3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(18):3429-3443. doi:10.7150/jca.89044 This issue Cite
Research Paper
APOBEC3A suppresses cervical cancer via apoptosis
1. Department of Epidemiology, Second Military Medical University, Shanghai, 200433, China.
2. Department of Obstetrics and Gynecology, the 1st Affiliated Hospital, Second Military Medical University, Shanghai 200433, China.
3. Department of Hepatic Surgery, the 3rd Affiliated Hospital, Second Military Medical University, Shanghai, 200438, China.
4. Shanghai East Hospital, Key Laboratory of Arrhythmias, Ministry of Education, Tongji University School of Medicine Tongji University, Shanghai 200120, China.
5. Shanghai Key Laboratory of Medical Bioprotection, Shanghai, 200433, China.
6. Key Laboratory of Biological Defense, Ministry of Education, Shanghai, 200433, China.
* These authors contributed equally to this work.
Abstract
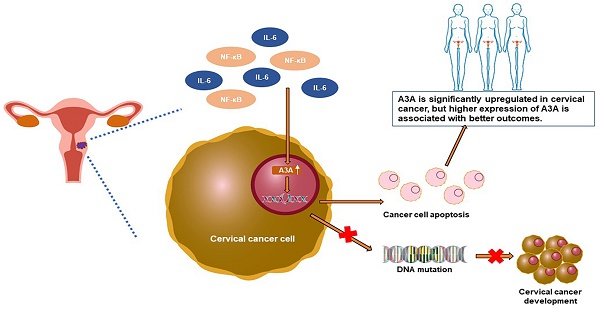
Background: Family members of Apolipoprotein B mRNA-editing enzyme catalytic 3 (APOBEC3) play critical roles in cancer evolution and development. However, the role of APOBEC3A in cervical cancer remains to be clarified.
Methods: We used bioinformatics to investigate APOBEC3A expression and outcomes using The Cancer Genome Atlas (TCGA)-cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC) dataset, GTEx, and GSE7803. Immunohistochemistry was then used to identify APOBEC3A's expression pattern. We performed Cell Counting Kit-8, wound-healing, Transwell, and flow cytometry assays to measure proliferation, migration, invasion, and apoptosis, respectively, using the SiHa and HeLa cell lines transfected with APOBEC3A. BALB/c nude mice were used to investigate the effects of APOBEC3A in vivo. The phosphorylated gamma-H2AX staining assay was applied to measure DNA damage. RNA sequencing (RNA-Seq) was applied to explore APOBEC3A-related signaling pathways.
Results: APOBEC3A was more significantly expressed in cancer tissues than in adjacent normal tissues. Higher expression of APOBEC3A was associated with better outcomes in TCGA-CESC and GTEx. Immunohistochemistry showed that the expression of APOBEC3A was significantly higher in cancer tissues than in normal tissues. Transfection experiments showed that APOBEC3A inhibited proliferation, upregulated S-phase cells, inhibited migration and invasion, induced DNA damage, and promoted apoptosis. Overexpression of APOBEC3A inhibited tumor formation in the mouse model. RNA-seq analysis showed that ectopic expression of APOBEC3A inhibited several cancer-associated signaling pathways.
Conclusions: APOBEC3A is significantly upregulated in cervical cancer, and higher expression of APOBEC3A is associated with better outcomes. APOBEC3A is a tumor suppressor whose overexpression induces apoptosis in cervical cancer.
Keywords: Cervical cancer, apolipoprotein B mRNA-editing enzyme catalytic 3 A (APOBEC3A), proliferation, apoptosis, outcomes

 Global reach, higher impact
Global reach, higher impact