Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(16):2998-3008. doi:10.7150/jca.87618 This issue Cite
Research Paper
Tumor stroma Siglec15 expression is a poor prognosis predictor in colon adenocarcinoma
1. Department of Oncology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
2. Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Disease, Guangdong Research Institute of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
3. Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
* These authors have contributed equally to this work.
Received 2023-6-29; Accepted 2023-9-7; Published 2023-9-18
Abstract
Sialic acid binding Ig-like lectin 15 (Siglec15) is considered a novel immune checkpoint and an emerging target for next-generation cancer immunotherapy. However, the significance of Siglec15 and its relationship with programmed death-ligand 1 (PD-L1) in colon adenocarcinoma (COAD) remain unknown. In this study, we analyzed Siglec15 expression within stromal area (SA) and tumor area (TA), and its relationship with tumor-infiltrating lymphocytes (TILs) in COAD and mismatch repair-proficient (MMR-p) COAD. Siglec15 expression was significantly higher in COAD tissues than in normal tissues, and elevated Siglec15(SA) expression, rather than Siglec15(TA) and Siglec15 (whole) expression, was correlated with poor prognosis and inversely correlated with the density of CD8+ T cell, both in COAD and MMR-p COAD. Moreover, there were no correlations between Siglec15(SA) and PD-L1(SA), and between Siglec15(TA) and PD-L1(TA), whereas there was positive correlation between Siglec15(whole) and PD-L1(whole). A new immune classification based on the Siglec15(SA)/PD-L1(SA) expression, indicated that patients with Siglec15(SA)Low/PD-L1(SA)+ status had the longest survival times in COAD. Our study highlights that Siglec15(SA) is an independent predictor of poor prognosis and has an immunosuppressive role in COAD and MMR-p COAD tissues. These findings may provide insights into improving responses to immunotherapy-included comprehensive treatments for COAD in the future.
Keywords: Siglec15, PD-L1, immune checkpoint, immunotherapy, colon adenocarcinoma
Introduction
Colorectal cancer (CRC) is the third most common cancer and second most frequent cause of cancer-related deaths worldwide[1]. CRC is classified into two major groups: mismatch repair-deficient (MMR-d) and mismatch repair-proficient (MMR-p). Treatments of CRC include surgery, chemotherapy, radiotherapy and emerging immunotherapy[2]. Immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1) and anti-programmed cell death ligand 1 (PD-L1) have exhibited a durable response and currently dominate the method of treatment for various tumor types[3-5]. However, anti-PD-1/PD-L1 therapy is considered ineffective in most patients with CRC, and only those with MMR-d tumors and a high tumor mutational burden (TMB) have been found to be responsive to anti-PD-1/PD-L1 therapy[6-9]. Most CRC patients with MMR-p, which comprise approximately 95% of metastatic CRC cases, often do not benefit from current immunotherapy approaches. Thus, a predictive immune biomarker is urgently needed to be identified, especially in MMR-p CRC.
Sialic acid-binding immunoglobulin-like lectin 15 (Siglec15), a member of the sialic acid-binding immunoglobulin-like lectin family, is one of the most evolutionarily conserved Siglecs in vertebrates and is phylogenetically distant from other family members[10]. Siglec15 is originally found to be overexpressed in giant cell tumors of the bone and was discovered to regulate osteoclast differentiation and bone remodeling through interaction with the signaling adaptor DAP12[11-14]. Recent research has found that Siglec15 is also broadly upregulated in human tumor area and tumor infiltrating myeloid cells, and its expression is mutually exclusive to PD-L1. Siglec15 is considered a novel anti-tumor target comparable to PD-L1, and has the ability to sustainably suppress T-cell responses and elicit immune evasion in the tumor microenvironment[15]. Therefore, Siglec15 is considered a promising new target for normalization cancer immunotherapy independent of the PD-1/PD-L1 pathway, and targeting Siglec15 may be an effective alternative therapy for patients who do not respond to PD-1/PD-L1 antibodies [16-18].
However, the significance of Siglec15 in cancer is uncertain. High Siglec15 levels are associated with poor prognosis in lung adenocarcinoma (LUAD) and kidney cancer, but are associated with better prognoses in bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), head and neck squamous cell carcinoma (HNSC), thyroid cancer (THCA) and uterine corpus endometrial carcinoma (UCEC) [19-23]. The relationship between Siglec15 and the immune microenvironment in colon adenocarcinoma (COAD), especially MMR-p COAD, has not been elucidated.
Moreover, PD-L1 expression is related to T-cell subpopulations in CRC immune microenvironment, and the PD-1/PD-L1 axis is considered a clinically relevant mediator of tumor immune escape[24-26]. Meanwhile, PD-L1 can enable tumor cells to evade immune elimination by negatively regulating T-cell immune responses[27]. The relationship between Siglec15 and PD-L1 in COAD remains unclear.
Here, we evaluated the relationship between Siglec15 expression (in the stromal area (SA), tumor area (TA) or whole cells), clinicopathological characteristics, and the immune microenvironment in patients with COAD. We found that Siglec15 was significantly higher expression in COAD than normal intestinal tissue, and Siglec15(SA)High, rather than Siglec15(TA)High and Siglec15(whole)High, correlated with poor prognosis. Notably, Siglec15(SA) served as an immune suppressor by suppressing CD8+ T cell, but not CD4+ T cell in COAD and MMR-p COAD. Besides, there were no correlations between Siglec15(SA) and PD-L1(SA), and between Siglec15(TA) and PD-L1(TA), whereas there was positive correlation between Siglec15(whole) and PD-L1(whole). A new immune classification, based on the expressions of Siglec15(SA)/PD-L1(SA), indicating that patients with Siglec15(SA)Low/PD-L1(SA)+ status had the longest survival. Our study may provide new insights into patients with COAD or MMR-p COAD to improve the ability to select the appropriate immunotherapy.
Material and methods
Patients and samples
Our clinical cohort was based on a tissue microarray (TMA) purchased from Shanghai Outdo Biotech. Patients were eligible for inclusion if they had histologically confirmed colon adenocarcinoma, and without any previous systemic anticancer therapy for colon cancer disease, and underwent initial resections. Paraffin-embedded pathological specimens were obtained from 102 patients with COAD, including 76 matched adjacent normal tissues. Patients were collected from July 2006 to May 2007, with 10-year follow-ups. Clinicopathological variables included general information, tumor location, tumor growth pattern, degree of differentiation, depth of tumor invasion, nodal status, metastatic status, mismatch repair (MMR) status, and the outcome of follow-up data. Tumor pT, pN, and pM statuses were assessed according to the criteria of the Seventh Edition of the American Joint Committee on Cancer (AJCC) staging standards. The overall survival (OS) was defined as the time from the date of surgery to the date of death.
Mismatch repair (MMR) status
Samples were stratified according to DNA MMR status, as described previously[28]. Briefly, paraffin sections were baked overnight, deparaffinized in xylene, rehydrated through graded ethanol, quenched for endogenous peroxidase activity in hydrogen peroxide and incubated with primary antibodies. Then, the sections were incubated with the primary antibody overnight and stained with diaminobenzidine. MMR-proficient tumors were defined as those simultaneously expressing MutL homolog 1 (MLH1), MutS homolog 2 (MSH2), PMS1 Homolog 2 (PMS2) and MutS homolog 6 (MSH6), whereas MMR-deficient tumors were defined as those lacking the expression of at least one of these markers.
Immunohistochemistry
We constructed a tissue microarray (TMA) from colon cancer blocks and performed an immunohistochemistry (IHC) analysis. IHC staining was performed according to the standard protocols. Siglec15 rabbit antibody (1:500; Novus; NBP2-41162), PD-L1 rabbit antibody (1:50; Cell Signaling Technology; CST #13684), CD8 rabbit antibody (1:50; Cell Signaling Technology; CST#85336) and CD4 (ZSGB-BIO; ZM-0418) were used for immunostaining. To score tumor cells as positive, both nuclear and cytoplasmic staining were counted. For quantitative analysis, the histochemistry score (H-score) was calculated based on the staining intensity and percentage of stained cells using the HALO image analysis platform. The intensity score was defined as follows: 0, no appreciable staining in cells; 1, weak staining in cells comparable to that in stromal cells; 2, intermediate staining; and 3, strong staining. The fraction of positive cells was scored as 0%-100%. The H-score was calculated by multiplying the intensity and fraction scores using the following formula:
[1× (% cells 1+) + 2× (% cells 2+) + 3× (% cells 3+)]
with a total range of 0-300. Tissue sections were examined and scored separately by two independent investigators who were blind to the clinicopathological data.
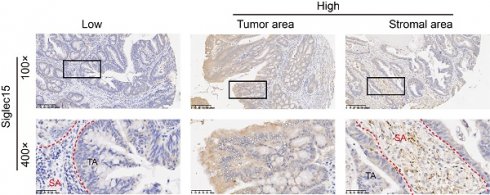
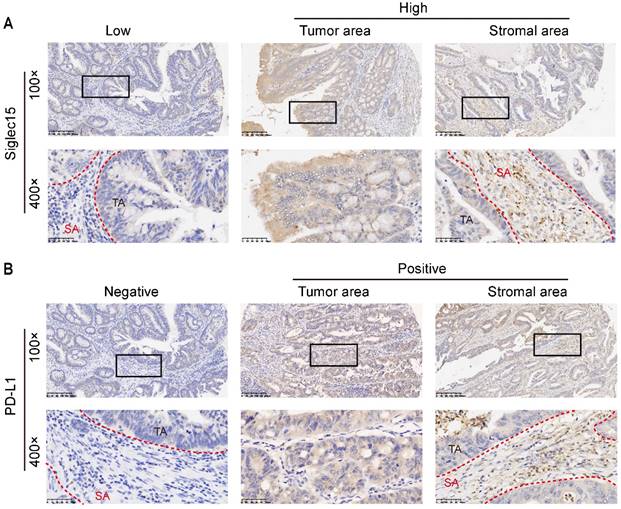
The Siglec15 and PD-L1 expression levels on TA and SA were evaluated by H-score. Siglec15 expression was evaluated based on immunostaining in the cytoplasm and membrane of cells, and the expression level (intensity) was scored as 0 (absent), 1 (weak), 2 (intermediate) or 3 (strong). The expression of Siglec15 was evaluated in the whole tumor tissue (whole), TA, SA cells on stained sections (Figure S1), and the median H-score was chosen as the cut-off value to define as high and low (<70, Siglec15(whole)Low and ≥70, Siglec15(whole)High; <120, Siglec15(TA)Low and ≥120, Siglec15(TA)High; <35, Siglec15(SA)Low and ≥35, Siglec15(SA)High; respectively). When detecting Siglec15 expression in SA, we analyzed all kinds of cells in stromal without selection (the green region). To some extent, we explored Siglec15 expression in TA and tumor infiltrating myeloid cells, respectively. Here, we are unable to distinguish the type of cells in stromal region without staining specified markers. The expression of PD-L1 was also evaluated in the whole tumor tissue (whole), TA, SA cells, and was defined as positive when PD-L1 staining was present on ≥1% of cells [26, 29]. For CD8 and CD4 evaluation, the number of CD8+ or CD4+ T cell in SA was counted, and the density (cells/mm2) of each T-cell population in the SA was determined.
Statistical analysis
Correlation analyses were performed using the Student's t-test and χ2 test, whereas survival analysis was performed using the Kaplan-Meier method to depict the survival curves of OS. A log-rank test was performed to examine intergroup differences. Univariate and multivariate analyses were performed using the Cox proportional hazard model. All statistical analyses were accomplished by SPSS software (version 26.0) and GraphPad Prism 8 software (La Jolla, California, USA). Statistical significance was set at p < 0.05. * p < 0.05, ** p < 0.01, and *** p < 0.001.
Results
Association of Siglec15 expression with the clinicopathological characteristics
The clinicopathological characteristics of the COAD cohort were illustrated (Table S1). In this study, the median age of all COAD patients was 68 years (range from 24-90 years) and 58 (56.9 %) and 44 (43.1 %) were male and female, respectively. The median overall survival (OS) time was 51 months (range from 1-108 months) and 61 patients died during the follow-up.
As Siglec15 is broadly upregulated in human cancer cells and tumor infiltrating myeloid cells, we detected the Siglec15 expression in TA, SA and whole cells. The same method was used to detect the PD-L1 expression in colon cancer (Figure 1). The positive rates of Siglec15 expression were 55.9% (57/102 in SA), 65.7% (67/102 in TA) and 58.8% (60/102 in whole), respectively (Table 1 and Supplementary Table S1).
Next, we explored the association between Siglec15 expression and clinicopathological characteristics. No significant associations were found between Siglec15 expression and clinicopathological characteristics, such as age, gender, tumor location, TNM stage, MMR status, and tumor growth pattern, excluding that Siglec15(SA) was correlated with age (p<0.05) (Table 1).
Prognostic significance of Siglec15 and PD-L1 on the overall survival
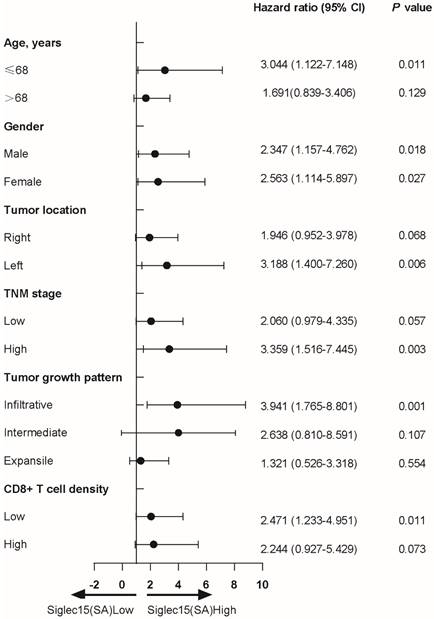
The univariate Cox regression analyses of patients revealed that age (HR=1.947, 95% CI: 1.152 to 3.291, p<0.01), TNM stage (HR=2.910, 95% CI: 1.746 to 4.850, p<0.001), PD-L1(SA) (HR=0.493, 95% CI: 0.278 to 0.874, p=0.015) and Siglec15(SA) (HR=2.481, 95% CI: 1.450 to 4.245, p<0.01) expression were associated with prognosis of COAD patients in terms of OS (Table 2). Multivariate Cox regression analysis indicated that only TNM stage (HR=3.059, 95% CI: 1.799 to 5.200, p<0.001), PD-L1(SA) (HR=0.352, 95% CI: 0.193 to 0.642, p=0.001) and Siglec15(SA) (HR=3.264, 95% CI: 1.808 to 5.890, p<0.001) expression were independent prognostic factors for OS in COAD patients, but not Siglec15(whole) and Siglec15(TA) (Table 2).
Siglec15 and PD-L1 expression in COAD cancer samples. Representative micrographs of Siglec15 (A) and PD-L1 (B) expression within the stromal area (SA) and tumor area (TA). Representative images are presented in 100× (upper) and 400× (below).
The association of Siglec15 expression with clinicopathological characteristics
| Siglec15(whole) | Siglec15(TA) | Siglec15(SA) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Low | High | p value | Low | High | p value | Low | High | p value | |
| Age (median 68) | 0.6185 | 0.6739 | 0.0064 | |||||||
| ≤68 | 48 | 21 | 27 | 16 | 32 | 28 | 20 | |||
| >68 | 54 | 21 | 33 | 19 | 35 | 17 | 37 | |||
| Gender | 0.1148 | 0.5718 | 0.5225 | |||||||
| Male | 58 | 20 | 38 | 19 | 39 | 24 | 34 | |||
| Female | 44 | 22 | 22 | 16 | 28 | 21 | 23 | |||
| Tumor location | 0.9433 | 0.8886 | 0.5185 | |||||||
| Right | 49 | 20 | 29 | 18 | 31 | 20 | 29 | |||
| Left | 53 | 22 | 31 | 17 | 36 | 25 | 28 | |||
| Tumor growth pattern | 0.5059 | 0.442 | 0.5355 | |||||||
| Expansile | 33 | 16 | 17 | 12 | 21 | 12 | 21 | |||
| Intermediate | 24 | 8 | 16 | 7 | 17 | 11 | 13 | |||
| Infiltrative | 45 | 18 | 27 | 16 | 29 | 22 | 23 | |||
| TNM stage | 0.4216 | 0.6656 | 0.3654 | |||||||
| Low | 63 | 24 | 39 | 22 | 41 | 30 | 33 | |||
| High | 39 | 18 | 21 | 13 | 26 | 15 | 24 | |||
Next, we analyzed the protein expression of Siglec15 in patients with COAD. Compared to adjacent normal tissue, Siglec15 expression was significantly higher in tumors (Figure 2A). Kaplan-Meier analysis also suggested that Siglec15(SA)High was significantly associated with poor OS (p<0.001), but not Siglec15(whole)High and Siglec15(TA)High (Figure 2B). We also examined the relationship of Siglec15(SA) expression levels with clinicopathological characteristics. Siglec15(SA) expression were poor prognosis for patients—under 68 years old, male, left side tumor, high stage, infiltrative tumor, and low CD8+ T cell density (Table 3).
Univariate and multivariate Cox proportional hazards analysis of OS in COAD patients
| Variables | Univariable OR (95% CI) | p Value | Multivariable OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (>68/≤68) | 1.947 (1.152 to 3.291) | 0.013 | 1.502 (0.869 to 2.595) | 0.145 |
| Gender (Male/Female) | 1.182 (0.707 to 1.977) | 0.524 | ||
| Tumor location (Right/Left) | 1.560 (0.942 to 2.584) | 0.084 | ||
| Tumor growth pattern | ||||
| Infiltrative/Expansile | 1.335 (0.664 to 2.685) | 0.418 | ||
| Intermediate/Expansile | 1.448 (0.750 to 2.797) | 0.270 | ||
| TNM stage (High/Low) | 2.910 (1.746 to 4.850) | <0.0001 | 3.059 (1.799 to 5.200) | <0.0001 |
| CD8+ T cell density (High/Low) | 0.736 (0.431 to 1.256) | 0.261 | ||
| PD-L1 (SA) (Positive/Negative) | 0.493 (0.278 to 0.874) | 0.015 | 0.352 (0.193 to 0.642) | 0.001 |
| PD-L1 (TA) (Positive/Negative) | 0.996 (0.563 to 1.763) | 0.989 | ||
| PD-L1 (whole) (Positive/Negative) | 0.782 (0.455 to 1.345) | 0.374 | ||
| Siglec15 (SA) (High/Low) | 2.481 (1.450 to 4.245) | 0.001 | 3.264(1.808 to 5.890) | <0.0001 |
| Siglec15 (TA) (High/Low) | 1.164 (0.682 to 1.987) | 0.578 | ||
| Siglec15 (whole) (High/Low) | 1.455 (0.862 to 2.456) | 0.160 |
Cox regression analysis to assess the relationship of Siglec15(SA) expression levels with clinicopathological characteristics
Prognostic significance of Siglec15 and PD-L1 expression on prognosis in COAD. (A) H-score of Siglec15 in the primary human COAD tissue microarray and adjacent normal tissues. Data are means ± SEM. p-values are according to Student's t-test. (B) Overall survival curves were generated based on the protein levels of Siglec15 in stromal area, tumor area and whole cells of COAD. (C) H-score of PD-L1 in the primary human COAD tissue microarray and adjacent normal tissues. Data are means ± SEM. p-values are according to Student's t-test. (D) Overall survival curves were generated based on the protein levels of PD-L1 in stromal area, tumor area and whole cells of COAD. p-values are according to Kaplan-Meier plots and are compared to the log-rank test.
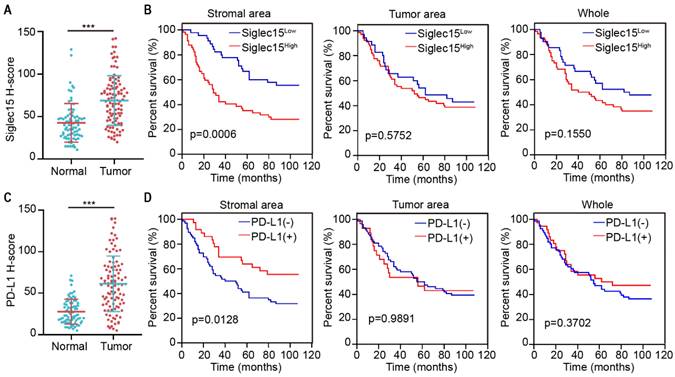
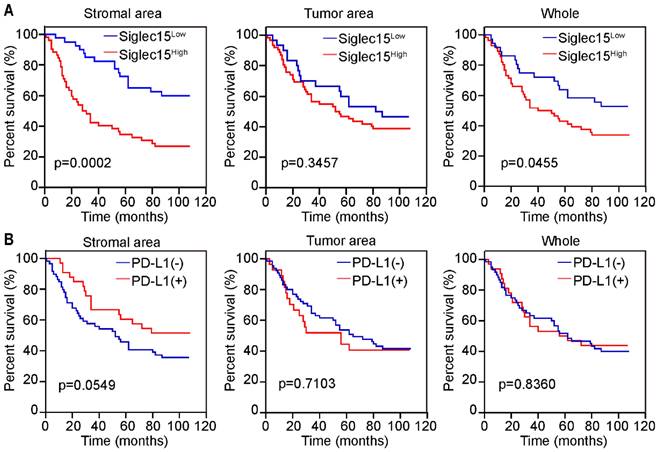
PD-L1 expression was significantly higher in the tumor tissues than in the normal tissues (Figure 2C). Kaplan-Meier analysis suggested that patients with PD-L1(SA)+ had longer OS than those with PD-L1(SA)-; however, there were no significant differences in survival between patients with PD-L1(whole)+ and those with PD-L1(whole)-, or between patients with PD-L1(TA)+ and those with PD-L1(TA)- (Figure 2D).
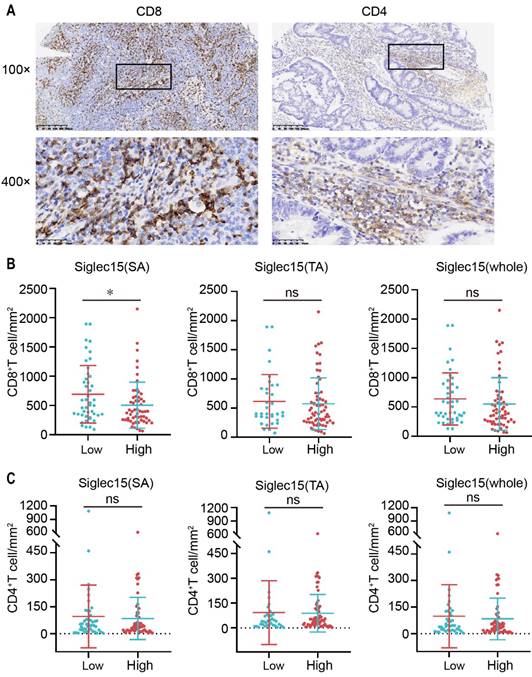
Relationship between Siglec15 or PD-L1 and infiltration of T cells in COAD
As the Siglec15 and PD-L1 expression levels influence the tumor microenvironment [15, 30], we were curious regarding CD4+ and CD8+ tumor infiltrating lymphocytes (TILs) in COAD (Figure 3A). To assess the number of CD4+ and CD8+ lymphocytes, we classified tissues into TA and SA and counted the number of CD4+ and CD8+ TILs in SA, which were classified to be positive or negative (Figure S1B). As shown, the density of CD8+ TILs was inversely associated with the expression level of Siglec15(SA), but was not significantly associated with the expression levels of Siglec15(TA) and Siglec15(whole) in COAD (Figure 3B). Regarding CD4+ TILs, there was no significant association between them and the Siglec15 expression level (Figure 3C). In addition, the densities of CD4+ and CD8+ TILs were not associated with PD-L1 expression (Figure S2, A and B).
Significance of Siglec15 and PD-L1 in the MMR-proficient COAD
Previous studies have indicated that patients with MMR-p COAD could not benefit from immunotherapy; therefore, we were curious regarding the role of Siglec15 and PD-L1 in MMR-p colon cancer. Kaplan-Meier analyses revealed that patients with high Siglec15(SA) levels had a significantly shorter OS (p<0.05) than those with low Siglec15(SA) levels in MMR-p COAD. Notably, Siglec15(whole), a factor without a significant prognostic value in all patients with COAD, showed a significant predictive value for the survival of patients with MMR-p COAD (Figure 4A). All types of PD-L1 expression status were not correlated with the survival of patients with MMR-p COAD (Figure 4B).
Association of Siglec15 and PD-L1 in COAD
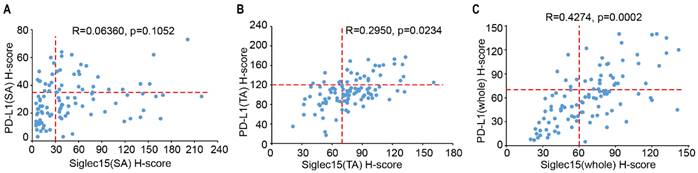
As Siglec15 is mutually exclusive to PD-L1, we analyzed the association between Siglec15 and PD-L1 expression in TA, SA and whole cells. In 102 samples, there was no correlation between Siglec15(SA) and PD-L1(SA) expression (R=0.06360, p=0.1052) (Figure 5A), with 12 of the Siglec15(SA)Low patients (33.3%) being PD-L1(SA)+, and 33 of the PD-L1(SA)- patients (50.0%) being Siglec15(SA)High. Similarly, there was no correlation between Siglec15(TA) and PD-L1(TA) expression (R=0.2950, p=0.0234) (Figure 5B). However, there was positive correlations between Siglec15(whole) and PD-L1(whole) expression (R=0.4274, p=0.0002) (Figure 5C). These data suggested that there were no correlations between Siglec15(SA) and PD-L1(SA), and between Siglec15(TA) and PD-L1(TA) in COAD, and there was positive correlation between Siglec15(whole) and PD-L1(whole).
Immune classification of Siglec15 and PD-L1 in COAD
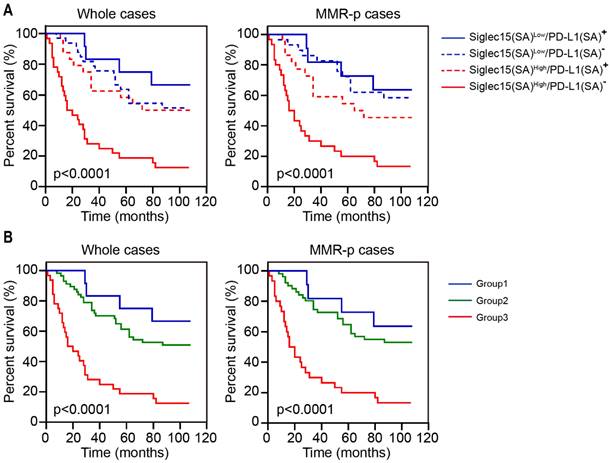
Given that Siglec15(SA) and PD-L1(SA) were significantly associated with OS, we explored the expression of Siglec15(SA) and PD-L1(SA) and analyzed their association with the survival of patients with COAD. Kaplan-Meier analysis demonstrated that patients with Siglec15(SA)Low/PD-L1(SA)+ had the best prognosis and those with Siglec15(SA)High/PD-L1(SA)- had the worst prognosis, both in MMR-p COAD and the whole COAD tissue (Figure 6A). Next, we classified the patients into three groups: group 1 (Siglec15(SA)Low/PD-L1(SA)+), group 2 (Siglec15(SA)Low/PD-L1(SA)- or Siglec15(SA)High/PD-L1(SA)+), group 3 (Siglec15(SA)High/PD-L1(SA)-), and group 1 was with low risk, group 2 with moderate risk and group 3 with high risk, both in MMR-p COAD and the whole COAD (Figure 6B).
Relationship between Siglec15 expression and the infiltration of CD4+/CD8+ TILs. (A) Representative micrographs of CD8 (left) and CD4 (right) expression in the COAD. Representative images are presented in 100× (upper) and 400× (below). (B and C) The correlation of Siglec15 expression and CD8+ T cells (B) and CD4+ T cells (C) in the COAD. Data are means ± SEM. p-values are according to Student's t-test.
Prognostic significance of Siglec15 and PD-L1 in MMR-p COAD. (A and B) Prognostic significance of Siglec15 (A) and PD-L1 (B) in stromal area, tumor area and whole cells of MMR-p COAD, respectively. p-values are according to Kaplan-Meier plots and compared to the log-rank test.
The association of Siglec15 and PD-L1 in COAD. (A to C) The correlation between Siglec15 and PD-L1 expression was assessed in stromal area (A), tumor area (B) and whole cells (C) on COAD tissue microarray. The Pearson R scores and p-values are shown.
Discussion
Siglec15, a novel immune checkpoint molecule, is an emerging target for next-generation cancer immunotherapy. In this study, we first found that Siglec15 protein levels were higher in tumor tissues than in normal tissues, and elevated Siglec15(SA)— but not Siglec15(TA) and Siglec15(whole)—correlated with poor prognosis in COAD and MMR-p COAD and served as an immune suppressor by suppressing CD8+ T cell, but not CD4+ T cell. In addition, there were no correlations between Siglec15 and PD-L1 in SA and TA, whereas Siglec15 was correlated with PD-L1 in whole COAD tissues.
Based on pan-cancer analysis from The Cancer Genome Atlas database, Siglec15 was upregulated across most cancer types, including COAD[23], which was consistent with our results by detecting protein expression. Considering Siglec15 is expressed in tumor cells and tumor infiltrating myeloid cells, we detected Siglec15 in SA, TA and whole cells. Our results showed that Siglec15(SA)High, rather than Siglec15(TA)High and Siglec15(whole)High, were correlated with poor prognosis.
Furthermore, the protein level of Siglec15 was positively correlated with that of PD-L1 in TA and whole cells. Siglec15 is broadly expression on human cancer cells and tumor infiltrating myeloid cells, and it has been reported that SIGLEC15+ tumor-associated macrophage, rather than SIGLEC15+ PDAC cells, correlated with poor prognosis in PDAC[31]. Different cells expressing Siglec15 may execute different functions, and it's important to classify Siglec15 expression. Tumor Siglec15 can be downregulated by IFN-γ, which is the dominant cytokine required for PD-L1 induction[15]. Moreover, it was reported that higher Siglec15 expression in EGFR-mutant lung cancers and EGFR-mutant lung cancer cells induced the expression of PD-L1[32, 33]. These contradictory results in different types of cancer reveal that the complexity and heterogeneity in tumors and that Siglec15 and PD-L1 may be simultaneously regulated by other molecules in COAD; this line of research needs to be further investigated in future studies.
Survival analysis for patients with COAD based on Siglec15 and PD-L1 expression in stromal area. (A) Kaplan-Meier survival curves for overall survival based on the expression status of Siglec15(SA)/PD-L1(SA) in COAD and MMR-p COAD. (B) Kaplan-Meier survival curves for OS in three groups: group 1, Siglec15(SA)Low /PD-L1(SA)+; group 2, Siglec15(SA)Low/PD-L1(SA)- or Siglec15(SA)High/PD-L1(SA)+ and group 3, Siglec15(SA)High/PD-L1(SA)-. p-values using Kaplan-Meier plots and compared with the log-rank test.
In addition, the expression of PD-L1 in TA or whole cells was not correlated with COAD patients' prognosis, which is consistent with previous large sample study[26]. In accordance with a previous study[34], patients with PD-L1(SA)+ had longer survival in COAD in our study. However, PD-L1(SA) expression was not correlated with MMR-p COAD patient prognosis, whereas Siglec15(SA)High was correlated with poor prognosis in MMR-p COAD. These data indicated that Siglec15(SA) may be an immune biomarker of MMR-p COAD. MMR-P COAD is considered as immune desert tumor, and several clinical trials have revealed that no responses were observed among the patients with MMR-p tumors treated with PD-L1 inhibitors. Similarly, Siglec15 was also overexpression in immune desert tumor—sarcoma, and was involved in immune-related pathways and predicted poor prognosis[23]. Our results indicated that Siglec15 may be an immune predicative biomarker in MMR-p COAD and combining Siglec15 antibody with PD-1/PD-L1 antibodies may benefit MMR-p COAD patients, which require further investigation and clinical trials to confirm.
Currently, the relationship between Siglec15 expression and immune cell infiltration in different types of cancers remains uncertain. Chen et al. demonstrated that Siglec15 on macrophages/myeloid cells suppresses CD8+ T cell responses[15]. Yang et al. reported that Siglec15 is significantly associated with Treg infiltration in cancers and positively associated with Foxp3 in LUAD[23]. Chen et al. found that Siglec15 expression in tumor cells was negatively correlated with the density of CD45RO T cells and Tregs and had no effect on the density of macrophage in PDCA[22]. Zu et al. reported that Siglec15 was negatively correlated with the infiltration levels of CD8+ T cells, natural killer cells, macrophages, type 1 T helper cells, and dendritic cells in bladder cancer[21]. In CRC sentinel lymph nodes, Siglec15 expression suppressed T-cell responses[35]. In this study, we found that Siglec15(SA)High was significantly associated with a low density of CD8+ TILs, but not with the infiltration of CD4+ TILs in COAD. Our data suggested that Siglec15(SA) expression suppressed immune by reducing CD8+ TILs in COAD. Similar phenomena were observed in MMR-p COAD. Interestingly, we observed a higher density of CD4+ and CD8+ T cells in normal tissues than in tumors (Figure S4). Siglec15(SA) may play a role in immunosuppression in MMR-p COAD by inhibiting T-cell proliferation. A possible mechanism is that Siglec15(SA)High cells are continuous secreting some inhibitory cytokines inhibiting T-cell proliferation, which needs further study. If the treatment with Siglec15 antibody could recruit more CD8+ T cells to the tumor, it may convert the MMR-p COAD from a “cold” to a “hot” tumor.
However, there are also some limitations in our study. First, it was a retrospective study in a medium sample size and a single central cohort; thus, selection bias was inevitable, which may limit the generalizability of results. Second, we evaluated the Siglec15 and PD-L1 expression and T-cell markers such as CD4 and CD8 using TMA IHC and focused on information within SA, which may have ignored some other information regarding the tumor. Therefore, clinical data from multicenter and prospective studies are required for further research.
In conclusion, this study showed that Siglec15 expression in COAD tissues was higher than in normal tissues, and high Siglec15 expression in SA, rather than in TA and whole cells, indicated poor prognosis by suppressing immune responses by reducing the density of CD8+ TILs in COAD and MMR-p COAD. These results may provide new insights into patients with COAD or MMR-p COAD when choosing appropriate immunotherapy.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This work was supported by the National Nature Science Foundation in China (NSFC) (81974369 and 82103080), the National Postdoctoral Program for Innovative Talents (BX2021392), the China Postdoctoral Science Foundation (2021M693652), the Province Natural Science Fund of Guangdong (2021A1515010568) and the program of Guangdong Provincial Clinical Research Center for Digestive Diseases (2020B1111170004).
Ethics Statement
Approval of the research protocol by an Institutional Reviewer Board: The human tissue specimen research was approved by the institutional ethics committee of Shanghai Outdo Biotech Co, Shanghai, China (Exp. number: YB M-05-02).
Informed Consent: All patients signed an informed consent before surgery that permitted the usage of resected tumors and clinical profiles in research, under the condition of anonymity.
Author Contributions
Conception and design: Y-HD and W-XZ. Performing experiments: W-XZ, FB, YC. Drafting of the article: W-XZ. Acquisition and interpretation of data: W-XZ, YC, FB, J-WZ, GQ, Y-QX; Review, editing and approval of the manuscript: all authors.
Data Availability Statement
Data are available upon reasonable request. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel R L. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249
2. Dekker E, Tanis P J, Vleugels J L A. et al. Colorectal cancer. Lancet. 2019;394:1467-1480
3. Luke J J, Flaherty K T, Ribas A. et al. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463-482
4. Zou W, Wolchok J D, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv324
5. Topalian S L, Hodi F S, Brahmer J R. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454
6. Le D T, Uram J N, Wang H. et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520
7. O'Neil B H, Wallmark J M, Lorente D. et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12:e0189848
8. Agarwal P, Le D T, Boland P M. Immunotherapy in colorectal cancer. Adv Cancer Res. 2021;151:137-196
9. Wu X, Li J, Zhang Y. et al. Identification of immune cell infiltration landscape for predicting prognosis of colorectal cancer. Gastroenterol Rep (Oxf). 2023;11:goad014
10. Angata T, Tabuchi Y, Nakamura K. et al. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838-846
11. Hiruma Y, Tsuda E, Maeda N. et al. Impaired osteoclast differentiation and function and mild osteopetrosis development in Siglec-15-deficient mice. Bone. 2013;53:87-93
12. Stuible M, Moraitis A, Fortin A. et al. Mechanism and function of monoclonal antibodies targeting siglec-15 for therapeutic inhibition of osteoclastic bone resorption. J Biol Chem. 2014;289:6498-6512
13. Ishida-Kitagawa N, Tanaka K, Bao X. et al. Siglec-15 protein regulates formation of functional osteoclasts in concert with DNAX-activating protein of 12 kDa (DAP12). J Biol Chem. 2012;287:17493-17502
14. Hiruma Y, Hirai T, Tsuda E. Siglec-15, a member of the sialic acid-binding lectin, is a novel regulator for osteoclast differentiation. Biochem Biophys Res Commun. 2011;409:424-429
15. Wang J, Sun J, Liu L N. et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med. 2019;25:656-666
16. Siglec-15. An Attractive Immunotherapy Target. Cancer Discov. 2020;10:7-8
17. Sun J, Lu Q, Sanmamed M F. et al. Siglec-15 as an Emerging Target for Next-generation Cancer Immunotherapy. Clin Cancer Res. 2021;27:680-688
18. Cao G, Xiao Z, Yin Z. Normalization cancer immunotherapy: blocking Siglec-15!. Signal Transduct Target Ther. 2019;4:10
19. Hao J Q, Nong J Y, Zhao D. et al. The significance of Siglec-15 expression in resectable non-small cell lung cancer. Neoplasma. 2020;67:1214-1222
20. Quirino M W L, Pereira M C, Deodato de Souza M F. et al. Immunopositivity for Siglec-15 in gastric cancer and its association with clinical and pathological parameters. Eur J Histochem. 2021 65
21. Hu J, Yu A, Othmane B. et al. Siglec15 shapes a non-inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer. Theranostics. 2021;11:3089-3108
22. Chen X, Mo S, Zhang Y. et al. Analysis of a novel immune checkpoint, Siglec-15, in pancreatic ductal adenocarcinoma. J Pathol Clin Res. 2022;8:268-278
23. Li B, Zhang B, Wang X. et al. Expression signature, prognosis value, and immune characteristics of Siglec-15 identified by pan-cancer analysis. Oncoimmunology. 2020;9:1807291
24. Noguchi T, Ward J P, Gubin M M. et al. Temporally Distinct PD-L1 Expression by Tumor and Host Cells Contributes to Immune Escape. Cancer Immunol Res. 2017;5:106-117
25. Llosa N J, Cruise M, Tam A. et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43-51
26. Masugi Y, Nishihara R, Yang J. et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66:1463-1473
27. Juneja V R, McGuire K A, Manguso R T. et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214:895-904
28. Baker K, Zlobec I, Tornillo L. et al. Differential significance of tumour infiltrating lymphocytes in sporadic mismatch repair deficient versus proficient colorectal cancers: a potential role for dysregulation of the transforming growth factor-beta pathway. Eur J Cancer. 2007;43:624-631
29. Li Y, Liang L, Dai W. et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15:55
30. Dong H, Strome S E, Salomao D R. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800
31. Li T J, Jin K Z, Li H. et al. SIGLEC15 amplifies immunosuppressive properties of tumor-associated macrophages in pancreatic cancer. Cancer Lett. 2022;530:142-155
32. Toki M,Zugazagoitia J,Altan M et al. Abstract 3151: Quantitative measurement of Siglec-15 expression in non-small cell lung cancer and its association with PD-L1, B7-H4 and tumor infiltrating lymphocytes.Cancer Res. 2019; 79: 3151
33. Akbay E A, Koyama S, Carretero J. et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355-1363
34. Peng Q H, Wang C H, Chen H M. et al. CMTM6 and PD-L1 coexpression is associated with an active immune microenvironment and a favorable prognosis in colorectal cancer. J Immunother Cancer. 2021;9:e001638
35. Du H, Tang J, Li X. et al. Siglec-15 Is an Immune Suppressor and Potential Target for Immunotherapy in the Pre-Metastatic Lymph Node of Colorectal Cancer. Front Cell Dev Biol. 2021;9:691937
Author contact
![]() Corresponding author: Yanhong Deng, Department of Medical Oncology, The Sixth Affiliated Hospital, Sun Yat-sen University, 26 YuanCun Er Heng Road, Guangzhou, Guangdong 510655, China. Tel: +86-020-38254084; Fax: +86-020-38254084; E-mail: dengyanhsysu.edu.cn
Corresponding author: Yanhong Deng, Department of Medical Oncology, The Sixth Affiliated Hospital, Sun Yat-sen University, 26 YuanCun Er Heng Road, Guangzhou, Guangdong 510655, China. Tel: +86-020-38254084; Fax: +86-020-38254084; E-mail: dengyanhsysu.edu.cn

 Global reach, higher impact
Global reach, higher impact