3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(15):2848-2866. doi:10.7150/jca.87590 This issue Cite
Research Paper
Comprehensive analysis of prognostic value and immune infiltration of IAPs family in hepatocellular carcinoma
1. Department of Pathology, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China.
2. Department of Oncology, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China.
3. Departments of Ultrasound Imaging, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China.
4. National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
† These authors have contributed equally to this work.
* These authors jointly supervised this work.
Received 2023-6-29; Accepted 2023-8-28; Published 2023-9-11
Abstract
Hepatocellular carcinoma (HCC) is a malignant tumor with high morbidity and mortality rates. The inhibitors of apoptosis (IAP) family act as oncogenes in various tumor types; however, their functions in HCC remain unclear. Here, we used integrated bioinformatics analysis and experimental verification to assess the expression and the prognostic and clinical value of the IAP family in HCC. Using the University of Alabama at Birmingham Cancer Data Analysis Portal (UALCAN) and the Tumor Immune Estimation Resource (TIMER), we analyzed the expression profiles of IAP family members in HCC tissue, normal tissues, and in patients with different stages and grades of HCC. We further verified the expression level of BIRC2 in 25 HCC samples and matched adjacent normal tissues using quantitative real-time PCR (qRT-PCR), and analyzed its correlation with the marker gene of T-helper type 1 cells (Th1)—STAT1. Meanwhile, the association between BIRC2 and the immunotherapeutic response or immunomodulators was confirmed using the Biomarker Exploration of Solid Tumors (BEST) database. The results showed that NAIP, BIRC2, BIRC3, XIAP, BIRC5, and BIRC6 mRNAs were overexpressed in HCC. The clinical stages, pathological grades, and other clinicopathological features of HCC were closely related to the expression levels of the IAP family members, especially the BIRC2 and BIRC5, which were found to be potential prognostic biomarkers for HCC. Expression of the IAPs was strongly associated with immune cell infiltration. Based on the infiltrative status of various immune cells, HCC patients with high BIRC2 and BIRC5 expression demonstrated poor overall survival (OS) rates. In patients with HCC, BIRC2 expression was noticeably elevated. Concurrently, the expression levels of BIRC2 and STAT1 showed a favorable correlation. BEST database analysis revealed that BIRC2 was a negative predictor of responsiveness to anti-programmed cell death ligand 1 (PD-L1)/cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) inhibitor treatment in HCC, and BIRC2 mRNA expression levels were positively correlated with the expression levels of the immune checkpoint genes programmed cell death protein 1 (PD-1), PD-L1, and CTLA-4 in HCC. Consequently, the IAP family may play a role in carcinogenesis and cancer-immune system interactions in HCC. Our results demonstrate that IAP family members may be viable predictive biomarkers and therapeutic targets for HCC.
Keywords: IAPs family, hepatocellular carcinoma, prognosis, methylation, immune cells, biomarkers, therapeutic target
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies worldwide [1, 2]. Patients often have a poor prognosis due to the tumor heterogeneity and distant metastasis of HCC. Over the last two decades, significant advancements have been made in the diagnostic modalities and standard therapies for HCC; however, its recurrence and death rates remain high [3]. Hence, new treatments and prognostic biomarkers are urgently needed to improve the survival rates of patients with HCC.
The inhibitors of apoptosis (IAP) family is made up of a highly conserved class of endogenous caspase inhibitors, present in Drosophila to vertebrates. There are eight proteins in this family: BIRC1 (also called NAIP), BIRC2, BIRC3, XIAP, BIRC5, BIRC6, BIRC7, and BIRC8. A defining characteristic of the IAP family is the presence of one or more baculoviral IAP repeats [4]. IAPs have a variety of biological functions, including the regulation of apoptosis, innate immunity, inflammation, cell migration, and proliferation [5]. IAPs are among the most thoroughly investigated molecular and therapeutic targets for cancer treatment, and dysregulation in their expression has been reported in some malignancies. Studies have shown that the IAP family members are crucial for the occurrence and development of many tumors, such as colorectal cancer (CRC) [6], renal cell carcinoma (RCC) [7], glioma [8], and cervical squamous cell carcinoma (SCC) [9]. The underlying molecular mechanisms of the IAP family in the progression of HCC have yet to be fully elucidated. In this study, we used research databases and bioinformatic tools to assess the mRNA expression levels of the IAP family members in HCC patient samples and analyzed their prognostic values, which may be useful for improving treatment outcomes for HCC patients.
Materials and Methods
The Cancer Genome Atlas (TCGA) HCC samples
The RNA-seq data and clinicopathological parameters of the HCC cohort sample were acquired from TCGA database [10]. HCC samples with missing information in the TCGA dataset were excluded from the study, resulting in a final selection of 342 HCC samples for analysis. To group the HCC samples, we categorized them based on the mean expression of IAPs, creating two distinct groups: one with high expression and another with low expression. Subsequently, by linking the TCGA data to the corresponding mRNA expression values, we conducted an investigation into the relevance of the IAP gene family concerning clinicopathological parameters in the cohort of 342 HCC patients.
Exclusion and inclusion criteria
Exclusion and inclusion criteria Eligible patients included in this article are in accordance with the following inclusion criteria: (1) pathological confirmation of the diagnosis; (2) prior to resection, none of the patients had received any type of therapy, including chemotherapy, radiation, or immunotherapy; (3) complete clinicopathological data. The detailed clinic parameters of enrolled patients were presented in Table. Exclusion criteria included the following: (1) other treatments were used after the operation; (2) vital organ dysfunction; (3) other organ tumors.
Patients' tissue samples
A total of 25 pairs of matched adjacent normal tissue samples and paraffin-embedded archival HCC specimens were collected from Xiangya Hospital (Changsha, P. R. China). The HCC tumor specimens were obtained after surgical resection within the period of June 2022 to March 2023. A total of 25 cases were included in this study, comprising 18 male patients and 7 female patients. The age of the patients ranged from 40 to 76 years, with a median age of 60 years. Among the cases, 14 had tumors smaller than 4cm, while 11 cases had tumors measuring 4cm or larger. Tumor grading revealed 5 cases with high differentiation, 16 cases with moderate differentiation, and 3 cases with low differentiation. 23 patients were diagnosed with neural invasion, all 25 patients showed no signs of neural invasion. Moreover, microvascular invasion (MVI) was present in 2 patients, while 13 patients did not exhibit any MVI. The collection of clinical HCC specimens was conducted with the necessary approval from the Research Ethics Board of Xiangya Hospital of Central South University.
Quantitative real-time polymerase chain reaction
RNA isolation and amplification and quantitative real-time polymerase chain reaction (qRT-PCR) were performed as described previously [11, 12]. The thermocycling program used was as follows: 95°C for 30 sec, followed by 40 cycles of 60°C for 30 sec and 72°C for 30 sec. Primer sequences for qRT-PCR are showed in Table 1.
Primer sequence for qRT-PCR
| Gene | Primer (Forward) | Primer (Reverse) |
|---|---|---|
| BIRC2 | AGCACGATCTTGTCAGATTGG | GGCGGGGAAAGTTGAATATGTA |
| STAT1 | CGGCTGAATTTCGGCACCT | CAGTAACGATGAGAGGACCCT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
TIMER
We utilized TIMER (https://cistrome.shinyapps.io/timer/) as it provides standardized cancer data sets that facilitate gene expression and survival analysis investigations[13]. We conducted an examination of the mRNA expression levels for IAP family members across multiple cancers using the TIMER database. To convert the data to logarithmic scale, we applied log2 transformations with a per million transcripts normalization method. In addition to this, using the TIMER database, we carried out an analysis focusing on the correlation between IAP family members and immune cell infiltration in the tumor microenvironment.
UALCAN
Users of the UALCAN (http://ualcan.path.uab.edu/) can compare data on mRNA expression in human malignant tumors based on the TCGA dataset [14]. To investigate the associations between 371 HCC samples and 50 normal liver samples' clinicopathological characteristics and different IAPs' mRNA expression, we employed the "individual cancer stages" model and "tumor grade" model. To determine the statistical significance of our findings, we conducted a Student's t-test, and results with P-values less than 0.05 were deemed significant.
cBioPortal
cBioPortal platform (http://cbioportal.org/) serves as an open, comprehensive resource for the analysis of extensive cancer genomics and clinical data sets [15]. In our study, we utilized cBioPortal to examine the genomic profiles of IAP family members in a dataset consisting of 178 HCC samples containing information on mutations and mRNA expression. We imposed a threshold of |log2 fold-change (FC)| set at 1, and established a P-value cutoff of 0.05 for our analyses.
STRING
The STRING database (https://cn.string-db.org/) was used to evaluate the associations between various IAP genes and their interactions.
Cytoscape
We compiled a list of 122 functionally related, co-expressed IAP family members chosen through cBioPortal analysis. Subsequently, we performed a protein-protein interaction (PPI) analysis utilizing the Cytoscape software. The size of each node in the PPI network was determined based on the degree values, which represent the connectivity between interacting proteins.
Metascape
Metscape (http://metascape.org) is a free tool for gene annotation and analysis that supports a wide range of enrichment analysis techniques and aids researchers in understanding multiple gene lists [16]. In our study, we employed this resource to perform Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses for gain insights into the functional characteristics and biological pathways associated with IAPs family members.
Kaplan-Meier database
Utilizing the Kaplan-Meier database (https://kmplot.com), we analyzed the prognostic significance of the IAP family in HCC [17]. The mRNA expression values were utilized to determine the optimal cutoff, thus separating the HCC samples into groups with high and low expression. By linking the TCGA data to the corresponding mRNA expression values using the plotter, we investigated the prognostic relevance of the IAP gene family in HCC patients.
TISIDB
The Tumor and Immune System Interaction Database (TISIDB) (http://cis.hku.hk/TISIDB) is an online resource designed to explore the connections between tumors and the immune system, incorporating a diverse range of data sources. In our study, we used the TISIDB to examine if the expression of IAPs varied among different immune subtypes of HCC patients.
BEST
Biomarker Exploration of Solid Tumors (BEST) portal (https://rookieutopia.com/app_direct/BEST/) was used for validation the association between BIRC2 and immunomodulators, as well as immunotherapeutic response [18].
Statistical analysis
SPSS (version 26.0) was used to conduct statistical analysis on the relationships between the mRNA expression of IAPs and the clinicopathological characteristics of patients with HCC. Student's t-tests were used for comparisons and P-values less than 0.05 were considered statistically significant.
RESULTS
Aberrant expression of members of the IAP family in HCC patients
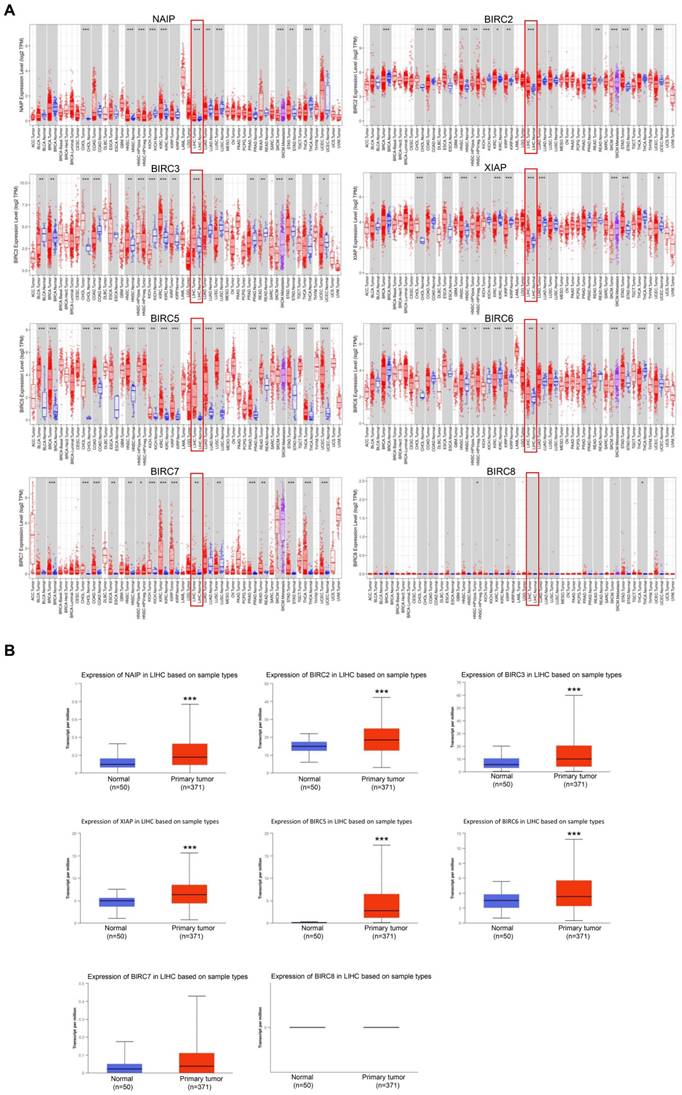
In our study, we utilized the TIMER database to examine the mRNA expression levels of the IAP family across various tumor and normal tissues. The analysis revealed that several members of the IAP family, including NAIP, BIRC2, BIRC3, XIAP, BIRC5, BIRC6, and BIRC7, exhibited higher expression levels in multiple cancers, including HCC (Figure 1A). However, the expression of BIRC8 did not show statistical significance. To further investigate the expression patterns and clinical significance of IAPs in HCC, we analyzed IAP expression in tumor and normal liver tissues using the UALCAN database. The results indicated that NAIP, BIRC2, BIRC3, XIAP, BIRC5, and BIRC6 showed significantly higher expression levels in tumor tissues than in normal tissues (Figure 1B). This observation suggests that IAPs may contribute to tumor progression in HCC.
Association between the mRNA levels of IAP family members and the clinicopathological features of patients with HCC
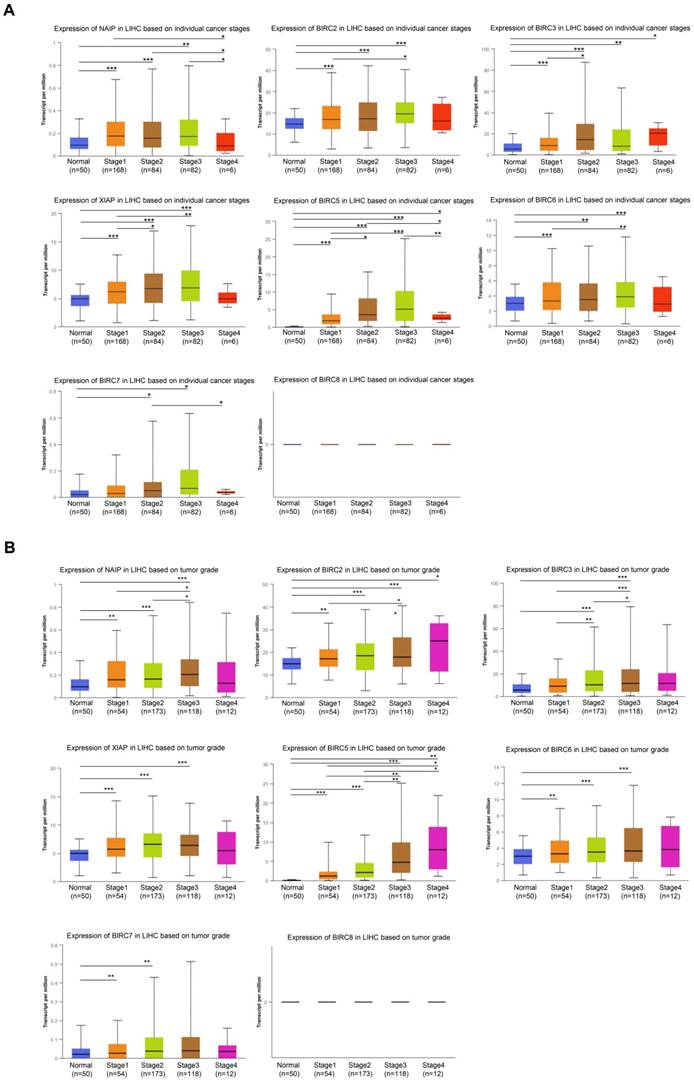
The expression of IAPs in relation to two crucial clinicopathological parameters, the tumor stage and grade of HCC, was further examined using patient samples from the UALCAN database. Notable changes were observed in the expression levels of BIRC3 and BIRC5 across different tumor stages. The levels of NAIP, BIRC2, XIAP, and BIRC6 were significantly elevated in the subgroups of tumor stages 1-3 compared to their levels in the normal subgroups (Figure 2A), with no discernible differences in their levels in the subgroup of tumor stage 4. BIRC2 and BIRC5 expression gradually and significantly increased from grades 1 to 4. The mRNA expression levels of NIAP, XIAP, and BIRC6 tended to be higher in grades 1 to 3 (Figure 2B).
Furthermore, we expanded our analysis by including 342 HCC patient samples from the TCGA database to investigate the correlations between the IAP gene family and the pathological characteristics of HCC (Table 2). The results revealed significant associations between the expression of IAP family members and various clinicopathological parameters in HCC patients. We observed that high levels of NIAP, BIRC5, BIRC6, and BIRC8 expression were significantly associated with the T stage of HCC patients. The expression of NIAP and BIRC6 showed a correlation with sex, while NIAP and BIRC5/6/7/8 expression demonstrated associations with the pathological stage of HCC. Additionally, high expression levels of all eight IAP members were significantly associated with the histological grade of HCC. These findings suggest that the differentiation of tumor cells may, to some extent, indicate the potential diagnostic value of IAP family members in HCC. The identified correlations between IAP expression and the clinicopathological parameters of HCC provide valuable insights into the potential role of IAPs as diagnostic markers for HCC.
Genetic alterations and functional enrichment analysis of members of the IAP family
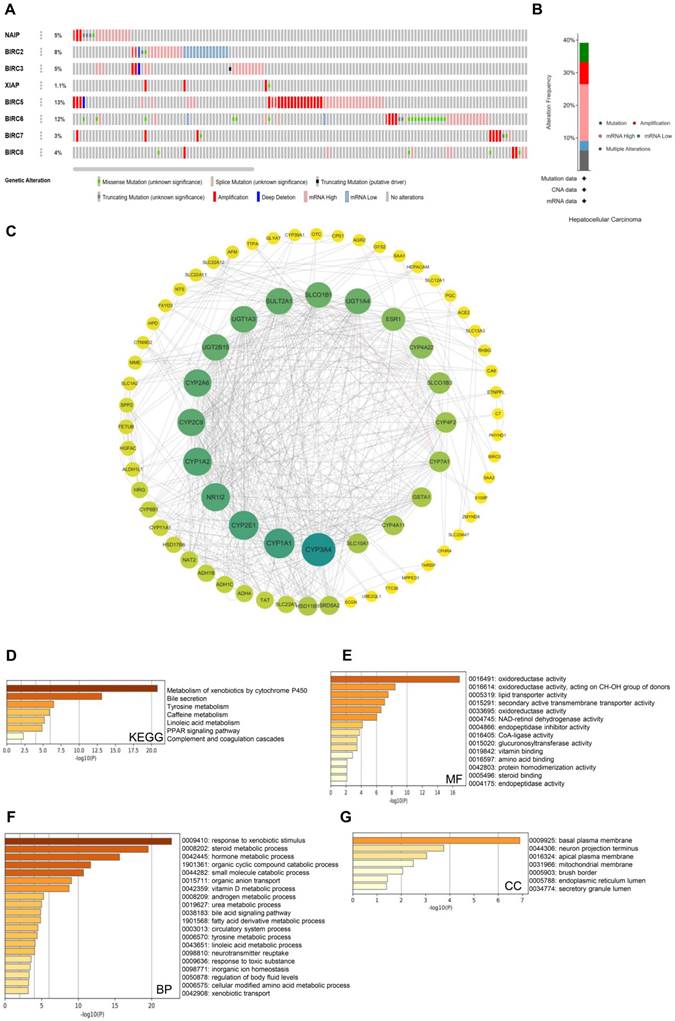
Genetic alterations are widely recognized as crucial factors in cancer development. To investigate the genetic alteration status of the IAP family members, we utilized the cBioPortal database for comprehensive analysis. Out of 178 HCC patient samples, alterations in IAP genes were observed in 80 samples (45%), with BIRC5 displaying the highest alteration rate (13%). The most common abnormalities within the IAP family were mRNA alterations, followed by amplifications and other mutations (Figure 3A and 3B). To delve deeper into the co-expression networks linked with the IAP family members, we identified co-expressed genes using the cBioPortal database with a threshold of |log2FC| ≥ 1 and P-value < 0.05. The resulting network of key genes related to the IAP family was visualized using Cytoscape_v.3.9.0 (Figure 3C). Notably, CYP3A4, CYP1A1, CYP2E1, CYP1A2, NR1I2, CYP2C9, CYP2A6, and UGT2B15 were identified to be primarily associated with the modulation and function of IAPs in HCC.
In addition, we employed the Metascape database to perform GO and KEGG pathway analyses on the co-expressed genes of IAP family members in HCC patient samples. The KEGG pathway analysis highlighted key pathways such as the metabolism of xenobiotics by cytochrome P450 enzymes, bile secretion, tyrosine metabolism, and complement and coagulation cascades (Figure 3D). Molecular function analysis revealed their involvement in oxidoreductase activity, specifically acting on the CH-OH group of donors, as well as lipid transporter activity (Figure 3E). Biological process analysis indicated their role in responding to xenobiotic stimuli, steroid metabolic processes, hormone metabolic processes, and organic cyclic compound and small molecule catabolic processes (Figure 3F). Cellular component analysis unveiled their frequent association with the basal plasma membrane, neuron projection terminus, apical plasma membrane, and mitochondrial membrane (Figure 3G). These results suggest that the IAP family may participate in drug metabolism and complement and coagulation cascades in HCC patients, potentially influencing their treatment outcomes and immune infiltration.
The inhibitors of apoptosis (IAP) gene family is overexpressed in hepatocellular carcinoma (HCC). (A) The mRNA expression levels of IAP family members across various cancers were detected by TIMER. (B) The relative expression of the IAP family in HCC. Statistical significance is indicated as * P < 0.05, ** P < 0.01, *** P < 0.001 compared to the control group.
The association between the mRNA expression of various IAP family members and the clinicopathological characteristics of HCC patients was examined using UALCAN. (A) The correlation between the mRNA expression of different IAP family members and the pathological stage of HCC in patients. (B) The relationship between tumor grade in HCC patients and the mRNA expression of IAP family members. Statistical significance is indicated as * P < 0.05, ** P < 0.01, *** P < 0.001 compared to the control group.
Relationship between clinicopathologic parameters and IAP family members expression in RCC
| Characteristics | N | NAIP | BIRC2 | BIRC3 | XIAP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | Low | High | P | Low | High | P | |||||
| Gender | ||||||||||||||||
| Male | 233 | 169 | 64 | 0.011 | 116 | 117 | 0.454 | 169 | 64 | 0.852 | 137 | 96 | 0.759 | |||
| Female | 109 | 64 | 45 | 59 | 50 | 78 | 31 | 66 | 43 | |||||||
| Age (year) | ||||||||||||||||
| ≤60 | 170 | 119 | 51 | 0.460 | 85 | 85 | 0.667 | 122 | 48 | 0.920 | 103 | 67 | 0.645 | |||
| >60 | 172 | 114 | 58 | 90 | 82 | 125 | 47 | 100 | 72 | |||||||
| T stage | ||||||||||||||||
| T1 + T2 | 256 | 167 | 89 | 0.048 | 129 | 127 | 0.619 | 186 | 70 | 0.757 | 153 | 103 | 0.791 | |||
| T3 + T4 | 86 | 66 | 20 | 46 | 40 | 61 | 25 | 50 | 36 | |||||||
| N stage | ||||||||||||||||
| Nx | 87 | 60 | 27 | 0.846 | 42 | 45 | 0.532 | 61 | 26 | 0.611 | 49 | 38 | 0.504 | |||
| N0 + N1 | 255 | 173 | 82 | 133 | 122 | 186 | 69 | 154 | 101 | |||||||
| M stage | ||||||||||||||||
| Mx | 77 | 49 | 28 | 0.337 | 37 | 40 | 0.534 | 58 | 19 | 0.490 | 38 | 39 | 0.045 | |||
| M0 + M1 | 265 | 184 | 81 | 138 | 127 | 189 | 76 | 167 | 102 | |||||||
| Pathologic stage | ||||||||||||||||
| Stage I + II | 252 | 164 | 88 | 0.043 | 127 | 125 | 0.632 | 183 | 69 | 0.784 | 151 | 101 | 0.243 | |||
| Stage III + IV | 90 | 69 | 21 | 48 | 42 | 64 | 26 | 52 | 46 | |||||||
| Histologic grade | ||||||||||||||||
| grade 1 + 2 | 213 | 147 | 66 | 0.000 | 105 | 108 | 0.000 | 163 | 50 | 0.000 | 121 | 92 | 0.000 | |||
| grade 3 + 4 | 129 | 86 | 43 | 70 | 59 | 84 | 45 | 82 | 47 | |||||||
| Characteristics | N | BIRC5 | BIRC6 | BIRC7 | BIRC8 | |||||||||||
| Low | High | P | Low | High | P | Low | High | P | Low | High | P | |||||
| Gender | ||||||||||||||||
| Male | 233 | 155 | 78 | 0.361 | 138 | 95 | 0.031 | 212 | 21 | 0.415 | 225 | 8 | 0.209 | |||
| Female | 109 | 67 | 42 | 51 | 58 | 102 | 7 | 102 | 7 | |||||||
| Age (year) | ||||||||||||||||
| ≤60 | 170 | 104 | 66 | 0.66 | 89 | 81 | 0.000 | 158 | 12 | 0.449 | 161 | 9 | 0.415 | |||
| >60 | 172 | 118 | 54 | 100 | 72 | 156 | 16 | 166 | 6 | |||||||
| T stage | ||||||||||||||||
| T1 + T2 | 256 | 179 | 77 | 0.001 | 143 | 113 | 0.000 | 235 | 21 | 0.985 | 248 | 8 | 0.049 | |||
| T3 + T4 | 86 | 43 | 43 | 169 | 40 | 79 | 7 | 79 | 7 | |||||||
| N stage | ||||||||||||||||
| Nx | 87 | 62 | 25 | 0.003 | 51 | 36 | 0.466 | 77 | 10 | 0.193 | 80 | 7 | 0.372 | |||
| N0 + N1 | 255 | 160 | 95 | 138 | 117 | 237 | 18 | 247 | 8 | |||||||
| M stage | ||||||||||||||||
| Mx | 77 | 60 | 17 | 0.007 | 42 | 35 | 0.886 | 69 | 8 | 0.423 | 69 | 8 | 0.003 | |||
| M0 + M1 | 265 | 162 | 103 | 147 | 118 | 245 | 20 | 258 | 7 | |||||||
| Pathologic stage | ||||||||||||||||
| Stage I + II | 252 | 176 | 76 | 0.000 | 142 | 110 | 0.000 | 231 | 21 | 0.000 | 244 | 8 | 0.000 | |||
| Stage III + IV | 90 | 46 | 44 | 47 | 43 | 83 | 7 | 83 | 7 | |||||||
| Histologic grade | ||||||||||||||||
| grade 1 + 2 | 213 | 155 | 58 | 0.000 | 117 | 96 | 0.000 | 201 | 12 | 0.000 | 205 | 8 | 0.000 | |||
| grade 3 + 4 | 129 | 67 | 62 | 72 | 57 | 113 | 16 | 122 | 7 | |||||||
Bold font indicates significant difference.
Association between the expression levels of IAPs and immune infiltration in HCC
To comprehend the functions of the IAP family members in HCC, we employed TIMER to investigate the molecular characteristics of tumor-immune interactions. Figure 4 illustrates that except of BIRC8, the transcriptional expression of the remaining IAPs exhibited a positive correlation with the expression of B cells, CD8+ T cells, CD4+ T cells, neutrophils, macrophages, and dendritic cells (DCs) (R> 0.1, P < 0.05). This evidence implies that members of the IAP family might influence the immune response within the tumor microenvironment of HCC.
Our study elucidated the associations between IAPs and the signature marker genes of various immune cells in HCC (Table 3). We observed a strong correlation between the expression of NAIP and the expression of several immune cells, including CD8+ T cells, M1 and M2 macrophages, tumor-associated macrophages (TAMs), DCs, natural killer cells (NKs), B cells, T-helper type 1 cells (Th1), Th2, neutrophils, regulatory T cells (Tregs), and monocytes. A close correlation was also observed between the expression of BIRC2 and the expression of B cells, neutrophils, TAM, M1 and M2 macrophages, Tfh, Th1, Th2, Tregs, Th17, DCs, and monocytes. A moderate correlation was identified between the expression of XIAP and the expression of neutrophils, TAM, Th1, Th2, DCs, and Treg markers. Correlations in expression levels were moderately close between BIRC5 and neutrophils, Th1, Th2, CD8+ T cells, TAM, B cells, and monocytes. In terms of expression levels, BIRC6 exhibited very strong correlations with DCs, TAMs, M1 and M2 macrophages, Th1, Th2, Tregs, neutrophils, and monocyte markers in HCC patients. BIRC7, on the other hand, showed relatively high correlations with TAMs, M1, B cells, Tfh, T cells, and Th17 cells. These findings suggest that IAP family members play pivotal roles in immune infiltration in HCC.
Genetic alterations and enrichment analysis of IAPs in HCC. (A-B) An overview of the mutation rates of IAPs in HCC cases. (C) A protein-protein interaction (PPI) network involving members of the IAP family and their partners was constructed using cBioPortal and Cytoscape. (D-G) KEGG enrichment pathway analysis examined molecular functions, biological processes, and cellular components related to the co-expressed genes of IAPs.
Association between the expression of IAPs and immune infiltration in HCC. (A) The effect of IAP family members on the immune cell infiltration of HCC was analyzed by TIMER.
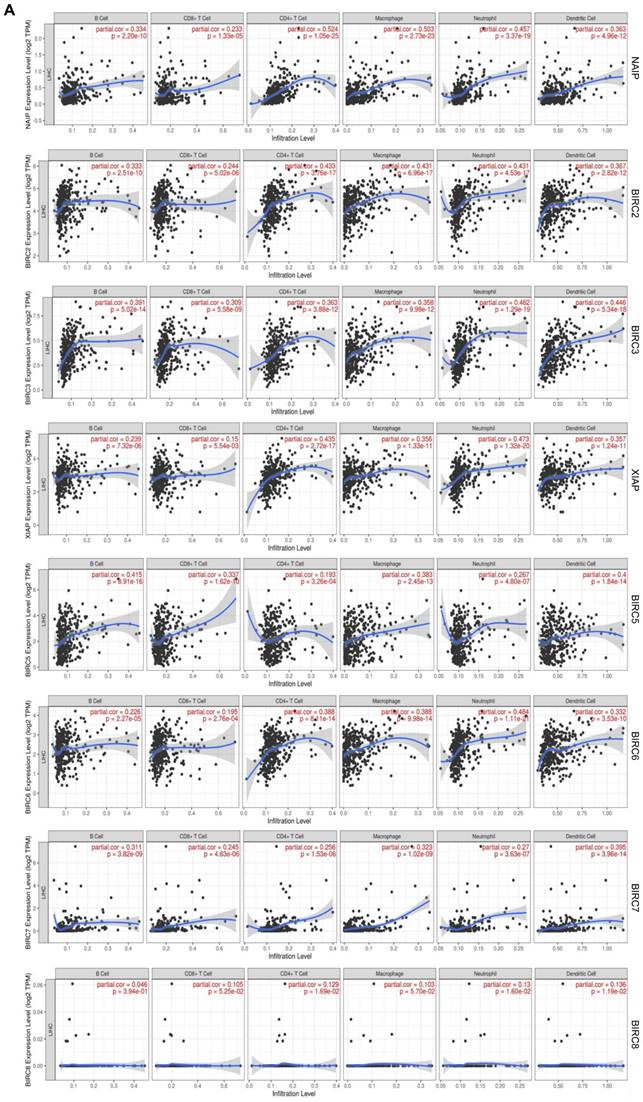
The relationship between immune cell markers and the expression of IAP family members
| NAIP | BIRC2 | BIRC3 | XIAP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| cor | p | cor | p | cor | p | cor | p | ||
| CD8+ T cell | CD8A | 0.298 | *** | 0.193 | *** | 0.368 | *** | 0.127 | * |
| CD8B | 0.157 | ** | 0.092 | 0.076 | 0.294 | *** | 0.009 | 0.860 | |
| GZMA | 0.197 | *** | 0.068 | 0.188 | 0.381 | *** | 0.008 | 0.884 | |
| B cell | CD19 | 0.227 | *** | 0.141 | *** | 0.294 | *** | 0.068 | 0.193 |
| CD79A | 0.272 | *** | 0.106 | ** | 0.309 | *** | 0.028 | 0.591 | |
| MS4A1 | 0.297 | *** | 0.144 | *** | 0.328 | *** | 0.079 | 0.129 | |
| T cell | CD3D | 0.209 | *** | 0.079 | 0.128 | 0.407 | *** | 0.011 | 0.827 |
| CD3E | 0.307 | *** | 0.150 | *** | 0.467 | *** | 0.092 | 0.076 | |
| CD2 | 0.301 | *** | 0.127 | * | 0.462 | *** | 0.071 | 0.170 | |
| TAM | CCL2 | 0.320 | *** | 0.218 | *** | 0.403 | *** | 0.216 | *** |
| CD68 | 0.288 | *** | 0.148 | *** | 0.340 | *** | 0.204 | *** | |
| IL10 | 0.404 | *** | 0.263 | *** | 0.378 | *** | 0.230 | *** | |
| M1 | IRF5 | 0.484 | *** | 0.394 | *** | 0.317 | *** | 0.359 | *** |
| PTGS2 | 0.438 | *** | 0.312 | *** | 0.445 | *** | 0.330 | *** | |
| NOS2 | 0.208 | *** | 0.188 | *** | 0.075 | 0.149 | 0.244 | *** | |
| M2 | MS4A4A | 0.339 | *** | 0.201 | *** | 0.470 | *** | 0.272 | *** |
| CD163 | 0.339 | *** | 0.239 | *** | 0.381 | *** | 0.292 | *** | |
| VSIG4 | 0.334 | *** | 0.202 | *** | 0.456 | *** | 0.276 | *** | |
| Neutrophils | ITGAM | 0.388 | *** | 0.276 | *** | 0.582 | *** | 0.362 | *** |
| CCR7 | 0.382 | *** | 0.190 | *** | 0.378 | *** | 0.167 | ** | |
| SIGLEC5 | 0.464 | *** | 0.391 | *** | 0.438 | *** | 0.389 | *** | |
| DC | HLA-DQB1 | 0.232 | *** | 0.098 | 0.059 | 0.391 | *** | 0.099 | 0.056 |
| HLA-DPB1 | 0.294 | *** | 0.177 | *** | 0.484 | *** | 0.195 | *** | |
| HLA-DRA | 0.320 | *** | 0.239 | *** | 0.553 | *** | 0.288 | *** | |
| HLA-DPA1 | 0.349 | *** | 0.244 | *** | 0.516 | *** | 0.289 | *** | |
| ITGAX | 0.505 | *** | 0.326 | *** | 0.512 | *** | 0.293 | *** | |
| CD1C | 0.343 | *** | 0.256 | *** | 0.339 | *** | 0.185 | *** | |
| NRP1 | 0.433 | *** | 0.530 | *** | 0.250 | *** | 0.564 | *** | |
| NK cell | KIR2DL1 | 0.050 | 0.333 | -0.001 | 0.981 | -0.001 | 0.977 | 0.065 | 0.209 |
| KIR2DL3 | 0.177 | *** | 0.181 | *** | 0.179 | *** | 0.164 | ** | |
| KIR2DL4 | 0.171 | *** | 0.103 | * | 0.263 | *** | 0.109 | * | |
| KIR3DL1 | 0.139 | ** | 0.118 | * | 0.047 | 0.369 | 0.164 | ** | |
| KIR3DL2 | 0.183 | *** | 0.126 | * | 0.148 | ** | 0.055 | 0.294 | |
| KIR3DL3 | 0.103 | * | 0.071 | 0.173 | 0.042 | 0.422 | 0.009 | 0.864 | |
| KIR2DS4 | 0.195 | *** | 0.115 | * | 0.064 | 0.222 | 0.092 | 0.076 | |
| Th1 | TBX21 | 0.248 | *** | 0.129 | * | 0.292 | *** | 0.104 | * |
| STAT1 | 0.487 | *** | 0.484 | *** | 0.522 | *** | 0.398 | *** | |
| STAT4 | 0.412 | *** | 0.234 | *** | 0.523 | *** | 0.162 | ** | |
| IFNG | 0.196 | *** | 0.110 | * | 0.292 | *** | 0.093 | 0.073 | |
| Th2 | STAT6 | 0.349 | *** | 0.399 | *** | 0.246 | *** | 0.516 | *** |
| GATA3 | 0.420 | *** | 0.226 | *** | 0.409 | *** | 0.215 | *** | |
| STAT5A | 0.386 | *** | 0.349 | *** | 0.361 | *** | 0.395 | *** | |
| IL13 | 0.236 | *** | 0.099 | 0.056 | 0.138 | ** | 0.118 | * | |
| Tfh | BCL6 | 0.294 | *** | 0.434 | *** | 0.089 | 0.085 | 0.426 | *** |
| IL21 | 0.196 | *** | 0.072 | 0.164 | 0.120 | * | 0.077 | 0.140 | |
| Th17 | STAT3 | 0.427 | *** | 0.418 | *** | 0.448 | *** | 0.503 | *** |
| IL17A | 0.147 | ** | 0.109 | * | 0.100 | 0.054 | 0.084 | 0.105 | |
| Treg | FOXP3 | 0.362 | *** | 0.235 | *** | 0.171 | *** | 0.289 | *** |
| STAT5B | 0.470 | *** | 0.643 | *** | 0.129 | * | 0.621 | *** | |
| CCR8 | 0.548 | *** | 0.386 | *** | 0.466 | *** | 0.424 | *** | |
| TGFB1 | 0.436 | *** | 0.307 | *** | 0.321 | *** | 0.219 | *** | |
| T -cell exhaustion | PDCD1 | 0.331 | *** | 0.185 | *** | 0.341 | *** | 0.101 | 0.051 |
| CTLA4 | 0.281 | *** | 0.108 | * | 0.415 | *** | 0.065 | 0.212 | |
| HAVCR2 | 0.421 | *** | 0.262 | *** | 0.512 | *** | 0.263 | *** | |
| LAG3 | 0.202 | *** | 0.058 | 0.267 | 0.139 | ** | -0.017 | 0.748 | |
| Monocyte | CD86 | 0.419 | *** | 0.285 | *** | 0.512 | *** | 0.268 | *** |
| C3AR1 | 0.411 | *** | 0.293 | *** | 0.499 | *** | 0.334 | *** | |
| CSF1R | 0.375 | *** | 0.242 | *** | 0.489 | *** | 0.284 | *** | |
| BIRC5 | BIRC6 | BIRC7 | BIRC8 | ||||||
| cor | p | cor | p | cor | p | cor | p | ||
| CD8+ T cell | CD8A | 0.144 | ** | 0.124 | * | 0.321 | *** | 0.038 | 0.461 |
| CD8B | 0.217 | *** | -0.010 | 0.843 | 0.368 | *** | 0.044 | 0.397 | |
| GZMA | 0.133 | * | -0.010 | 0.845 | 0.339 | *** | -0.016 | 0.753 | |
| B cell | CD19 | 0.229 | *** | 0.059 | 0.256 | 0.303 | *** | 0.095 | 0.068 |
| CD79A | 0.118 | * | 0.031 | 0.551 | 0.330 | *** | 0.060 | 0.251 | |
| MS4A1 | 0.001 | 0.980 | 0.089 | 0.087 | 0.225 | *** | 0.055 | 0.291 | |
| T cell | CD3D | 0.324 | *** | -0.031 | 0.552 | 0.454 | *** | 0.118 | * |
| CD3E | 0.153 | ** | 0.075 | 0.150 | 0.395 | *** | 0.041 | 0.427 | |
| CD2 | 0.185 | *** | 0.049 | 0.343 | 0.408 | *** | 0.042 | 0.420 | |
| TAM | CCL2 | -0.022 | 0.674 | 0.165 | ** | 0.308 | *** | -0.004 | 0.946 |
| CD68 | 0.141 | ** | 0.170 | ** | 0.458 | *** | 0.047 | 0.365 | |
| IL10 | 0.145 | ** | 0.220 | *** | 0.429 | *** | 0.030 | 0.560 | |
| M1 | IRF5 | 0.294 | *** | 0.405 | *** | 0.186 | *** | 0.032 | 0.545 |
| PTGS2 | -0.028 | 0.595 | 0.336 | *** | 0.286 | *** | 0.047 | 0.369 | |
| NOS2 | -0.138 | ** | 0.292 | *** | -0.081 | 0.118 | 0.021 | 0.681 | |
| M2 | MS4A4A | 0.009 | 0.862 | 0.208 | *** | 0.369 | *** | -0.027 | 0.603 |
| CD163 | -0.032 | 0.542 | 0.289 | *** | 0.291 | *** | -0.011 | 0.838 | |
| VSIG4 | 0.025 | 0.631 | 0.182 | *** | 0.297 | *** | -0.018 | 0.735 | |
| Neutrophils | ITGAM | 0.251 | *** | 0.261 | *** | 0.373 | *** | 0.090 | 0.085 |
| CCR7 | -0.028 | 0.588 | 0.166 | ** | 0.283 | *** | 0.001 | 0.986 | |
| SIGLEC5 | 0.123 | * | 0.397 | *** | 0.340 | *** | 0.049 | 0.343 | |
| DC | HLA-DQB1 | 0.147 | ** | 0.033 | 0.526 | 0.378 | *** | -0.022 | 0.669 |
| HLA-DPB1 | 0.133 | * | 0.127 | * | 0.341 | *** | -0.009 | 0.858 | |
| HLA-DRA | 0.085 | 0.101 | 0.207 | *** | 0.295 | *** | -0.018 | 0.726 | |
| HLA-DPA1 | 0.064 | 0.221 | 0.223 | *** | 0.329 | *** | -0.011 | 0.829 | |
| ITGAX | 0.248 | *** | 0.270 | *** | 0.538 | *** | 0.063 | 0.229 | |
| CD1C | 0.016 | 0.760 | 0.241 | *** | 0.145 | ** | 0.095 | 0.066 | |
| NRP1 | 0.049 | 0.351 | 0.585 | *** | 0.178 | *** | 0.039 | 0.457 | |
| NK cell | KIR2DL1 | -0.047 | 0.364 | 0.072 | 0.168 | 0.021 | 0.688 | -0.004 | 0.939 |
| KIR2DL3 | 0.123 | * | 0.124 | * | 0.130 | * | -0.044 | 0.400 | |
| KIR2DL4 | 0.185 | *** | 0.053 | 0.311 | 0.224 | *** | 0.047 | 0.372 | |
| KIR3DL1 | -0.039 | 0.457 | 0.154 | ** | 0.118 | * | -0.051 | 0.327 | |
| KIR3DL2 | 0.061 | 0.241 | 0.047 | 0.371 | 0.139 | ** | -0.030 | 0.563 | |
| KIR3DL3 | 0.042 | 0.418 | 0.012 | 0.822 | 0.030 | 0.564 | -0.050 | 0.338 | |
| KIR2DS4 | -0.002 | 0.968 | 0.118 | * | 0.081 | 0.121 | -0.096 | 0.064 | |
| Th1 | TBX21 | -0.005 | 0.929 | 0.096 | 0.065 | 0.276 | *** | -0.041 | 0.429 |
| STAT1 | 0.215 | *** | 0.500 | *** | 0.230 | *** | 0.085 | 0.100 | |
| STAT4 | 0.207 | *** | 0.190 | *** | 0.278 | *** | 0.030 | 0.564 | |
| IFNG | 0.257 | *** | 0.058 | 0.269 | 0.381 | *** | 0.051 | 0.323 | |
| Th2 | STAT6 | -0.082 | 0.115 | 0.496 | *** | 0.013 | 0.806 | -0.019 | 0.717 |
| GATA3 | 0.145 | ** | 0.212 | *** | 0.388 | *** | 0.062 | 0.236 | |
| STAT5A | 0.230 | *** | 0.329 | *** | 0.387 | *** | 0.080 | 0.125 | |
| IL13 | 0.055 | 0.290 | 0.114 | * | 0.063 | 0.226 | -0.001 | 0.981 | |
| Tfh | BCL6 | -0.018 | 0.734 | 0.407 | *** | 0.046 | 0.373 | 0.078 | 0.136 |
| IL21 | 0.095 | 0.067 | 0.144 | ** | 0.095 | 0.067 | 0.058 | 0.263 | |
| Th17 | STAT3 | -0.047 | 0.362 | 0.508 | *** | 0.153 | ** | 0.127 | * |
| IL17A | -0.006 | 0.912 | 0.127 | * | 0.054 | 0.303 | 0.101 | 0.052 | |
| Treg | FOXP3 | -0.001 | 0.991 | 0.335 | *** | 0.163 | ** | 0.099 | 0.056 |
| STAT5B | 0.029 | 0.583 | 0.739 | *** | -0.072 | 0.166 | 0.104 | * | |
| CCR8 | 0.188 | *** | 0.447 | *** | 0.332 | *** | 0.130 | * | |
| TGFB1 | 0.218 | *** | 0.238 | *** | 0.427 | *** | 0.013 | 0.800 | |
| T -cell exhaustion | PDCD1 | 0.312 | *** | 0.047 | 0.365 | 0.421 | *** | 0.070 | 0.179 |
| CTLA4 | 0.355 | *** | -0.002 | 0.972 | 0.484 | *** | 0.079 | 0.129 | |
| HAVCR2 | 0.275 | *** | 0.206 | *** | 0.542 | *** | 0.065 | 0.214 | |
| LAG3 | 0.344 | *** | 0.013 | 0.801 | 0.333 | *** | 0.106 | * | |
| Monocyte | CD86 | 0.248 | *** | 0.224 | *** | 0.476 | *** | 0.035 | 0.504 |
| C3AR1 | 0.139 | ** | 0.272 | *** | 0.431 | *** | 0.019 | 0.717 | |
| CSF1R | 0.119 | * | 0.208 | *** | 0.387 | *** | 0.003 | 0.958 | |
Correlation R value was calculated by Spearman's algorithm and adjusted by tumor purity.*P < 0.05, **P< 0.01, *** P < 0.001.
Prognostic value of mRNA expression of IAPs in HCC patients
To assess the prognostic value of the mRNA expression of IAP family members in HCC patients, we utilized the Kaplan-Meier plotter to analyze both overall survival (OS) and relapse-free survival (RFS) rates. We found that elevated expression of BIRC2, XIAP, BIRC5, BIRC6, and BIRC7 was significantly associated with poor OS rates (P < 0.05; Figure 5A). Furthermore, patients with higher transcription levels of BIRC2 and BIRC5 experienced shorter RFS rates (P < 0.05; Figure 5B). Patients with higher transcript levels of BIRC8 had longer OS and RFS rates (P < 0.05). Based on these findings, we proposed that BIRC2 and BIRC5 may potentially serve as prognostic biomarkers for HCC patients.
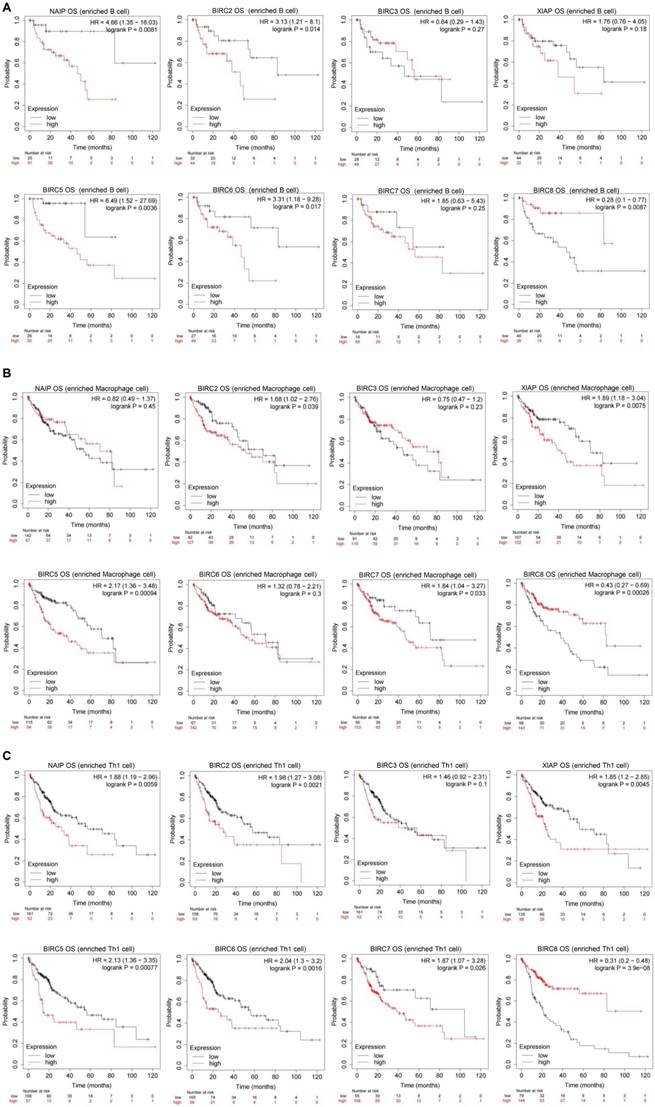
We conducted further prognostic analysis using the Kaplan-Meier plotter, focusing on the expression levels of IAPs in HCC patients within the relevant immune cell subgroups (Figure 6). The findings revealed that upregulation of BIRC2 and BIRC5 was correlated with poor OS rates and RFS rates when patients with HCC were enriched with B cells, macrophages, and Th1 cells. In patients with B cell enrichment, NAIP and BIRC6 expression showed a significant negative correlation with OS rates, and high levels of XIAP and BIRC7 were associated with shorter OS rates when HCC patients were enriched with macrophages. Similarly, high NAIP, XIAP, BIRC6, and BIRC7 expression in the enriched Th1 cells cohort was associated with a worse prognosis. The above analyses indicate that high expression of IAPs in HCC may affect the prognosis due in part to immune infiltration of HCC.
Prognostic significance of the IAP family in HCC. (A-B) The relationship between mRNA expression of IAP family members and overall survival (OS) and progression-free survival (RFS) rates in HCC patients was examined using the Kaplan-Meier plotter.
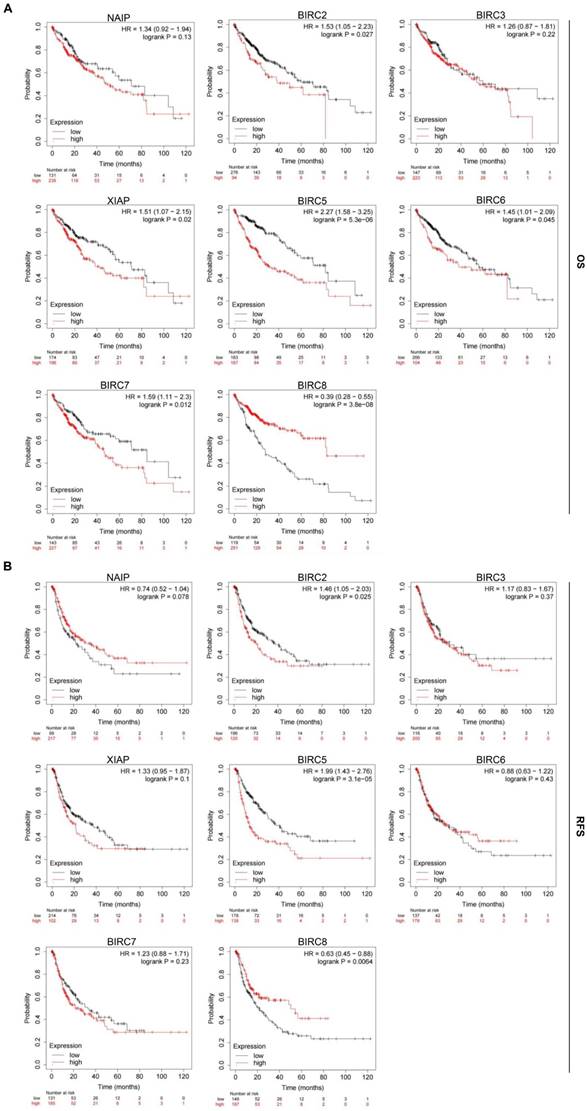
The mRNA gene expression levels of the IAP family members in HCC are correlated with the overall survival (OS) rates of HCC patients. Associations between mRNA gene expression levels of IAPs and the OS rates of HCC patients who had (A) B cells, (B) macrophages, and (C) Th1 cells enriched.
Relationship between BIRC2 expression in HCC and tumor immune subtypes and immunotherapy
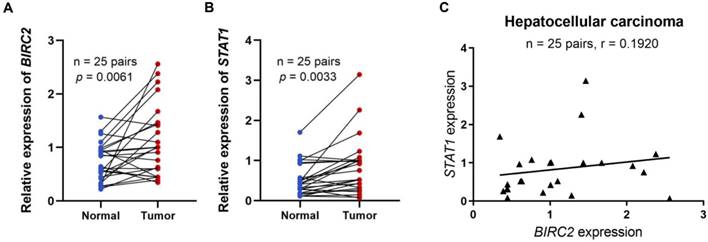
Based on the above analysis, we found that BIRC2 may play an important role in promoting the progression of HCC. To further explore the role of BIRC2 in HCC, we verified the differential expression of BIRC2 in HCC tissues and its correlation with immune cell-associated molecules using qRT-PCR. The results revealed that the relative expression levels of BIRC2 in the 25 HCC tissue samples were significantly higher than the BIRC2 expression levels in the matched adjacent non-tumor tissue samples (P < 0.05; Figure 7A). Moreover, we demonstrated a relationship between BIRC2 and STAT1 [19]. Using qRT-PCR analysis, we found that the relative expression levels of STAT1 were significantly higher in the 25 HCC tissues than the expression of STAT1 in the matched paracancerous normal tissues (P < 0.05, Figure 7B). After comparing the expression of BIRC2 and STAT1 in the 25 HCC samples, we found a weak positive correlation (R= 0.1920; Figure 7C). Moreover, we further analyzed the relationship between the expression levels of BIRC2 or STAT1 and the clinicopathological characteristics of HCC patients, and found that the expression levels of BIRC2 or STAT1 were significantly associated with the differentiation degree of HCC in patients (P < 0.05, Table 4).
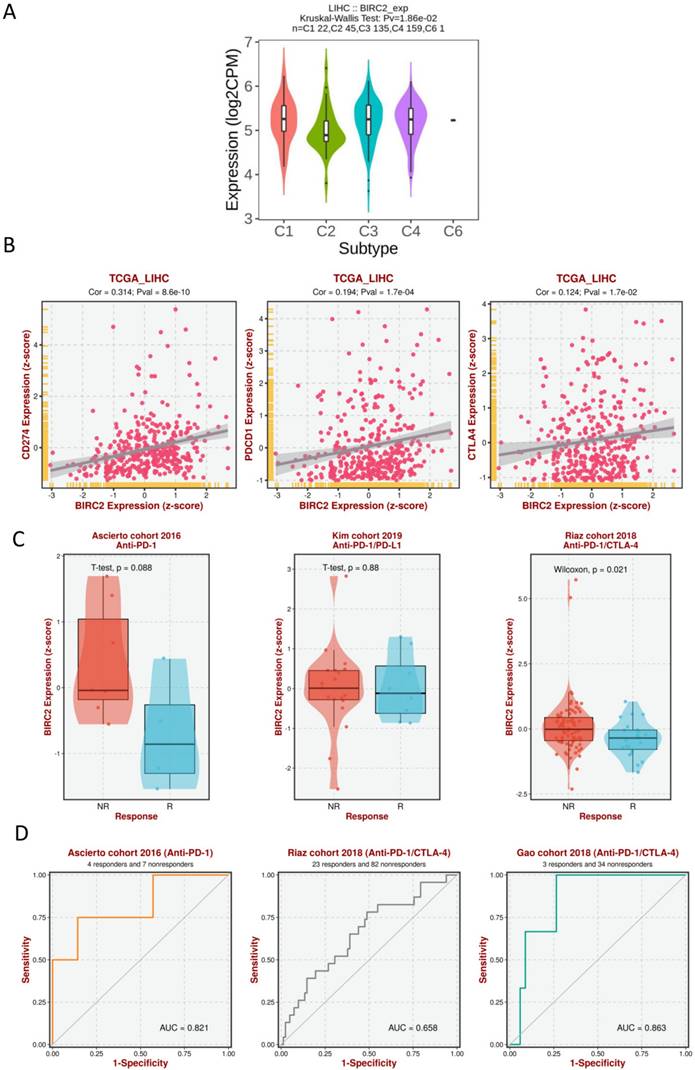
We investigated whether the expression of IAPs differed between different immune subtypes of HCC (Figure 8A). The results showed that BIRC2 expression differed significantly between the four immune subtypes (C1, C2, C3, and C4), indicating that BIRC2 plays a vital role in the immune infiltration of HCC. To further test the correlation between BIRC2 and immunotherapy, the association of the expression of known immune checkpoint genes [20] with BIRC2 in HCC tissue was analyzed using BEST. As shown in Figure 8B, a positive correlation was observed between the mRNA expression levels of BIRC2 and those of PDCD1 (programmed cell death protein 1, PD-1), CD274 (programmed death ligand 1, PD-L1), and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4). Moreover, using BEST analysis, we wanted to determine whether aberrant BIRC2 expression could affect the immunotherapeutic response to HCC. As shown in Figure 8C, the expression of BIRC2 was elevated in anti-PD-1 nonresponders in the Ascierto cohort and in the anti-PD-1/PD-L1 nonresponders in the Kim cohort. Similarly, anti-PD-1/CTLA-4 nonresponders in the Riaz cohort expressed BIRC2 mRNA in abundance. The area under the receiver operating characteristic curve values for the Ascierto, Riaz, and Gao cohorts were 0.821, 0.658, and 0.863, respectively, indicating that BIRC2 can distinguish between anti-PD-1 and anti-PD-1/CTLA-4 responders and nonresponders (Figure 8D).
Relationship between BIRC2 or STAT1 expression and clinicopathologic parameters in HCC
| Characteristics | N | BIRC2 | STAT1 | ||||
|---|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | ||
| Gender | |||||||
| Male | 18 | 11 | 7 | 0.152 | 11 | 7 | 0.152 |
| Female | 7 | 2 | 5 | 2 | 5 | ||
| Age (year) | |||||||
| ≤60 | 14 | 8 | 6 | 0.569 | 7 | 7 | 0.821 |
| >60 | 11 | 5 | 6 | 6 | 5 | ||
| MVI | |||||||
| M0 | 23 | 13 | 10 | 0.133 | 13 | 10 | 0.133 |
| M1 | 2 | 0 | 2 | 0 | 2 | ||
| Differentiation degree | |||||||
| Poor | 4 | 1 | 3 | 0.025 | 1 | 3 | 0.025 |
| Moderate | 16 | 9 | 7 | 9 | 7 | ||
| Well | 5 | 3 | 2 | 3 | 2 | ||
| Diameter (cm) | |||||||
| <4 | 14 | 7 | 7 | 0.825 | 6 | 8 | 0.312 |
| ≥4 | 11 | 6 | 5 | 7 | 4 | ||
Bold font indicates significant difference.
The mRNA expression levels of BIRC2 and STAT1 in HCC tissues. (A) The mRNA expression levels of BIRC2 in HCC. (B) The mRNA expression levels of STAT1 in HCC tissues. (C) The relationships between the mRNA expression levels of BIRC2 and STAT1 in HCC tissues.
The association of BIRC2 mRNA expression and the immunotherapeutic response. (A) The relationship between BIRC2 mRNA expression and the different immune subtypes of HCC (TISIDB). (B) The correlation between the expression of immune checkpoint genes PDCD1 (Programmed cell death protein 1, PD-1), CD274 (Programmed cell death protein 1, PD-L1), and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) with the mRNA expression of BIRC2 in HCC tissue (BEST). (C) A comparison in the mRNA expression of BIRC2 in anti-PD-1, anti-PD-1/PD-L1, and anti-PD-1/CTLA-4 responders and nonresponders based on the Asicerto, Kim, and Riaz cohorts (BEST). (D) ROC curve of BIRC2 expression for patients in the Asicerto, Riaz, and Gao cohorts (BEST).
Discussion
IAP family members are frequently overexpressed and associated with poor prognosis in human cancers. Lin et al. demonstrated that miR-143-3p sensitized the response of α7-HPV-related cervical SCC to chemotherapy by targeting BIRC2, an IAP family member associated with the poor prognosis of many cancers. This indicates that BIRC2 may be used as a novel prognostic factor and therapeutic target [9]. Moreover, another IAP family member, XIAP regulates cancer initiation, promotion, and progression. Several drugs targeting XIAP are currently under development, mainly containing small-molecule antagonists (SMAC mimetics) and antisense oligonucleotides (AEG35156) [21]. BIRC5 expression is significantly higher in cancer tissue than in normal tissue in 16 different cancer types [22], and several studies have implicated BIRC5 in chemoresistance to platinum- or taxane-based chemotherapy in ovarian cancer [23, 24]. Additionally, BIRC6 is believed to play an important role in the progression and chemoresistance of several cancers [25-27]. Increased BIRC6 expression in non-small cell lung cancer (NSCLC) is linked to an advanced pathological T stage, poor differentiation, and lymph node metastasis, and may be associated with tumor progression. Therefore, targeting BIRC6 may be useful for the treatment of NSCLC [28]. Although the roles of IAPs in tumor development and progression have been partially confirmed, the specific roles of the IAP family members in HCC remain to be determined. This is the first study to comprehensively explore the mRNA expression levels, prognostic values, functional enrichment, and immunotherapeutic relationships of IAPs in HCC. We explored the differential mRNA expression of each IAP family member in HCC tissue compared to their expression in normal tissue using the TIMER and UALCAN databases. NAIP, BIRC2, BIRC3, XIAP, BIRC5, and BIRC6 mRNAs were overexpressed in HCC cells compared to their expression within normal cells. Another novel finding was that the IAPs were closely associated with the individual clinicopathological stages, tumor grades, and T stage of HCC. These data suggest that IAPs may be associated with HCC progression and that members of the IAP family may act as potential diagnostic markers for HCC. Moreover, the IAP family members exhibited frequent genetic alterations during HCC. Alterations in mRNA expression are among the most frequent mutations. These findings strongly imply that the differential expression of IAP family members may be essential for HCC progression.
Next, we explored the molecular and biological functions of the members of the IAP family, for their capacity to metabolize xenobiotics and other foreign entities as they are eliminated from the body. Cytochrome P450 and other families of drug-metabolizing enzymes were commonly considered and investigated [29]. KEGG pathway analysis of IAP family members and their co-expressing genes revealed that the metabolism of xenobiotics through the cytochrome P450 pathways was significantly enriched, indicating that the members of the IAP family may serve as therapeutic drug targets for HCC. Meanwhile, according to KEGG pathway analyses, complement and coagulation cascades were especially associated with IAPs. The three main outcomes of complement activation are the opsonization of pathogens, recruitment of inflammatory and immune-competent cells, and direct pathogen killing [30]. Our results suggest that IAP-associated signaling is essential for antitumor immunity as it influences the recruitment of immunocompetent cells.
Increasing evidence suggests that immune cell infiltration may significantly affect tumor development and recurrence, and may play a significant role in determining the effectiveness of immunotherapy and clinical outcomes [31, 32]. We discovered a significant relationship between the mRNA expression of IAPs and the infiltration of six different immune cell types: B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils, and DCs. Therefore, we investigated the relationship between the mRNA expression of IAPs and the mRNA expression of immune infiltration markers in patients with HCC; various immune cells demonstrated significant relationships with the mRNA expression of IAPs. Many studies have also found that immune cell infiltration is closely associated with OS [33, 34]. Therefore, we investigated the association between the mRNA expression of IAP family members and the prognosis of patients with HCC. Elevated expression levels of BIRC2 and BIRC5 were associated with worse OS and RFS rates in patients with HCC. When HCC patients were enriched with B cells, macrophages, and Th1 cells, overexpression of BIRC2 and BIRC5 was associated with poor OS rates. These findings imply that BIRC2 and BIRC5 are important oncogenes in HCC. Furthermore, the expression of BIRC2 was significantly correlated with the expression of the Th1 cell marker gene STAT1 and the prognosis of HCC patients enriched with Th1 cells. To further confirm the differential expression of BIRC2 in HCC and its association with immune cells, we collected 25 pairs of paraffin-embedded archived HCC specimens to detect BIRC2 expression and matched them against the BIRC2 expression in adjacent normal tissue samples. BIRC2 expression was considerably upregulated in patients with HCC. BIRC2 expression positively correlated with the expression of the signature marker gene of Th1 cells, STAT1.
BIRC2, a member of the IAP family, also known as cIAP1, serves as a therapeutic target for chemotherapy against various tumors. Zhen et al. discovered that AT406, an IAP antagonist, induced degradation of IAPs (cIAP-1 and XIAP) and exhibited cytotoxic and pro-apoptotic effects on HepG2, SMMC-7721 cell lines, and primary HCC cells [35]. Similarly, Tian et al. found that the small-molecule IAP inhibitor LCL16 targeted cIAP1, cIAP2, and XIAP, which are upregulated in HCC tumors, suggesting that LCL161 may be an effective agent in combination with paclitaxel for treating liver tumors [36]. The first tyrosine kinase inhibitor, sorafenib, was the only drug approved for first-line treatment of HCC. Experimental evidence suggests that sorafenib weakens the translation of c-IAP1 mRNA by targeting its internal ribosome entry site. Additionally, ectopic expression of c-IAP1 alleviates sorafenib-induced cancer cell apoptosis [37]. BIRC2 plays an important role in tumor chemotherapy, and it can also be used as a target for tumor immunotherapy. Cancer immunotherapy, which was recently approved for HCC, has emerged as an effective treatment strategy that targets the immune system [38]. However, tumor immunotherapy is effective only in a small number of individuals with HCC and other solid tumors. Therefore, it is crucial to understand the composition of various immune cell types in the tumor microenvironment and their interactions with the tumor cells. Recent trials have demonstrated that the development of new therapeutic options has improved the survival of patients with HCC [39]. The response rate to immune checkpoint inhibitors (ICIs) or targeted drugs is low. Thus, a combination of different ICIs or immunotherapies with other treatments (targeted and locoregional therapies) is effective for HCC. Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) is a B7/CD28 family member and inhibits T cell function. Ipilimumab, an anti-CTLA-4 blocking antibody, was the first immune checkpoint inhibitor to be tested and has been approved for treating cancer patients. The most widely used immunotherapeutic drugs are PD-1/PD-L1 checkpoint inhibitors. They are antibodies against the membrane receptors PD-1 and PD-L1 and control cell migration, proliferation, and secretion of cytotoxic mediators [40, 41]. A combination of the anti-PD-1 antibody nivolumab and the anti-CTLA-4 antibody ipilimumab has been approved for treating melanoma, RCC, CRC with high microsatellite instability, and non-small cell lung cancer [42]. In the area of HCC, nivolumab plus ipilimumab has been granted accelerated approval by the FDA as a second-line therapy after sorafenib [43], and a phase 3 trial using this combination of drugs is currently underway. Atezolizumab is a humanized monoclonal antibody against PD-L1. The combination of atezolizumab and bevacizumab is currently the preferred first-line therapy for patients with HCC who are not at risk of bleeding [44]. In October 2020, the tremelimumab (a CTLA-4 inhibitor) plus durvalumab (a PD-L1 inhibitor) regimen was the first combination immunotherapy with anti-PD-L1 and anti-CTLA-4 antibodies approved for the treatment of adult patients with unresectable HCC [45]. Samanta et al. demonstrated BIRC2-mediated immune evasion and immune checkpoint blockade (ICB) resistance in cancer cells [46]. They discovered that mouse melanoma and breast tumors were significantly more sensitive to anti-CTLA4 and/or anti-PD1 ICB when there was a deficiency in the expression of BIRC2. In this study, we aimed to determine whether aberrant BIRC2 expression influences the immunotherapeutic response to HCC. We found that BIRC2 is a negative marker for anti-PD-L1/CTLA4 inhibitor therapy in HCC. Our results support the immunosuppressive functions of BIRC2 by demonstrating a strong association between the expression of BIRC2 and the expression of immunological molecules in HCC, which indicates that BIRC2 plays a crucial role in tumor immunity and is a promising biomarker for predicting the prognosis and effectiveness of immunotherapy in patients with HCC.
In summary, this is the first study to analyze the relationship between the mRNA expression of IAPs and tumor immune infiltration in HCC, which may provide a better comprehension on the crucial functions of these genes in the development of tumors and in the immune system of HCC patients. Moreover, we identified the potential of IAPs as useful biomarkers and therapeutic targets that can be used to develop diagnostic and prognostic approaches to improve treatment outcomes for HCC patients. However, this study had significant limitations that need to be considered. To validate the probable processes behind the actions of multiple IAP family members in HCC, as well as the molecular connections between them and the clinical applications of these genes, more studies are required, including both in vitro and clinical studies.
Supplementary Material
Supplementary table.
Acknowledgements
Funding
This study was supported by the National Natural Science Foundation of China (82373062), the China Postdoctoral Science Foundation (2020M672520), the Outstanding Youth Foundation of Hunan Provincial Natural Science Foundation of China (2022JJ20098), the Natural Science Foundation of Hunan Province (2021JJ41013 and 2022JJ40784), the Changsha Municipal Natural Science Foundation (kq2202374), the Central South University Innovation-Driven Research Programme (2023CXQD075) and the Student Innovation Project of Central South University (2022ZZTS0938).
Author Contributions
Xuejie Yang and Xiaoqian Yu conducted experimental operations, sample processing, data analysis, and performed the experiments. All authors participated in writing the paper. Chunlin Ou and Xiaoyun He conceived and designed the experiments. All authors read and approved the final manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: a cancer journal for clinicians. 2023;73:17-48
2. Nie H, Wang Y, Yang X, Liao Z, He X, Zhou J. et al. Clinical Significance and Integrative Analysis of the SMC Family in Hepatocellular Carcinoma. Frontiers in medicine. 2021;8:727965
3. Li X, Zhang Z, Liu M, Fu X, A J, Chen G. et al. Establishment of a lncRNA-Based Prognostic Gene Signature Associated With Altered Immune Responses in HCC. Frontiers in immunology. 2022;13:880288
4. Kumar S, Fairmichael C, Longley DB, Turkington RC. The Multiple Roles of the IAP Super-family in cancer. Pharmacology & therapeutics. 2020;214:107610
5. LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252-75
6. Faruk M, Ibrahim S, Aminu SM, Adamu A, Abdullahi A, Suleiman AM. et al. Prognostic significance of BIRC7/Livin, Bcl-2, p53, Annexin V, PD-L1, DARC, MSH2 and PMS2 in colorectal cancer treated with FOLFOX chemotherapy with or without aspirin. PloS one. 2021;16:e0245581
7. Li F, Aljahdali IAM, Zhang R, Nastiuk KL, Krolewski JJ, Ling X. Kidney cancer biomarkers and targets for therapeutics: survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma. Journal of experimental & clinical cancer research: CR. 2021;40:254
8. Yang J, Yan DM, Xhu LX, Si DM, Liang QH. MiR-5195-3p inhibits the proliferation of glioma cells by targeting BIRC2. European review for medical and pharmacological sciences. 2020;24:267-73
9. Lin CY, Wang CC, Wu RC, Yang LY, Chang CB, Pan YB. et al. Inhibition of BIRC2 Sensitizes α7-HPV-Related Cervical Squamous Cell Carcinoma to Chemotherapy. International journal of molecular sciences. 2021 22
10. Liu L, Wang Q, Wu L, Zhang L, Huang Y, Yang H. et al. Overexpression of POLA2 in hepatocellular carcinoma is involved in immune infiltration and predicts a poor prognosis. Cancer cell international. 2023;23:138
11. He X, Yu B, Kuang G, Wu Y, Zhang M, Cao P. et al. Long noncoding RNA DLEU2 affects the proliferative and invasive ability of colorectal cancer cells. Journal of Cancer. 2021;12:428-37
12. Ou C, Sun Z, He X, Li X, Fan S, Zheng X. et al. Targeting YAP1/LINC00152/FSCN1 Signaling Axis Prevents the Progression of Colorectal Cancer. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2020;7:1901380
13. Wu G, Yan Y, Cai Y, Peng B, Li J, Huang J. et al. ALKBH1-8 and FTO: Potential Therapeutic Targets and Prognostic Biomarkers in Lung Adenocarcinoma Pathogenesis. Frontiers in cell and developmental biology. 2021;9:633927
14. Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M. et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia (New York, NY). 2022;25:18-27
15. Wu P, Heins ZJ, Muller JT, Katsnelson L, de Bruijn I, Abeshouse AA. et al. Integration and Analysis of CPTAC Proteomics Data in the Context of Cancer Genomics in the cBioPortal. Molecular & cellular proteomics: MCP. 2019;18:1893-8
16. Gao J, Tarcea VG, Karnovsky A, Mirel BR, Weymouth TE, Beecher CW. et al. Metscape: a Cytoscape plug-in for visualizing and interpreting metabolomic data in the context of human metabolic networks. Bioinformatics (Oxford, England). 2010;26:971-3
17. Hu J, Qiu D, Yu A, Hu J, Deng H, Li H. et al. YTHDF1 Is a Potential Pan-Cancer Biomarker for Prognosis and Immunotherapy. Frontiers in oncology. 2021;11:607224
18. Yan Y, Liang Q, Liu Y, Zhou S, Xu Z. COL12A1 as a prognostic biomarker links immunotherapy response in breast cancer. Endocrine-related cancer. 2023 30
19. Yang X, Mei C, Nie H, Zhou J, Ou C, He X. Expression profile and prognostic values of GATA family members in kidney renal clear cell carcinoma. Aging. 2023;15:2170-88
20. Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S. et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. Journal of hepatology. 2023;78:770-82
21. Tu H, Costa M. XIAP's Profile in Human Cancer. Biomolecules. 2020 10
22. Fäldt Beding A, Larsson P, Helou K, Einbeigi Z, Parris TZ. Pan-cancer analysis identifies BIRC5 as a prognostic biomarker. BMC cancer. 2022;22:322
23. Chen L, Liang L, Yan X, Liu N, Gong L, Pan S. et al. Survivin status affects prognosis and chemosensitivity in epithelial ovarian cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2013;23:256-63
24. Du J, Li B, Fang Y, Liu Y, Wang Y, Li J. et al. Overexpression of Class III β-tubulin, Sox2, and nuclear Survivin is predictive of taxane resistance in patients with stage III ovarian epithelial cancer. BMC cancer. 2015;15:536
25. Chen Z, Naito M, Hori S, Mashima T, Yamori T, Tsuruo T. A human IAP-family gene, apollon, expressed in human brain cancer cells. Biochemical and biophysical research communications. 1999;264:847-54
26. Lopergolo A, Pennati M, Gandellini P, Orlotti NI, Poma P, Daidone MG. et al. Apollon gene silencing induces apoptosis in breast cancer cells through p53 stabilisation and caspase-3 activation. British journal of cancer. 2009;100:739-46
27. Sung KW, Choi J, Hwang YK, Lee SJ, Kim HJ, Lee SH. et al. Overexpression of Apollon, an antiapoptotic protein, is associated with poor prognosis in childhood de novo acute myeloid leukemia. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:5109-14
28. Dong X, Lin D, Low C, Vucic EA, English JC, Yee J. et al. Elevated expression of BIRC6 protein in non-small-cell lung cancers is associated with cancer recurrence and chemoresistance. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013;8:161-70
29. Foti RS. Cytochrome P450 and Other Drug-Metabolizing Enzymes As Therapeutic Targets. Drug metabolism and disposition: the biological fate of chemicals. 2023;51:936-49
30. Yanase Y, Takahagi S, Ozawa K, Hide M. The Role of Coagulation and Complement Factors for Mast Cell Activation in the Pathogenesis of Chronic Spontaneous Urticaria. Cells. 2021 10
31. Li B, Chen H, Yang S, Chen F, Xu L, Li Y. et al. Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers. Molecular cancer. 2023;22:71
32. Han Y, Wang D, Peng L, Huang T, He X, Wang J. et al. Single-cell sequencing: a promising approach for uncovering the mechanisms of tumor metastasis. Journal of hematology & oncology. 2022;15:59
33. Yang J, Li H, Hu S, Zhou Y. ACE2 correlated with immune infiltration serves as a prognostic biomarker in endometrial carcinoma and renal papillary cell carcinoma: implication for COVID-19. Aging. 2020;12:6518-35
34. Nie H, Wu Y, Ou C, He X. Comprehensive Analysis of SMC Gene Family Prognostic Value and Immune Infiltration in Patients With Pancreatic Adenocarcinoma. Frontiers in medicine. 2022;9:832312
35. Zhen MC, Wang FQ, Wu SF, Zhao YL, Liu PG, Yin ZY. Identification of mTOR as a primary resistance factor of the IAP antagonist AT406 in hepatocellular carcinoma cells. Oncotarget. 2017;8:9466-75
36. Tian A, Wilson GS, Lie S, Wu G, Hu Z, Hebbard L. et al. Synergistic effects of IAP inhibitor LCL161 and paclitaxel on hepatocellular carcinoma cells. Cancer letters. 2014;351:232-41
37. Li XF, Gong RY, Wang M, Yan ZL, Yuan B, Wang K. et al. Sorafenib down-regulates c-IAP expression post-transcriptionally in hepatic carcinoma cells to suppress apoptosis. Biochemical and biophysical research communications. 2012;418:531-6
38. Ruf B, Heinrich B, Greten TF. Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells. Cellular & molecular immunology. 2021;18:112-27
39. Okusaka T, Morimoto M, Eguchi Y, Nakamura S, Iino S, Kageyama R. A Phase I Study to Investigate the Safety, Tolerability and Pharmacokinetics of Napabucasin Combined with Sorafenib in Japanese Patients with Unresectable Hepatocellular Carcinoma. Drugs in R&D. 2023;23:99-107
40. Dibajnia P, Cardenas LM, Lalani AA. The emerging landscape of neo/adjuvant immunotherapy in renal cell carcinoma. Human vaccines & immunotherapeutics. 2023;19:2178217
41. Longuespée R, Theile D, Zörnig I, Hassel JC, Lindner JR, Haefeli WE. et al. Molecular prediction of clinical response to anti-PD-1/anti-PD-L1 immune checkpoint inhibitors: New perspectives for precision medicine and mass spectrometry-based investigations. International journal of cancer. 2023;153:252-64
42. Kudo M. Durvalumab Plus Tremelimumab: A Novel Combination Immunotherapy for Unresectable Hepatocellular Carcinoma. Liver cancer. 2022;11:87-93
43. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B. et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA oncology. 2020;6:e204564
44. Jost-Brinkmann F, Demir M, Wree A, Luedde T, Loosen SH, Müller T. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma: Results from a German real-world cohort. Alimentary pharmacology & therapeutics. 2023;57:1313-25
45. Keam SJ. Tremelimumab: First Approval. Drugs. 2023;83:93-102
46. Samanta D, Huang TY, Shah R, Yang Y, Pan F, Semenza GL. BIRC2 Expression Impairs Anti-Cancer Immunity and Immunotherapy Efficacy. Cell reports. 2020;32:108073
Author contact
![]() Corresponding authors: Xiaoyun He. Department of Pathology, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China. Email: hexiaoyunedu.cn; Chunlin Ou. Department of Pathology, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China. Email: ouchunlinedu.cn.
Corresponding authors: Xiaoyun He. Department of Pathology, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China. Email: hexiaoyunedu.cn; Chunlin Ou. Department of Pathology, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China. Email: ouchunlinedu.cn.

 Global reach, higher impact
Global reach, higher impact