknown | 0.503 (0.071-3.571) | 0.492 | 0.669 (0.094-4.754) | 0.688 | |||||||
| Tumor size | <0.001 | 0.048 | <0.001 | 0.069 | |||||||
| ≤1 cm | Reference | Reference | Reference | Reference | |||||||
| >1, ≤2 cm | 0.000 (0.000-2.900E+) | 0.934 | 0.000 (0.000-5.934E+) | 0.945 | 0.000 (0.000-6.954E+) | 0.942 | 0.000 (0.000-2.382E+) | 0.954 | |||
| >2, ≤3 cm | 0.000 (0.000-1.615E+) | 0.883 | 0.000 (0.000-6.192E+) | 0.892 | 0.000 (0.000-1.346E+) | 0.903 | 0.000 (0.000-2.732E+) | 0.923 | |||
| >3, ≤4 cm | 0.425 (0.060-3.019) | 0.392 | 0.346 (0.048-2.500) | 0.293 | 0.589 (0.083-4.184) | 0.596 | 0.467 (0.064-3.384) | 0.451 | |||
| Unknown | 1.740 (1.362-2.222) | <0.001 | 1.468 (1.133-1.902) | 0.004 | 1.953 (1.490-2.559) | <0.001 | 1.522 (1.140-2.031) | 0.004 | |||
| Diagnosis year | <0.001 | 0.003 | <0.001 | 0.017 | |||||||
| 2004-2007 | Reference | Reference | Reference | Reference | |||||||
| 2008-2011 | 0.881 (0.778-0.999) | 0.048 | 0.933 (0.771-1.131) | 0.481 | 0.857 (0.742-0.990) | 0.035 | 0.934 (0.750-1.165) | 0.546 | |||
| 2012-2015 | 0.636 (0.529-0.765) | <0.001 | 0.696 (0.549-0.881) | 0.003 | 0.614 (0.499-0.756) | <0.001 | 0.706 (0.540-0.923) | 0.011 | |||
| Tumor location | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Main bronchus | Reference | Reference | Reference | Reference | |||||||
| Upper lobe | 0.401 (0.283-0.568) | <0.001 | 0.490 (0.342-0.701) | <0.001 | 0.366 (0.249-0.539) | <0.001 | 0.446 (0.299-0.664) | <0.001 | |||
| Middle lobe | 0.496 (0.316-0.777) | 0.002 | 0.565 (0.356-0.897) | 0.016 | 0.403 (0.240-0.678) | 0.001 | 0.471 (0.276-0.805) | 0.006 | |||
| Lower lobe | 0.447 (0.313-0.638) | <0.001 | 0.585 (0.406-0.844) | 0.004 | 0.413 (0.278-0.613) | <0.001 | 0.550 (0.366-0.827) | 0.004 | |||
| Overlapping lesion | 0.746 (0.424-1.311) | 0.308 | 0.733 (0.409-1.314) | 0.297 | 0.727 (0.387-1.367) | 0.322 | 0.714 (0.372-1.374) | 0.313 | |||
| NOS | 0.425 (0.257-0.703) | 0.001 | 0.614 (0.367-1.027) | 0.063 | 0.396 (0.224-0.701) | 0.001 | 0.569 (0.317-1.022) | 0.059 | |||
| Grade | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Well differentiated | Reference | Reference | Reference | Reference | |||||||
| Moderate differentiated | 1.427 (1.160-1.754) | 0.001 | 1.314 (1.061-1.628) | 0.012 | 1.522 (1.186-1.954) | 0.001 | 1.400 (1.081-1.812) | 0.011 | |||
| Poorly differentiated | 2.145 (1.757-2.619) | <0.001 | 1.728 (1.397-2.137) | <0.001 | 2.377 (1.868-3.025) | <0.001 | 1.869 (1.447-2.414) | <0.001 | |||
| Undifferentiated | 2.847 (1.953-4.151) | <0.001 | 2.861 (1.800-4.549) | <0.001 | 3.379 (2.197-5.197) | <0.001 | 3.477 (2.078-5.818) | <0.001 | |||
| Unknown | 1.815 (1.441-2.287) | <.001 | 1.383 (1.085-1.764) | 0.009 | 2.189 (1.667-2.875) | <0.001 | 1.588 (1.193-2.114) | 0.002 | |||
| Laterality | 0.120 | 0.163 | |||||||||
| Left-origin of primary | Reference | Reference | |||||||||
| Right-origin of primary | 0.888 (0.793-0.995) | 0.040 | 0.884 (0.775-1.007) | 0.064 | |||||||
| Paired site | 1.148 (0.161-8.167) | 0.890 | 1.478 (0.208-10.518) | 0.697 | |||||||
| Histologic type | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Adenocarcinoma | Reference | Reference | Reference | Reference | |||||||
| Squamous cell carcinoma | 1.738 (1.521-1.986) | <0.001 | 1.231 (1.065-1.422) | 0.005 | 1.790 (1.531-2.092) | <0.001 | 1.220 (1.030-1.445) | 0.021 | |||
| Adenosquamous | 1.898 (1.388-2.595) | <0.001 | 1.644 (1.192-2.267) | 0.002 | 2.347 (1.677-3.284) | <0.001 | 1.959 (1.385-2.772) | <0.001 | |||
| Large cell carcinoma | 1.813 (1.297-2.536) | 0.001 | 0.837 (0.544-1.287) | 0.417 | 1.793 (1.203-2.674) | 0.004 | 0.723 (0.439-1.191) | 0.203 | |||
| Other types of NSCLC | 0.969 (0.835-1.126) | 0.685 | 0.957 (0.816-1.123) | 0.591 | 1.062 (0.894-1.262) | 0.494 | 1.053 (0.877-1.265) | 0.581 | |||
| RLNs removed | 0.003 | 0.088 | 0.004 | 0.179 | |||||||
| ≥4 RLNs removed | Reference | Reference | Reference | Reference | |||||||
| 1-3 RLNs removed | 1.212 (1.069-1.347) | 1.118 (0.983-1.271) | 1.234 (1.068-1.426) | 1.107 (0.954-1.284) | |||||||
| Radiotherapy record | <0.001 | 0.304 | <0.001 | 0.022 | |||||||
| Beam radiation | Reference | Reference | Reference | Reference | |||||||
| Radiation, but not specified | 0.846 (0.400-1.787) | 0.661 | 0.770 (0.358-1.657) | 0.504 | 0.916 (0.408-2.054) | 0.831 | 0.845 (0.369-1.937) | 0.691 | |||
| Radioactive implants | 0.740 (0.276-1.983) | 0.550 | 0.429 (0.136-1.355) | 0.149 | 0.435 (0.108-1.749) | 0.241 | 0.352 (0.086-1.436) | 0.145 | |||
| Recommended, unknown | 1.079 (0.593-1.966) | 0.803 | 0.996 (0.519-1.911) | 0.991 | 1.316 (0.721-2.401) | 0.371 | 1.265 (0.648-2.470) | 0.492 | |||
| Refused | 1.611 (0.667-3.895) | 0.289 | 1.618 (0.651-4.024) | 0.300 | 1.920 (0.794-4.647) | 0.148 | 1.928 (0.762-4.878) | 0.166 | |||
| None/Unknown | 0.584 (0.517-0.660) | <0.001 | 0.850 (0.670-1.079) | 0.182 | 0.506 (0.441-0.581) | <0.001 | 0.732 (0.551-0.973) | 0.032 | |||
| Radiation sequence | <0.001 | 0.001 | <0.001 | 0.004 | |||||||
| Prior to surgery | Reference | Reference | Reference | Reference | |||||||
| After surgery | 1.503 (1.186-1.906) | 0.001 | 1.387 (1.082-1.779) | 0.010 | 1.489 (1.143-1.940) | 0.003 | 1.393 (1.056-1.838) | 0.019 | |||
| Before and after surgery | 1.147 (0.597-2.204) | 0.681 | 1.695 (0.867-3.314) | 0.123 | 1.416 (0.731-2.745) | 0.302 | 1.984 (1.004-3.922) | 0.049 | |||
| Intraoperative radiation | 4.900 (0.682-35.225) | 0.114 | 22.610 (1.839-278.002) | 0.015 | 0.001 (0.000-1.284E+) | 0.922 | 0.000 (0.000-8.411E+) | 0.979 | |||
| Unknown, but both given | 7.228 (2.278-22.931) | 0.001 | 5.759 (1.755-18.896) | 0.004 | 8.550 (2.680-27.280) | <0.001 | 7.453 (2.244-24.756) | 0.001 | |||
| No radiation and/or surgery | 0.800 (0.643-0.994) | 0.044 | 0.701 (0.549-0.895) | 0.004 | |||||||
| Chemotherapy record | 0.834 | 0.009 | 0.407 | ||||||||
| No | Reference | Reference | Reference | ||||||||
| Yes | 1.012 (0.903-1.135) | 0.002 | 1.192 (1.046-1.358) | 0.001 | 0.934 (0.794-1.098) | 0.001 | |||||
| Marital status | 0.490 | 0.851 | |||||||||
| Married | Reference | Reference | |||||||||
| Separated | 0.987 (0.581-1.675) | 0.960 | 1.114 (0.628-1.974) | 0.712 | |||||||
| Single | 1.014 (0.843-1.220) | 0.884 | 0.948 (0.762-1.179) | 0.631 | |||||||
| Divorced | 1.041 (0.879-1.233) | 0.644 | 1.035 (0.852-1.257) | 0.730 | |||||||
| Widowed | 1.110 (0.949-1.298) | 0.190 | 1.003 (0.833-1.209) | 0.971 | |||||||
| Unknown | 0.739 (0.496-1.101) | 0.137 | 0.764 (0.489-1.105) | 0.239 | |||||||
| Median family income (dollar, tens) | 0.105 | 0.133 | |||||||||
| ≤5000 | Reference | Reference | |||||||||
| >5000, ≤7000 | 0.914 (0.802-1.042) | 0.179 | 0.937 (0.804-1.093) | 0.409 | |||||||
| >7000, ≤9000 | 0.812 (0.685-0.962) | 0.016 | 0.884 (0.727-1.075) | 0.217 | |||||||
| >9000 | 1.007 (0.728-1.391) | 0.968 | 1.183 (0.831-1.685) | 0.351 | |||||||
Abbreviations: OS, overall survival; LCSS, lung cancer-specific survival; NSCLC, non-small cell lung cancer; HR, hazard ratio; CI, confidence interval; RLN, regional lymph node.
4. Discussion
Surgical treatment with RLNs removed is the mostly common applied treatment of NSCLC patients especially for stage I-IIIA [6, 11]. Although the research about the number of RLNs are keeping increasing these years, there is still no specific recommendation for the number of LN removed in any guideline. Only few articles are about this topic, whose conclusions are still controversial. Dai et al. found that for gradually elevated T stage (mainly stage I), examination of more and more LNs seems to be crucial for survival outcomes [12]. David et al. concluded that compared with the ≥10 LNs removed, <10 LNs removed was associated with poor overall survival for stage I NSCLC patients [13]. Liang et al. reported that 16 examined LNs could be the cut point for prognostic stratification postoperatively for NSCLC patients with declared node-negative disease [14]. Cao et al. reported that LN dissection, especially more extensive RLN removed (≥4 RLNs) is associated with a higher survival rate in patients at stage IA NSCLC tumors ≤2 cm underwent sublobar resection [15]. The accuracy of staging is often affected by the number of LNs examined and different stages correspond to different treatment and different prognosis [12]. So, the exploration of the optimal number of LN removed needs to be studied after classify different stage of NSCLC. National Comprehensive Cancer Network Guidelines (Version 1.2020, 2019) only recommended suitable patients at stage IIIA NSCLC may be considered to have a surgery to cure which may be the only way to cure NSCLC, but there was no recommendation on the number of LNs removed. Unfortunately, there are few studies specifically evaluated the survival benefits of removing different number of RLNs for patients with IIIA NSCLC.
Forest plot of HRs of factors that can influence OS and LCSS in patients by multivariable Cox regression: (a) HRs of factors that can influence OS in all patients among our cohort; (b) HRs of factors that can influence LCSS in all patients among our cohort; (c) HRs of factors that can influence OS in patients with RLNs removed among our cohort; (d) HRs of factors that can influence LCSS in patients with RLNs removed among our cohort.
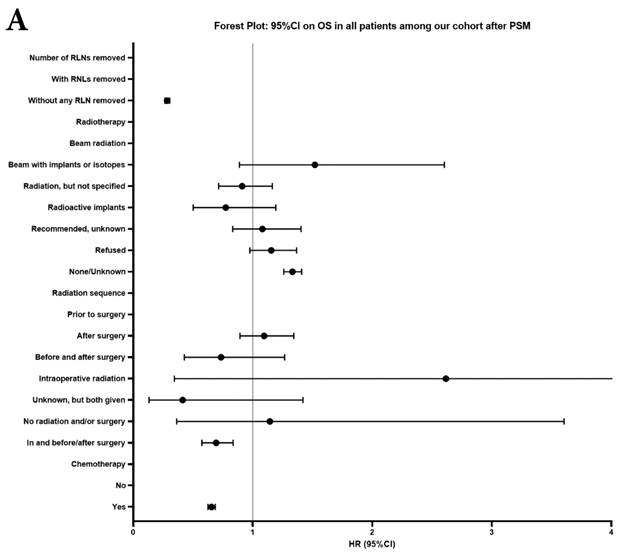
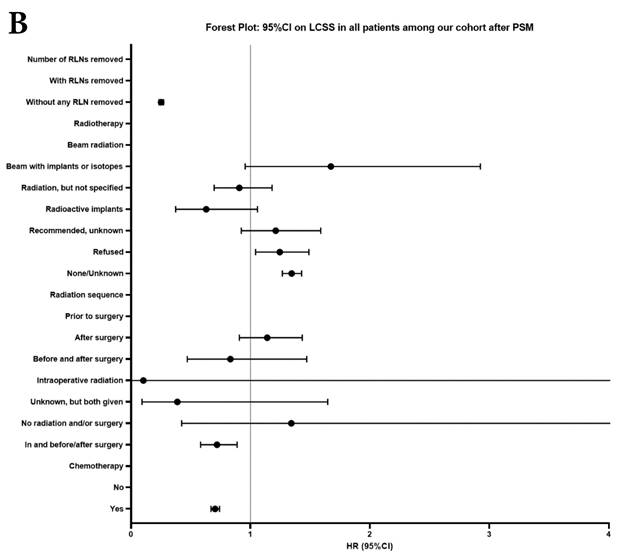
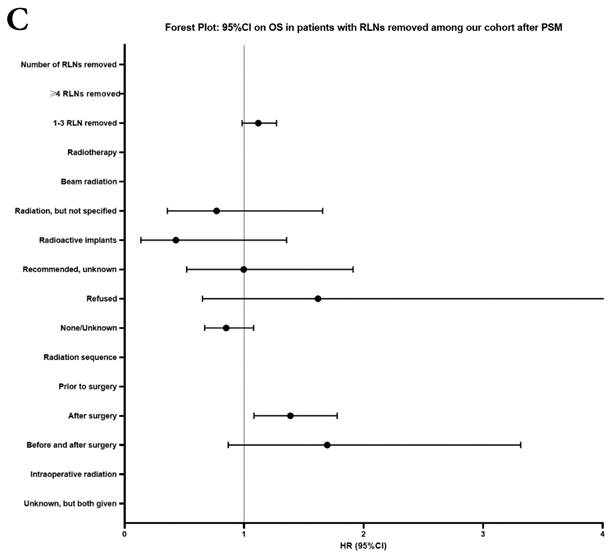
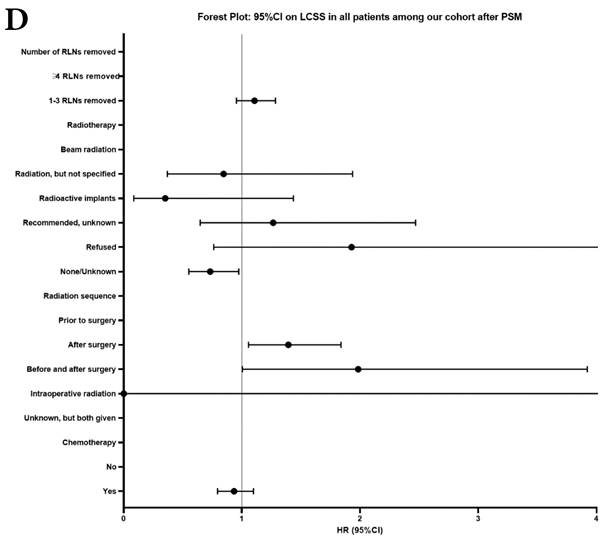
In our research, we found that there was better prognosis in RLNs removed group compared with none RLN removed group. In terms of 1-3 RLNs removed group and ≥4 RLNs removed group, survival indicators (1-year mortality rate, 5-year survival rate, MDST and MST), Kalan-Meier survival analyses and univariate Cox regression analyses on OS and LCSS all revealed that there was a better prognosis for ≥4 RLNs removed group. But, multivariable Cox regression analyses on OS and LCSS showed there was no statistical difference between two groups above (Figure 4). Here we found an interesting conclusion that under the premise of RLNs removed, the number of RLNs removed was not an independent prognostic factor, but ≥4 RLNs removed can indeed improve the prognosis. We can conclude that RLNs removed is an important treatment for stage IIIA N0 NSCLCN patients, and ≥4 RLNs removed seems to bring a better survival time but not an independent prognostic factor, compared with 1-3 RLNs removed. Besides, when we focused on the cohort only including 1-3 RLNs removed group and ≥4 RLNs removed group, the independent prognostic factors (age, sex, tumor location, histologic type and insurance) became much less compared with the cohort including with and without RLNs removed group, indicating that unchangeable factors (age, sex, tumor location and histologic type) are more important for patients choosing RLNs removed. In addition to paying more attention to the insurance situation, which can be changed, manual intervention cannot bring better effects, which maybe it will bring harm, considering the inconsistent effects of CT and RT in survival analysis (further discussion below).
It is well known that surgery with CT is an optimal treatment for stage IA-IIIA NSCLC patients [11, 16]. Even aggressive consolidative therapy may appear to improve survival in patients with persistent or high nodal burden disease [17]. Ito et al. reported that there was a significant difference in the OS and disease-free survival rates in the intralobar group which is opposite in the and hilar group according to adjuvant CT [18]. In our research, we found that whether in the whole cohort or in patients with RLNs removed, less patients chose CT than did not and the proportion of CT applied was reduced on the premise of RLN removed and CT was a controversial factor for the prognosis. Only between without and with RLNs removed group, CT is definitely good for prognosis on OS. Between 1-3 RLNs removed group and ≥4 RLNs removed group, there were no statistically significance of CT on OS. In terms of LCSS, the effects CT between without and with RLNs removed group, 1-3 RLNs removed group and ≥4 RLNs removed group seem to be indeterminate, whose survival curves crossing, demonstrating that CT has no clear benefits, and may also bring harm instead, under the premise of RLNs removed. The National Comprehensive Cancer Network guidelines (Version 3.2023) recommended conventionally fractionated RT for locally advanced IIIA N0 NSCLC. The role of RT in the treatment seems more non-uniform. RT may yield a negative effect on survival outcomes in surgical patients with IIA NSCLC, which is consistent with the National Comprehensive Cancer Network guidelines (Version 1.2020, 2019), which recommends CT, but not RT, for patients at stage IIA NSCLC [19]. In terms of stage IIIA NSCLC, the conclusions are still controversial. Shinde et al. concluded that there was no benefit observed for adjuvant CT or RT in the entire cohort which include patients with non-metastatic, cN2 (IIIA or IIIB) NSCLC diagnosed from 2004 to 2015 [17]. However, Liu et al. reported that patients can benefit from adjuvant RT if they have more than 5 positive RLNs, larger tumors (≥3cm), and older age (≥65 years old) [20]. In our research, whether in the whole cohort or in patients with RLNs removed, less patients chose RT than did not and the proportion of RT applied was keeping reducing with the number of RLNs removed increasing. And, all survival curves in terms of RT crossed, demonstrating that RT has no clear benefits, and may bring harm instead, under the premise of RLNs removed. The same as exploration of the number of LN removed, the explorations of CT or RT need be done based on detailed classification, considering that even if there is only a very small difference in stage, the treatment will be different or even the opposite.
The latest edition of the International Union Against Cancer TNM staging standard for lung cancer was announced in January 2017 [9]. The 8th TNM classification changed definitions in terms of tumor size, which provides a higher level of differentiation based on global database, extensive internal validation, sophisticated analyses and multiple evaluations that confirm generalizability [19]. Ashwin et al. found that the therapeutic effect of CT and RT was different in different LN ratio which meat number of nodes involved by tumor divided by number of nodes examined of LNs, highlighting the importance of LN biopsy and new TNM classification [17]. LN biopsy is important for staging, which is important for the choice of treatment, and the 8th TNM classification makes a more accurate and personalized treatment opportunity for stage IIIA NSCLC patients, considering that it provides a standard for the scope of LN removed. At present, the significance of LN removed in radical surgery of NSCLC mainly includes two aspects: one is to ensure complete tumor resection, which directly reduce the probability of recurrence; another is biopsy, ensuring the accuracy of LN staging so as to help develop a better treatment plan for patients, that is, for prognosis [21, 22]. Studies have found that even if the tumor is completely removed in the early stage of the disease, a considerable number of patients still have the risk of recurrence, suggesting that it is related to LN micrometastasis, and once again confirmed the importance of RLNs removed. In this study, our findings are consistent with the opinions above, which may provide a theoretical basis for the necessity of RLNs removed.
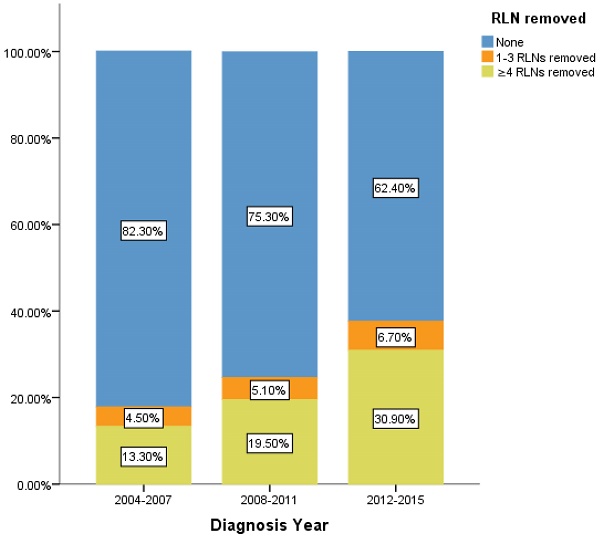
Percentage of IIIA N0 NSCLC patients of different number of RLNs removed over time (2004-2015). RLN, regional lymph node.
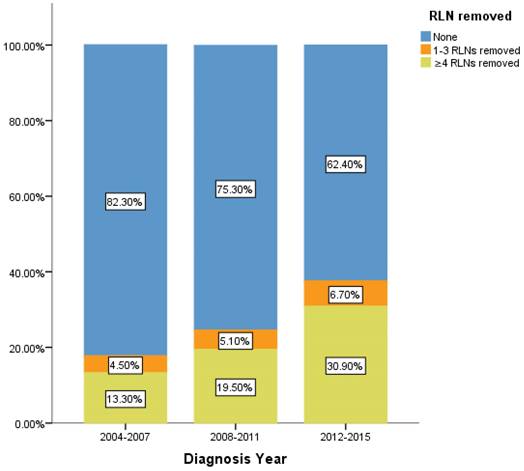
However, there is still limitation in our research. The data of RLNs are limited by the inherent flaw of the SEER database, which did not record specific number of RLNs removed. Our research found compared with 1-3 RLNs removed, ≥4 RLNs removed brings a better survival time but not an independent prognostic factor. But it is well known that infinite removal of RLNs cannot be sustained conducive to prognosis, on the contrary, there may be side effects. The topic of what specific number RLNs removed specifically are enough and best seems to be important [23]. Limited by the fact that the SEER database does not provide specific numbers, we cannot determine specific cut point number of RLNs in ≥4RLNs group. This information could be included in further research. However, our research has reported the positive effect of RLNs removed for the prognosis based on 11,583 patients and 17 variables among our cohort. All the comparisons were made after PSM which eliminated the baseline difference basically. Therefore, in the background of absent large-scale data from prospective trials and clinical guideline, our conclusion not only is highly reliable, but also brings a conclusion which could provide accurate reference about RLNs removed treatment for the patients at stage IIIA N0 NSCLC. We also found that 24.45% of patients at stage IIIA N0 NSCLC had RLNs removed, 5.2% and 19.26% of them had 1-3 RLNs and ≥4 RLNs removed, respectively between 2004 to 2015. In recent 12 years, there is a sustained growth trend for the number of RLNs removed (Figure 5). The proportion of 1-3 RLNs removed and ≥4 RLNs removed group was increased from 4.5% to 6.7% and 13.3% to 30.9%, respectively, dedicating that more and more clinicians are aware of the benefits of having more RLNs removed. The phenomenon mentioned above also was consistent with the finding that diagnosis year was an independent prognostic factor and the later the diagnosis year, the better the prognosis. 6.7% and 30.9% indicates the clinical significance that such benefit option still needs to be applied for the better prognosis of IIIA N0 NSCLC patients, which dedicated the significance of our conclusion again. Considering an interesting conclusion that under the premise of RLNs removed, the number of RLNs removed was not an independent prognostic factor, but ≥4 RLNs removed can indeed improve the prognosis, we predicted that the number of RLNs removed and diagnosis year were associated variables. This prediction will be researched in future research.
5. Conclusions
Removing RLNs was beneficial to survival outcomes of patients at stage IIIA N0 NSCLC. Compared with 1-3 RLNs removed, ≥4 RLNs removed brought a better survival time but not an independent prognostic factor (P>0.05).
Supplementary Material
Supplementary figures.
Author Contributions
Conceptualization, S.S.X. and Y.K.W.; methodology, S.S.X. and Y.K.W.; software, Y.K.W., Z.X.L. and H.Y.R.; validation, Z.H.Z.; formal analysis, Z.H.Z.; investigation, N/A; resources, S.S.X.; data curation, S.S.X. and Y.K.W.; writing—original draft preparation, Y.K.W. and Z.X.L.; writing—review and editing, Z.H.Z., Y.K.W. and S.S.X.; visualization, Y.K.W.; supervision, S.S.X.; project administration, S.S.X.; funding acquisition, S.S.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 82272673.
Informed Consent Statement
Patient consent was waived due to this article is from SEER database, which is publicly available deidentified patients' data from National Cancer Institute (NCI), USA.
Data Availability Statement
All data generated during this study are included in this article. The datasets supporting the conclusions of this article are available in SEER database: https://seer.cancer.gov/, accessed on 20 July 2022.
Acknowledgements
We would like to thank all the patients who donated their statistical data.
Abbreviations
LC: lung cancer; NSCLC: non-small cell lung cancer; RLN: regional lymph node; SEER: Surveillance, Epidemiology and End Results; LCSS: lung cancer-specific survival; OS: overall survival; PSM: propensity score method; RT: radiotherapy; CT: chemotherapy; HR: hazard ratio; CI: confidence interval; MDST: median survival time; MST: mean survival time.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-54
4. Pirker R. Conquering lung cancer: current status and prospects for the future. Pulmonology. 2020;26:283-90
5. Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther. 2018;18:63-70
6. Miller M, Hanna N. Advances in systemic therapy for non-small cell lung cancer. Bmj. 2021;375:n2363
7. Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI. et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest. 2011;139:1124-9
8. Dennett EJ, Janjua S, Stovold E, Harrison SL, McDonnell MJ, Holland AE. Tailored or adapted interventions for adults with chronic obstructive pulmonary disease and at least one other long-term condition: a mixed methods review. Cochrane Database Syst Rev. 2021;7:Cd013384
9. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151:193-203
10. Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am J Surg Pathol. 2016;40:e94-e102
11. Salazar MC, Rosen JE, Wang Z, Arnold BN, Thomas DC, Herbst RS. et al. Association of Delayed Adjuvant Chemotherapy With Survival After Lung Cancer Surgery. JAMA Oncol. 2017;3:610-9
12. Dai J, Liu M, Yang Y, Li Q, Song N, Rocco G. et al. Optimal Lymph Node Examination and Adjuvant Chemotherapy for Stage I Lung Cancer. J Thorac Oncol. 2019;14:1277-85
13. David EA, Cooke DT, Chen Y, Nijar K, Canter RJ, Cress RD. Does Lymph Node Count Influence Survival in Surgically Resected Non-Small Cell Lung Cancer? Ann Thorac Surg. 2017;103:226-35
14. Liang W, He J, Shen Y, Shen J, He Q, Zhang J. et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol. 2017;35:1162-70
15. Cao J, Xu J, He Z, Yuan P, Huang S, Lv W. et al. Prognostic impact of lymphadenectomy on outcomes of sublobar resection for stage IA non-small cell lung cancer ≤2 cm. J Thorac Cardiovasc Surg. 2018;156:796-805.e4
16. Li R, Suo R, Zhang J. Lymph Node Dissection and Adjuvant Chemotherapy for Early Lung Cancer. Ann Thorac Surg. 2022;114:1095-6
17. Shinde A, Horne ZD, Li R, Glaser S, Massarelli E, Koczywas M. et al. Optimal adjuvant therapy in clinically N2 non-small cell lung cancer patients undergoing neoadjuvant chemotherapy and surgery: The importance of pathological response and lymph node ratio. Lung Cancer. 2019;133:136-43
18. Ito R, Tsukioka T, Izumi N, Komatsu H, Inoue H, Kimura T. et al. Lymph Node Metastasis Location and Postoperative Adjuvant Chemotherapy in Patients With pN1 Stage IIB Non-small Cell Lung Cancer. In Vivo. 2022;36:355-60
19. Li X, Li G, Wang Y, Tan M, Wang C. Removing different number of regional lymph nodes affects survival outcomes of operable patients at stage IIA non-small cell lung cancer (according to the 8th edition staging). J Thorac Dis. 2023;15:552-67
20. Liu B, Wang Z, Zhao H, Gao S, Wang H, Zhang Y. et al. The Value of Radiotherapy in Patients With Resectable Stage IIIA Non-Small-Cell Lung Cancer in the Era of Individualized Treatment: A Population-Based Analysis. Clin Lung Cancer. 2023;24:18-28
21. Smeltzer MP, Faris NR, Ray MA, Osarogiagbon RU. Association of Pathologic Nodal Staging Quality With Survival Among Patients With Non-Small Cell Lung Cancer After Resection With Curative Intent. JAMA Oncol. 2018;4:80-7
22. Sun J, Wu S, Jin Z, Ren S, Cho WC, Zhu C. et al. Lymph node micrometastasis in non-small cell lung cancer. Biomed Pharmacother. 2022;149:112817
23. Sachs TE. Too Many or Too Few? How Many Lymph Nodes Are Enough? Ann Surg Oncol. 2022;29:1496-7
Author contact
![]() Corresponding authors: xieshuanshuancom; Tel.: +86-021-66302517.
Corresponding authors: xieshuanshuancom; Tel.: +86-021-66302517.

 Global reach, higher impact
Global reach, higher impact