Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(13):2552-2561. doi:10.7150/jca.84460 This issue Cite
Research Paper
HOXC8 mediates osteopontin expression in gastric cancer cells
1. Division of General Surgery, Department of Surgery, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
2. Department of Pathology and Laboratory Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
3. Department of Anatomy, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
4. Department of Medical Education and Research, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan.
Received 2023-3-20; Accepted 2023-7-26; Published 2023-8-21
Abstract
Genes of the homeobox (HOX) family encode transcription factors, which play a role in cancer progression. However, their role in gastric cancer has not been adequately evaluated. Herein, we evaluated the genetic changes and mRNA of target genes of the HOX family in gastric cancer patients using publicly available online datasets. We found that HOXC8 was amplified in gastric cancer tissues, and mRNA expression levels were significantly associated with tumor status (P=0.044) and poor overall survival (P<0.01). HOXC8 knockdown significantly reduced the viability of gastric cancer cell lines. HOXC8 modulated the expression of secreted phosphoprotein 1 (SPP1, osteopontin) and phosphorylation of AKT/ERK in gastric cancer cells. Survival analysis demonstrated a decrease in overall survival rates among the high HOXC8/high SPP1 expression group compared with the low HOXC8/low SPP1 expression group. In conclusion, HOXC8 may be an independent prognostic factor and serve as a useful predictive biomarker for gastric cancer.
Keywords: HOXC8, osteopontin, SPP1, and gastric cancer
Introduction
The survival and prognosis of cancer patients are associated with stage status development. Gastric cancer is the sixth most common malignant cancer (1.09 million cases in 2020, World Health Organization) and the leading cause of cancer-related death in the world [1-4]. Among the deaths in Taiwan in 2020, 50,161 were due to cancers, 2,339 (5.2%) of which occurred from gastric cancer (from the Ministry of Health and Welfare of Taiwan). Unfortunately, the lack of highly sensitive and specific biomarkers to diagnose gastric cancer leads to most patients being in the advanced stage before diagnosis [5-7]. Therefore, investigating and improving the diagnostic sensitivity of cancer biomarkers for early-stage tumors is beneficial for improving the survival rate of gastric patients.
The human homeobox (HOX) genes have four chromosomal clusters and 39 HOX genes: HOXA (chromosome 7), HOXB (chromosome 17), HOXC (chromosome 12), and HOXD (chromosome 2) [8]. The HOX genes encode transcription factors that are involved in a number of biological processes, including embryonic development, cell proliferation, and differentiation, among others [9, 10]. There has been substantial evidence of aberrant HOX gene expression in multiple cancers such as prostate, pancreatic, lung, and breast cancer [8, 11-13]. Numerous studies have shown that diverse HOX genes can either enhance or inhibit the cancer progression process on their abnormal expression in different organs. For example, the HOXB gene is a prognostic factor in breast cancer [14], HOXA13 expression impaired the chemotherapy in gastric cancer cells [15], HOXB5 promotes metastasis in hepatocellular carcinoma [16] and HOXA13, HOXD13, and HOXC6 were promoted colorectal cancer [17-19]. Furthermore, lymphoblastic leukemia with mixed-lineage leukemia translocations shows increased expression of HOXA4, HOXA5, HOXA7, HOXA9, and HOXB5, which is related to poor prognosis [20-24]. HOXC4-6 and HOXC8 are upregulated in primary tumor and cancer cell lines and are not normally expressed in normal prostate tissues or cell lines [25, 26]. HOX protein can modulate other proteins to enhance or repress gene expression [27] and it also can be expressed in cancer stem cells [28]. In gastric cancer and HOXC6 promotes invasion ability [29]. However, downstream targets of HOX genes have still not been fully identified.
Secreted phosphoprotein 1 (SPP1), also known as osteopontin (OPN) protein was upregulated in primary and metastatic lesions of gastric cancer, which indicates that OPN may play a role in gastric cancer [30, 31]. OPN was first described as a glyco-phosphoprotein that is secreted from malignant epithelial cells [32]. The functions of OPN in cancer progression, include cell adhesion, chemotaxis, invasion, migration, and the anchorage-independent growth of tumor cells [33]. A previous study showed that OPN was downregulated in HOXC8-silenced cells [11]. In our study, the highest expression of HOXC8 was observed in gastric cancer tissues. However, the mechanism by which HOXC8 modulates OPN expression and regulates the malignant phenotype of gastric cancer cells has not been clearly elucidated.
Materials and Methods
In silico mRNA profiles and Kaplan-Meier analysis of HOXC8
HOXC8 mRNA expression in stomach adenocarcinoma tissues was determined by using The Cancer Genome Atlas (TCGA) dataset and TNMplot (https://tnmplot.com/analysis/). Kaplan-Meier analysis (overall survival) was performed using a publicly available gastric cancer microarray dataset (http://kmplot.com/analysis/).
Cell lines and culture conditions
TSGH, AZ521, and HR cell lines (human gastric carcinoma) from Dr. Kuo-Wang Tsai at Taipei Tzu Chi Hospital in Taiwan. Cells were cultured in Dulbecco's Modified Eagle Medium with 10% fetal bovine serum and 1% penicillin-streptomycin-glutamine.
Lentivirus infection
Lentivirus vector control (pLKO-1-shLuc967) and shHOXC8 shRNA viral supernatant (TRCN0000019564, TRCN0000019565) were purchased from the National RNAi Core Facility (Taipei, Taiwan). The viral supernatants were used to infect AZ521 or HR cell lines with 8 μg/mL of polybrene at 72 h. After infection, cells were selected by using 2 μg/mL of puromycin.
Real-time-polymerase chain reaction (PCR)
Total RNA was extracted using TRI Reagent (Sigma-Aldrich, #T9424), cDNA was synthesized by TaKaRa PrimeScript™ RT reagent Kit (Cat. #RR037A), and PCR reactions were conducted using SYBR system (PCR Biosystems qPCRBIO SyGreen Mix Lo-ROX). The following primer sequences were used: HOXC8, forward: 5'TCAAAACTCGTCTCCCAGCC-3'; HOXC8, reverse: 5' TTCCAAGGTCTGATACCGGC-3'; SPP1, forward: 5'ATGATGGCCGAGGTGATAGTG; SPP1, reverse: GAGGTGATGTCCTCGTCTGTAGC; 5'ACTB, forward: 5' AGAAAATCTGGCACCACACC-3' and ACTB, reverse: 5' AGAGGCGTACAGGGATAGCA-3'.
Growth curve assay
Cells (2,000 cells/well for AZ-521/shluc, AZ-521/shHOXC8-1, AZ-521/shHOXC8-2, HR/shluc, HR/shHOXC8-1, and HR/shHOXC8-2) were seeded in a 96-well plate for 24-72 h (incubated at 37°C with 5% CO2). The cell growth curve was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
Colony formation assay
The stable lines (1,000 cells/well) were plated in a 6-well plate. After a 7-day incubation at 37°C with 5% CO2, the cells were fixed and stained using crystal violet. The number of colonies was counted using the NIH Image J software.
Western blot analysis
Western blot analysis was performed using the following primary antibodies: anti-OPN (IBL, #18625,1:1000), anti-pAKT (Cell Signaling, #4060, 1:1000), anti-AKT (Cell Signaling, #4691,1:1000), anti-pERK (Cell Signaling, #9101,1:1000), anti-ERK (Cell Signaling, #9102,1:1000), and β-actin (Sigma-Aldrich, #A5441, 1:5000).
Results
In silico genetic alterations of HOXC family members in stomach adenocarcinoma
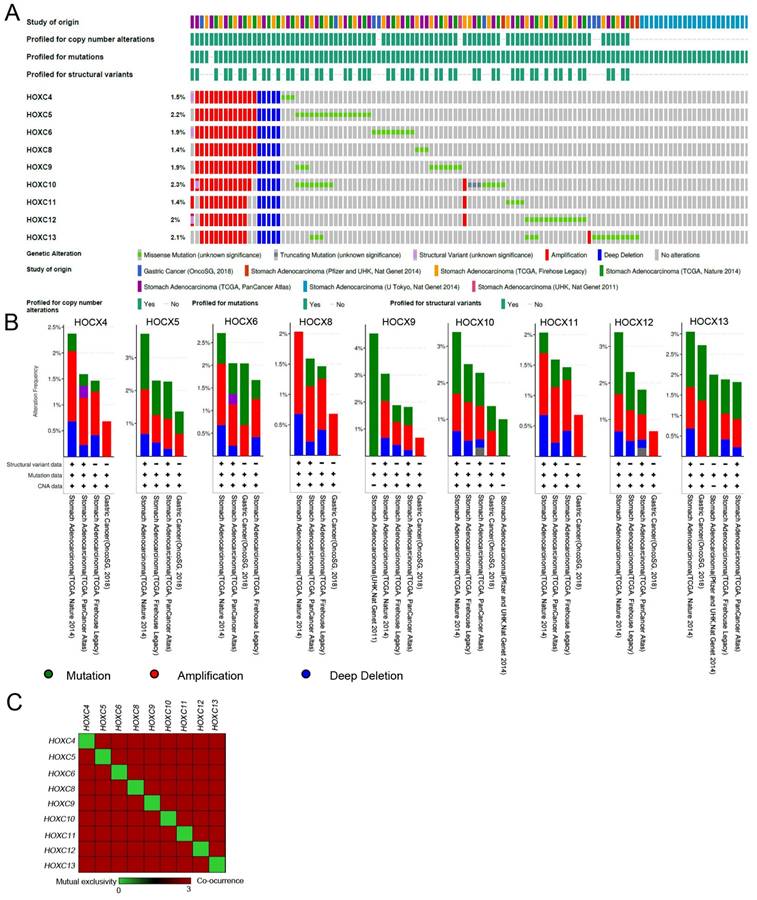
cBioPortal was used to examine the genetic alterations of the HOXC family in stomach adenocarcinoma (STAD) patients. HOXC4~HOXC13 had a 1.5-2.3% amplification, and HOXC9 had a 4% mutation ratio in STAD patients (Figure 1A-B). Further analyzing the frequency of co-occurrence of the HOXC family genetically altered in the same tumor tissues. Co-occurrence analysis of the STAD sample identified the HOXC family genetically altered significant co-occurrence in the same tumor sample (Figure 1C and Table 1). Previous studies showed several members of the HOXC family have been well-investigated for their role in gastric cancer [34, 35]. Moreover, HOXC8 knockdown reduced cell proliferation in gastric cancer [36]. However, the molecular mechanism of its action remains unclear. Therefore, in this study, we focused on HOXC8 to further investigate gastric cancer.
The HOXC8 expression level was significantly increased and correlates with poor survival rates in STAD patients
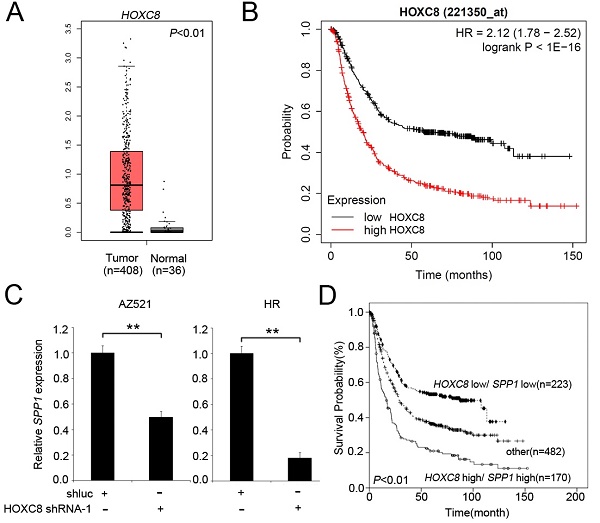
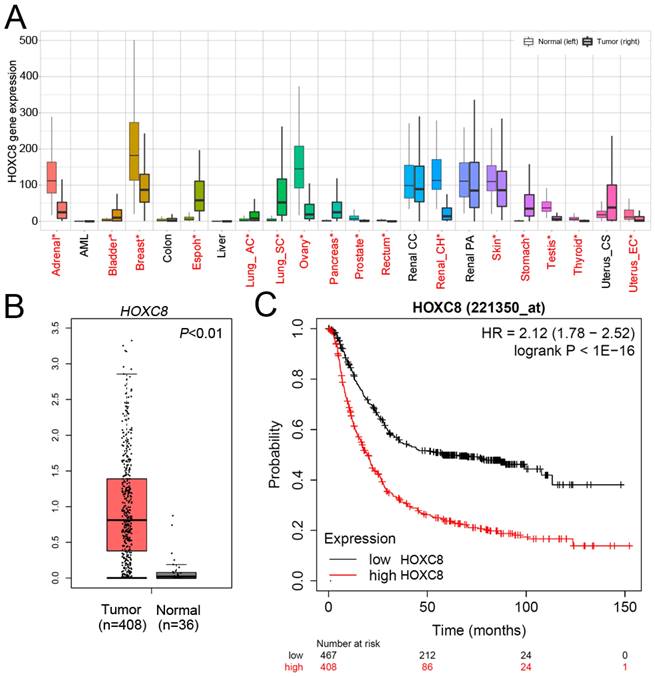
We next evaluated the mRNA levels of HOXC8 in multiple cancer types using TNMplot, which showed that HOXC8 was upregulated in many cancer types, including STAD (Figure 2A). We further analyzed the TCGA dataset to understand the clinical prognostic value of HOXC8 in STAD patients. Expression profiles were downloaded from TCGA, including 408 STAD tissues and 36 adjacent normal tissues. As shown in Figure 2B, the expression level of HOXC8 was significantly increased in STAD patients compared to adjacent normal tissues. The expression levels of HOXC8 in human STAD tissues were further evaluated in 95 patients with stage I-II STAD and 272 with stage III-IV STAD. HOXC8 expression analysis was based on the expression of HOXC8 mRNA. Using the clarified expression criteria, we classified patients into the HOXC8-low and HOXC8-high groups. The data showed that HOXC8 expression was significantly associated with tumor stage (P=0.044) (Table 2). Next, we detected the overall survival rate of HOXC8 in a clinical cohort using Kaplan-Meier analysis. A high level of HOXC8 expression correlated with the overall survival rate in patients with gastric cancer (Figure 2C). However, the detailed role of HOXC8 in STAD progression remains unclear.
Co-occurring of HOXC family genetic alterations in stomach adenocarcinoma.
| A | B | Neither | A Not B | B Not A | Both | Log2 Odds Ratio | p-Value | q-Value | Tendency |
|---|---|---|---|---|---|---|---|---|---|
| HOXC5 | HOXC10 | 1385 | 9 | 10 | 24 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC4 | HOXC8 | 1404 | 4 | 3 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC4 | HOXC6 | 1399 | 3 | 8 | 18 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC5 | HOXC9 | 1388 | 13 | 7 | 20 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC6 | HOXC8 | 1399 | 9 | 3 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC8 | HOXC9 | 1398 | 3 | 10 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC4 | HOXC9 | 1397 | 4 | 10 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC9 | HOXC10 | 1386 | 8 | 15 | 19 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC5 | HOXC8 | 1392 | 16 | 3 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC4 | HOXC11 | 1402 | 6 | 5 | 15 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC4 | HOXC5 | 1391 | 4 | 16 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC4 | HOXC10 | 1390 | 4 | 17 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC6 | HOXC9 | 1392 | 9 | 10 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC11 | HOXC12 | 1395 | 4 | 13 | 16 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC10 | HOXC11 | 1390 | 18 | 4 | 16 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC8 | HOXC10 | 1390 | 4 | 18 | 16 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC6 | HOXC11 | 1397 | 11 | 5 | 15 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC8 | HOXC11 | 1402 | 6 | 6 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC12 | HOXC13 | 1387 | 12 | 12 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC5 | HOXC6 | 1386 | 16 | 9 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC6 | HOXC10 | 1385 | 9 | 17 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC4 | HOXC12 | 1393 | 6 | 14 | 15 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC5 | HOXC13 | 1383 | 16 | 12 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC10 | HOXC13 | 1382 | 17 | 12 | 17 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC9 | HOXC11 | 1395 | 13 | 6 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC8 | HOXC12 | 1393 | 6 | 15 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC8 | HOXC13 | 1393 | 6 | 15 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC11 | HOXC13 | 1393 | 6 | 15 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC6 | HOXC12 | 1388 | 11 | 14 | 15 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC4 | HOXC13 | 1392 | 7 | 15 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC10 | HOXC12 | 1381 | 18 | 13 | 16 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC5 | HOXC11 | 1389 | 19 | 6 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC6 | HOXC13 | 1387 | 12 | 15 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC9 | HOXC12 | 1386 | 13 | 15 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC9 | HOXC13 | 1386 | 13 | 15 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
| HOXC5 | HOXC12 | 1380 | 19 | 15 | 14 | >3 | <0.001 | <0.001 | Co-occurrence |
HOXC family amplification in stomach adenocarcinoma (STAD). (A) Oncoprint showing HOXC4, HOXC5, HOXC6, HOXC8, HOXC9, HOXC10, HOXC11, HOXC12, and HOXC13 in STAD patients. (B) Cancer type summary of HOXC4, HOXC5, HOXC6, HOXC8, HOXC9, HOXC10, HOXC11, HOXC12, and HOXC13 by different STAD cohorts. (C) Co-occurrence analysis of HOXC family members in STAD tumors.
HOXC8 knockdown reduced proliferation and colony formation in gastric cancer cells
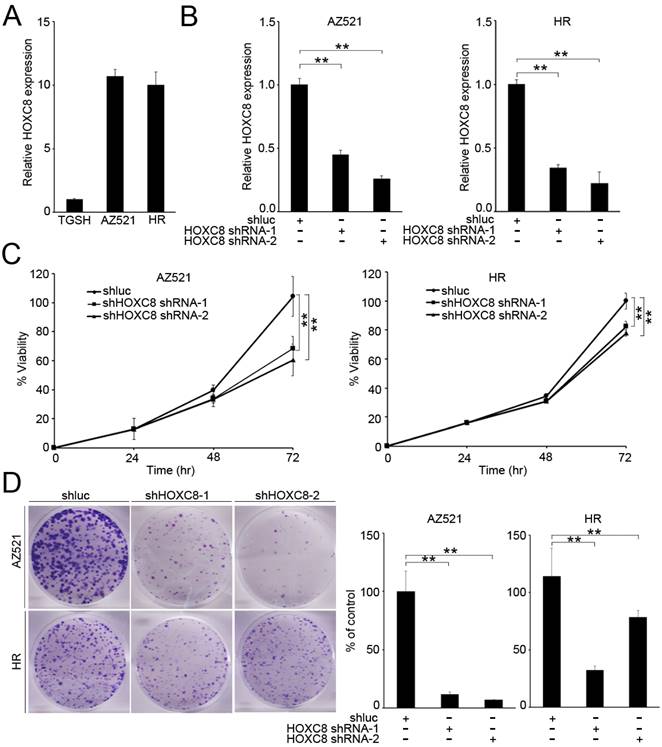
According to the clinical data, we found that HOXC8 expression was associated with tumor size (Table 2). Therefore, we evaluated the effect of HOXC8 on proliferation and colony formation in gastric cancer cells. First, we examined the expression levels of HOXC8 in gastric cancer cell lines. The results revealed that HOXC8 was upregulated in AZ521 and HR cancer cell lines compared with that in a TGCH cell line (Figure 3A). Next, we evaluated the effect of HOXC8 on cell viability and colony formation in gastric cancer cells. HOXC8 knockdown significantly reduced the cell growth and colony formation in AZ521 and HR cells (Figure 3B-D).
Association of HOXC8 expression with clinicopathological characteristics in 367 gastric cancer patients.
| HOXC8 | ||||||
|---|---|---|---|---|---|---|
| Low | High | |||||
| Variables | Item | Patient No. | No. (%) | No. (%) | p value* | |
| 126 | 241 | |||||
| Sex | Female | 41 | 148 | 0.304 | ||
| Male | 85 | 93 | ||||
| Stage | I/II | 57 | 110 | 1.000 | ||
| III/IV | 69 | 131 | ||||
| T status | T1/T2 | 41 | 54 | 0.044* | ||
| T3/T4 | 85 | 187 | ||||
| M status | Negative | 118 | 223 | 0.831 | ||
| Positive | 8 | 18 | ||||
| Lymph node status | Negative | 36 | 81 | 0.347 | ||
| Positive | 90 | 160 | ||||
*P value < 0.05 was considered statistically significant (chi-square test for categorical variables).
HOXC8 is overexpressed in gastric cancer and is correlated with poor survival. (A) HOXC8 mRNA expression in multiple cancer types (TNMplot). (B) The expression of HOXC8 is significantly upregulated in STAD tumors compared to that in adjacent normal tissue, as determined using TCGA dataset. (C) Kaplan-Meier graph of overall survival in publicly available gastric cancer microarray datasets, stratified according to HOXC8 expression (Kaplan-Meier Plotter).
HOXC8 downregulated the inhibitory OPN pathway in gastric cancer cells
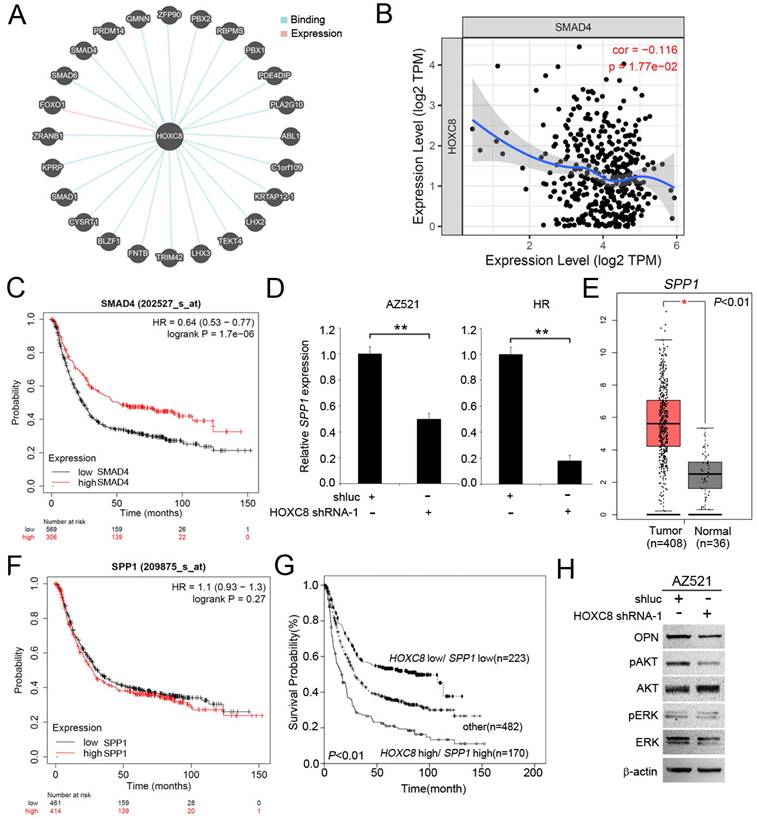
The SPP1 gene contains a HOXC8 responsive element in the promoter region [37]. We also used the pathway commons database (https://www.pathwaycommons.org/) to identify the associated molecular targets of HOXC8. As show in Figure 4A, HOXC8 interaction with 24 genes, including SMAD4, PRDM4, GMNN, ZFP90, PBX2, RBPMS, PBX1, PDE4DIP, PLA2G10, ABL1, C1orf109, KRTAP12-1, LHX2, TEKT4, LHX3, TRIM42, FNTB, BLZF1, CYSRT1, SMAD1, KPRP, ZRANB1 and FOXO1. In this study, we focus on SMAD4 which has been reported to mediate OPN expression in cancer cells [38]. We examined the correlation between HOXC8 and SMAD4 in patients with STAD using the TIMER. We found that HOXC8 levels was significantly negatively correlated with SMAD4 levels in STAD patients (Figure 4B). SMAD4 upregulated level associated prolong overall survival in gastric cancer patients (Figure 4C). We further confirmed whether there was HOXC8-mediated SPP1 expression in gastric cancer cells. The results showed that the expression of SPP1 was significantly reduced in gastric cancer cells (Figure 4D). We then examined SPP1 expression in gastric cancer patients using the TCGA database. SPP1 was upregulated in STAD tissues compared to normal tissues (Figure 4E). Although SPP1 expression has not significantly associated with overall survival in gastric cancer patients (Figure 4F). The high HOXC8/high SPP1 group was associated with shorter overall survival than the low HOXC8/low SPP1 group was among gastric cancer patients (Figure 4G). Moreover, OPN modulated the phosphorylation of AKT and ERK in lung cancer cells [39]. HOXC8 knockdown significantly reduced the phosphorylation of AKT and ERK in gastric cancer cells (Figure 4H).
HOXC8 knockdown inhibits cell proliferation. (A) Real-time polymerase chain reaction (PCR) analysis of HOXC8 expression in gastric cancer cell lines. (B) Real-time PCR analysis of HOXC8 expression after infection with shRNA. (C) Effect of HOXC8 knockdown on proliferation of AZ521 and HR cell lines. (D) Downregulation of HOXC8 reduced colony formation of gastric cancer cells.
HOXC8 mediates the OPN pathway in gastric cancer cells. (A) Putative binding interaction targets of HOXC8 were identified from Pathway Commons. (B) Analysis of the correlation between HOXC8 and SMAD4 mRNA expression by using TIMER. (C) Kaplan-Meier analysis of overall survival, according to SMAD4 mRNA expression using publicly available gastric cancer microarray datasets (Kaplan-Meier Plotter). (D) Expression of SPP1 mRNA in HOXC8 knockdown cells compared with shluc control. (E) Relative mRNA levels of SPP1 in stomach adenocarcinoma tissues (The Cancer Genome Atlas dataset). (F) According to SPP1 mRNA expression to analysis overall survival, using publicly available gastric cancer microarray datasets (Kaplan-Meier Plotter). (G) Kaplan-Meier analysis of overall survival in patients with gastric cancer is achieved by examining the combination of HOXC8 and SPP1 mRNA levels (Kaplan-Meier Plotter). Other: low HOXC8/high SPP1 and high HOXC8/low SPP1. (H) Comparison of OPN, pAKT, AKT, pERK, ERK, and β-actin levels between HOXC8-knockdown AZ521 cells and shluc control cells.
Discussion
In this study, the HOXC family was evaluated in patients with STAD, and HOXC8 expression was upregulated in STAD tumor tissues and associated with worse overall survival. High levels of HOXC8 mRNA expression also correlated with tumor stats, indicating that HOXC8 plays a role in STAD progression. Although HOXC8 reportedly mediate cell viability in gastric cancer cell lines [36], the role of HOXC8 in gastric cancer has not been fully evaluated. We revealed that HOXC8 knockdown reduced the expression of OPN and phosphorylation of AKT/ERK in gastric cancer cells.
Accumulating evidence shows that deregulated HOX gene expression promotes malignant transformation in many cancers, including leukemias, breast, cervical, ovarian, prostate, colorectal, melanoma, and squamous cell carcinoma. HOX genes reportedly have both oncogenic and tumor suppressing functions in cancer [25, 40, 41]. Furthermore, HOXC8 mediates cancer progression in multiple cancers, including lung, cervical, and gastric cancer [13, 36, 42]. In our study, the HOXC family exhibited genetic alterations and co-occurrence in the same STAD patients. HOXC8 was upregulated in STAD tissues and was associated with worse overall survival. High expression of HOXC8 was associated with the tumor grades in STAD patients. Our data also revealed that HOXC8 knockdown reduced the cell growth and colony formation ability in AZ521 and HR cells.
OPN is frequently observed in multiple human cancers, which contributes to tumor formation and progression. OPN expression is significantly upregulated in most gastric cancer patients at both the RNA and protein levels. Furthermore, OPN expression is significantly associated with a low apoptotic index, high proliferative index, low grade, high stage, lymph node and vascular invasion, and distal metastasis in the clinicopathology of gastric cancer patients [30, 43-47]. An earlier study showed OPN as a direct target of HOXC8 in C57BL/6J mouse embryo fibroblast cells. OPN was downregulated in HOXC8-overexpressed cells [37]. Adwan et al. showed that HOXC8 knockdown induced OPN expression in Suit2-007 cells [11]. In our study, HOXC8 knockdown significantly inhibited the cell growth and colony formation, and decreased the expression of OPN in gastric cancer cells. The high HOXC8/high OPN expression group was associated with shorter overall survival compared to that of the low HOXC8/high OPN expression group among gastric cancer patients. These findings suggest that the HOXC8/OPN axis may play a role in gastric cancer progression.
An earlier study reported that OPN is responsible for modulating the AKT and MAPK pathways and cell proliferation in vitro [48]. In this study, we further evaluated the effect of HOXC8 on the expression of OPN and the AKT/ERK pathway in gastric cancer cells. HOXC8 knockdown significantly inhibited the expression of OPN and the phosphorylation of AKT/ERK in gastric cancer cell lines. We could not exclude the possibility that HOXC8 directly or indirectly modulates OPN expression; however, this is the first study to evaluate alterations in HOXC8 and OPN expression in gastric cancer cells.
Conclusions
After evaluating the genetic alteration of the HOXC family, we focused on HOXC8 and its upregulation was associated with the tumor grade in patients with STAD. This study is the first to investigate the molecular mechanism by which HOXC8 modulates cell growth, colony formation, and the OPN-related pathway of gastric cancer cells. We found that HOXC8 expression correlates with poor overall survival in gastric cancer patients, indicating that HOXC8 is an independent prognostic factor.
Abbreviations
HOX: homeobox; SPP1: secreted phosphoprotein 1; OPN: osteopontin; TCGA: The Cancer Genome Atlas; STAD: stomach adenocarcinoma; SMAD4: SMAD Family Member 4; PRDM4: PR Domain Zinc Finger Protein 4; GMNN: Geminin DNA Replication Inhibitor; ZFP90: ZFP90 Zinc Finger Protein; PBX2: PBX Homeobox 2; RBPMS: RNA Binding Protein, MRNA Processing Factor; PBX1: PBX Homeobox 1; PDE4DIP: Phosphodiesterase 4D Interacting Protein; PLA2G10: Phospholipase A2 Group X; ABL1: ABL Proto-Oncogene 1, Non-Receptor Tyrosine Kinase; C1orf109: Chromosome 1 Open Reading Frame 109; KRTAP12-1: Keratin Associated Protein 12-1; LHX2: LIM Homeobox 2; TEKT4: Tektin 4; LHX3: LIM Homeobox 3; TRIM42: Tripartite Motif Containing 42; FNTB: Farnesyltransferase, CAAX Box, Beta; BLZF1: Basic Leucine Zipper Nuclear Factor 1; CYSRT1: Cysteine Rich Tail 1; SMAD1: SMAD Family Member 1; KPRP: Keratinocyte Proline Rich Protein; ZRANB1: Zinc Finger RANBP2-Type Containing 1; FOXO1: Forkhead Box O1.
Acknowledgements
We thank Dr. Kuo-Wang Tsai (Taipei Tzu Chi Hospital in Taiwan) and laboratory research staff for their assistance. This study was supported by grants from Kaohsiung Veterans General Hospital, Taiwan [grant number VGHKS109-014, KSVGH110-010, KSVGH111-001 and KSVGH112-007] and National Science Council [MOST 110-2314-B-075B-009 -MY3].
Availability of online data
In this study, we used publicly available online datasets from TCGA, TNMplot, TIMER and Kaplan-Meier analysis database.
Availability of data and materials
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Author contributions
Designed and wrote the manuscript: Y.-F. Y and C.-Y. T; performed experiments: Y.-C. L and Y.-F. Y; interpreted data: Y.-F. Y, J.-B. L and C.-Y. T.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends-An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27
2. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248
3. Collaborators GBDSC. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42-54
4. Zhang XY, Zhang PY. Gastric cancer: somatic genetics as a guide to therapy. J Med Genet. 2017;54:305-312
5. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221
6. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393
7. Park JY, von Karsa L, Herrero R. Prevention strategies for gastric cancer: a global perspective. Clin Endosc. 2014;47:478-489
8. Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10:361-371
9. Gehring WJ, Hiromi Y. Homeotic genes and the homeobox. Annu Rev Genet. 1986;20:147-173
10. Brotto DB, Siena ADD, de B, II, Carvalho S, Muys BR, Goedert L, Cardoso C, Placa JR, Ramao A, Squire JA. et al. Contributions of HOX genes to cancer hallmarks: Enrichment pathway analysis and review. Tumour Biol. 2020;42:1010428320918050
11. Adwan H, Zhivkova-Galunska M, Georges R, Eyol E, Kleeff J, Giese NA, Friess H, Bergmann F, Berger MR. Expression of HOXC8 is inversely related to the progression and metastasis of pancreatic ductal adenocarcinoma. Br J Cancer. 2011;105:288-295
12. Axlund SD, Lambert JR, Nordeen SK. HOXC8 inhibits androgen receptor signaling in human prostate cancer cells by inhibiting SRC-3 recruitment to direct androgen target genes. Mol Cancer Res. 2010;8:1643-1655
13. Liu H, Zhang M, Xu S, Zhang J, Zou J, Yang C, Zhang Y, Gong C, Kai Y, Li Y. HOXC8 promotes proliferation and migration through transcriptional up-regulation of TGFbeta1 in non-small cell lung cancer. Oncogenesis. 2018;7:1
14. de Bessa Garcia SA, Araujo M, Pereira T, Mouta J, Freitas R. HOX genes function in Breast Cancer development. Biochim Biophys Acta Rev Cancer. 2020;1873:188358
15. Chen Z, Qin Z, Li L, Wo Q, Chen X. HOXA13, Negatively Regulated by miR-139-5p, Decreases the Sensitivity of Gastric Cancer to 5-Fluorouracil Possibly by Targeting ABCC4. Front Oncol. 2021;11:645979
16. He Q, Huang W, Liu D, Zhang T, Wang Y, Ji X, Xie M, Sun M, Tian D, Liu M. et al. Homeobox B5 promotes metastasis and poor prognosis in Hepatocellular Carcinoma, via FGFR4 and CXCL1 upregulation. Theranostics. 2021;11:5759-5777
17. Ji M, Feng Q, He G, Yang L, Tang W, Lao X, Zhu D, Lin Q, Xu P, Wei Y. et al. Silencing homeobox C6 inhibits colorectal cancer cell proliferation. Oncotarget. 2016;7:29216-29227
18. Yin J, Guo Y. HOXD13 promotes the malignant progression of colon cancer by upregulating PTPRN2. Cancer Med. 2021;10:5524-5533
19. Qiao C, Huang W, Chen J, Feng W, Zhang T, Wang Y, Liu D, Ji X, Xie M, Sun M. et al. IGF1-mediated HOXA13 overexpression promotes colorectal cancer metastasis through upregulating ACLY and IGF1R. Cell Death Dis. 2021;12:564
20. Ferrando AA, Armstrong SA, Neuberg DS, Sallan SE, Silverman LB, Korsmeyer SJ, Look AT. Gene expression signatures in MLL-rearranged T-lineage and B-precursor acute leukemias: dominance of HOX dysregulation. Blood. 2003;102:262-268
21. Armstrong SA, Golub TR, Korsmeyer SJ. MLL-rearranged leukemias: insights from gene expression profiling. Semin Hematol. 2003;40:268-273
22. Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA. et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531-537
23. Chen M, Qu Y, Yue P, Yan X. The Prognostic Value and Function of HOXB5 in Acute Myeloid Leukemia. Front Genet. 2021;12:678368
24. Bhatlekar S, Viswanathan V, Fields JZ, Boman BM. Overexpression of HOXA4 and HOXA9 genes promotes self-renewal and contributes to colon cancer stem cell overpopulation. J Cell Physiol. 2018;233:727-735
25. Miller GJ, Miller HL, van Bokhoven A, Lambert JR, Werahera PN, Schirripa O, Lucia MS, Nordeen SK. Aberrant HOXC expression accompanies the malignant phenotype in human prostate. Cancer Res. 2003;63:5879-5888
26. Hamid AR, Hoogland AM, Smit F, Jannink S, van Rijt-van de Westerlo C, Jansen CF, van Leenders GJ, Verhaegh GW, Schalken JA. The role of HOXC6 in prostate cancer development. Prostate. 2015;75:1868-1876
27. Shen WF, Krishnan K, Lawrence HJ, Largman C. The HOX homeodomain proteins block CBP histone acetyltransferase activity. Mol Cell Biol. 2001;21:7509-7522
28. Bhatlekar S, Fields JZ, Boman BM. Role of HOX Genes in Stem Cell Differentiation and Cancer. Stem Cells Int. 2018;2018:3569493
29. Chen SW, Zhang Q, Xu ZF, Wang HP, Shi Y, Xu F, Zhang WJ, Wang P, Li Y. HOXC6 promotes gastric cancer cell invasion by upregulating the expression of MMP9. Mol Med Rep. 2016;14:3261-3268
30. Junnila S, Kokkola A, Mizuguchi T, Hirata K, Karjalainen-Lindsberg ML, Puolakkainen P, Monni O. Gene expression analysis identifies over-expression of CXCL1, SPARC, SPP1, and SULF1 in gastric cancer. Genes Chromosomes Cancer. 2010;49:28-39
31. Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, Chi NH, Chen GH, Lin JT. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782-789
32. Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16:885-893
33. El-Tanani MK. Role of osteopontin in cellular signaling and metastatic phenotype. Frontiers in bioscience: a journal and virtual library. 2008;13:4276-4284
34. Ghafouri-Fard S, Dashti S, Farsi M, Taheri M. HOX transcript antisense RNA: An oncogenic lncRNA in diverse malignancies. Exp Mol Pathol. 2021;118:104578
35. Wang MQ, Yin QY, Chen YR, Zhu SL. Diagnostic and prognostic value of HOXC family members in gastric cancer. Future Oncol. 2021;17:4907-4923
36. Gu H, Zhong Y, Liu J, Shen Q, Wei R, Zhu H, Zhang X, Xia X, Yao M, Ni M. The Role of miR-4256/HOXC8 Signaling Axis in the Gastric Cancer Progression: Evidence From lncRNA-miRNA-mRNA Network Analysis. Front Oncol. 2021;11:793678
37. Lei H, Wang H, Juan AH, Ruddle FH. The identification of Hoxc8 target genes. Proc Natl Acad Sci U S A. 2005;102:2420-2424
38. Zhang J, Yamada O, Kida S, Matsushita Y, Murase S, Hattori T, Kubohara Y, Kikuchi H, Oshima Y. Identification of brefelamide as a novel inhibitor of osteopontin that suppresses invasion of A549 lung cancer cells. Oncol Rep. 2016;36:2357-2364
39. Yang YF, Chang YC, Jan YH, Yang CJ, Huang MS, Hsiao M. Squalene synthase promotes the invasion of lung cancer cells via the osteopontin/ERK pathway. Oncogenesis. 2020;9:78
40. Flatte S. Calculations of wave propagation through statistical random media, with and without a waveguide. Opt Express. 2002;10:777-804
41. Hung YC, Ueda M, Terai Y, Kumagai K, Ueki K, Kanda K, Yamaguchi H, Akise D, Ueki M. Homeobox gene expression and mutation in cervical carcinoma cells. Cancer Sci. 2003;94:437-441
42. Huang Y, Chen L, Guo A. Upregulated expression of HOXC8 is associated with poor prognosis of cervical cancer. Oncol Lett. 2018;15:7291-7296
43. Ue T, Yokozaki H, Kitadai Y, Yamamoto S, Yasui W, Ishikawa T, Tahara E. Co-expression of osteopontin and CD44v9 in gastric cancer. Int J Cancer. 1998;79:127-132
44. Dai N, Bao Q, Lu A, Li J. Protein expression of osteopontin in tumor tissues is an independent prognostic indicator in gastric cancer. Oncology. 2007;72:89-96
45. Higashiyama M, Ito T, Tanaka E, Shimada Y. Prognostic significance of osteopontin expression in human gastric carcinoma. Ann Surg Oncol. 2007;14:3419-3427
46. Imano M, Satou T, Itoh T, Sakai K, Ishimaru E, Yasuda A, Peng YF, Shinkai M, Akai F, Yasuda T. et al. Immunohistochemical expression of osteopontin in gastric cancer. J Gastrointest Surg. 2009;13:1577-1582
47. Zhang X, Tsukamoto T, Mizoshita T, Ban H, Suzuki H, Toyoda T, Tatematsu M. Expression of osteopontin and CDX2: indications of phenotypes and prognosis in advanced gastric cancer. Oncol Rep. 2009;21:609-613
48. Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E, Lee NP, Wu GH, Luk JM. Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers. World J Gastroenterol. 2012;18:3923-3930
Author contact
![]() Corresponding author: Yi-Fang Yang, Department of Medical Education and Research, Kaohsiung Veterans General Hospital, No. 386, Dajhong 1st Rd., Zuoying Dist., Kaohsiung City 813414, Taiwan. Phone: 886-7-342-2121 #71592; Fax: 886-7-342-2288; E-mail: yvonne845040com. https://orcid.org/0000-0001-7425-3156.
Corresponding author: Yi-Fang Yang, Department of Medical Education and Research, Kaohsiung Veterans General Hospital, No. 386, Dajhong 1st Rd., Zuoying Dist., Kaohsiung City 813414, Taiwan. Phone: 886-7-342-2121 #71592; Fax: 886-7-342-2288; E-mail: yvonne845040com. https://orcid.org/0000-0001-7425-3156.

 Global reach, higher impact
Global reach, higher impact