Impact Factor
ISSN: 1837-9664
J Cancer 2023; 14(18):3532-3538. doi:10.7150/jca.90130 This issue Cite
Research Paper
The impacts of MACC1 gene polymorphisms on urothelial cell carcinoma susceptibility and clinicopathologic characteristics in Taiwan
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2. Division of Urology, Department of Surgery, Taichung Veterans General Hospital, Taichung, Taiwan.
3. Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan.
4. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
5. Department of Medicine and Nursing, Hungkuang University, Taichung, Taiwan.
6. School of Medicine, National Yang Ming University, Taipei, Taiwan.
7. Department of Applied Chemistry, National Chi Nan University, Nantou, Taiwan.
8. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
Received 2023-9-13; Accepted 2023-10-14; Published 2023-10-24
Abstract
Urothelial cell carcinoma (UCC) is a common malignancy of the urinary tract in Taiwan. Metastasis-Associated in Colon Cancer 1 (MACC1), a newly identified oncogene and regulator of the HGF/Met signaling pathway, has been shown to play a critical role in the development and progression of several types of cancer. Our study aims to investigate the impact of MACC1 gene polymorphisms on the clinicopathological features of patients with UCC. In this study, we included a total of 719 patients with UCC and 719 healthy controls. The genotyping of five MACC1 gene polymorphisms (rs1990172, rs975263, rs3095007, rs4721888, and rs3735615) was performed using real-time PCR with TaqMan assays. Our findings indicate that urothelial cancer patients with MACC1 rs3095007 A allele had a decreased risk of >T2 stage [Odds ratio (OR)=0.619, 95% CI=0.394-0.971, p=0.036] and lymph node invasion (OR=0.448, 95% CI=0.201-0.998, p=0.044). Additionally, these individuals were associated with longer relapse-free survival (p=0.007) and overall survival (p=0.028). In conclusion, our findings demonstrate that urothelial cancer patients with MACC1 (rs3095007) CA and AA genotypes have a lower risk of advanced T stage and lymph node metastasis. Additionally, these genotypes were associated with longer relapse-free survival and overall survival, highlighting the potential of these biomarkers as predictors of UCC prognosis.
Keywords: MACC1, single nucleotide polymorphism, urothelial cell carcinoma
Introduction
Bladder cancer (BC) is the fourth most common cancer in males and sixth in both genders, accounting for 81,180 new diagnosis cases and 17,100 deaths in the USA in 2022 [1]. 95% of BC cases develop from the urothelium and are called urothelial cell carcinoma (UCC), which is categorized into non-muscle invasive bladder cancer and muscle invasive bladder cancer based on the depth of tumor invasion [2, 3]. About 75% of UCC patients initially present with non-muscle invasive tumors, which are confined to the mucosa as stage Ta or carcinoma in situ (CIS) or confined to the submucosa as stage T1. The remaining patients present with muscle invasive tumors, defined as stage T2-4 disease, and have a higher risk of cancer-specific death [4, 5].
Tobacco smoking is the most significant risk factor for UCC and accounts for approximately half of all UCC cases [6]. Environmental or occupational exposure is the second most important risk factor and accounts for 10% of cases [7]. Genetic predisposition may also increase the susceptibility to UCC [8-12]. For example, TP53 mutation is found in almost 50% of bladder cancer patients and is associated with disease progression and drug selection [13]. Currently, data from genome-wide association studies have also identified three single nucleotide polymorphisms (SNPs) associated with aggressive UCC and a higher risk of progression [14]. However, there is still insufficient evidence to endorse genetic screening for UCCs.
Metastasis associated in colon cancer 1 (MACC1) gene was first identified in 2009 as a key regulator of hepatocyte growth factor-mesenchymal-epithelial transition factor (HGF-MET) signaling and the expression of MACC1 in tumor specimens is an independent prognostic factor for colon cancer [15]. The HGF-MET signal pathway activates the ERK/MAPK pathway and PI3K/Akt/mTOR pathway for cell proliferation and survival, leading to cancer proliferation, angiogenesis, tumor invasion, and metastasis [16, 17]. Upon binding of HGF to its receptor MET, MACC1 undergoes translocation from the cytoplasm to the nucleus [15]. MACC1 then binds to the promoter of the MET gene to increase the transcription and generate more MET protein as a receptor for HGF. Thus, the HGF-MET-MACC1 axis results in abnormal cell proliferation and an increased ability of cancer migration, invasion, and metastasis [18-20]. MACC1 overexpression could contribute to colorectal cancer progression and metastasis [18]. Similar results have been found in lung, gastric, liver, and ovarian cancer [21-24]. MACC1 expression is highly associated with lymphatic metastasis in oral cancer, and downregulation of MACC1 inhibits the migration, and proliferation of tumors [25]. MACC1 expression is also an independent predictor of more advanced tumor stage, grade of differentiation, and lymph node metastasis in infiltrating urothelial cell carcinoma of the bladder [26].
Variations in a solitary nucleotide base of the DNA sequence, known as single nucleotide polymorphisms (SNPs), are present in the population at a frequency of at least 1% [27, 28]. These genetic variations can result in amino acid substitutions that affect protein function and contribute to disease development [29-31]. For instance, the G allele of rs1990172 at MACC1 has been linked to significantly decreased overall survival in colorectal cancer, while heterozygous carriers of SNPs rs1990172 and rs975263 showed a significantly higher risk of disease relapse in hepatocellular carcinoma recurrence in liver transplant patients [32, 33]. Similarly, carriers of the G allele of SNP rs1990172 had an increased risk of progression and death in HER2-positive breast cancer patients, whereas the C allele of SNP rs3735615 conferred significant protective impact on overall survival [34].
Despite the known association of MACC1 SNPs with various cancers, their role in patients with UCC has not been established. In this study, we examined five MACC1 SNPs (rs1990172 [intron], rs975263 [exon 5], rs3095007 [intron], rs4721888 [exon 4], rs3735615 [exon 7]) to investigate their predictive role in UCC patients.
Material and methods
Subjects and Specimen Collection
Between 2011 and 2022, Taichung Veteran General Hospital in Taichung, Taiwan, conducted a case-control study that enrolled a total of 719 patients with UCC, consisting of 441 men and 278 women. To serve as a control group, 719 individuals of the same ethnic background but without a history of cancer of any sites were also enrolled. The patients were diagnosed with UCC based on the TNM staging system of the American Joint Committee on Cancer (AJCC) Staging Manual (7th ed.), which was confirmed by pathologists [35]. Tumor histopathologic grading was based on the 2004 WHO grading system, which classified tumors as either high grade or low-grade papillary tumors [36]. Lymph node and metastasis assessments were carried out using regular computer tomography (CT) scans. Personal information and patient characteristics were obtained through interviewer-administered questionnaires, which included questions about demographics and cigarette smoking status. The Institutional Review Board (IRB) of Taichung Veterans General Hospital approved the study, and all participants provided informed written consent (IRB No. CE19106A). Ethylenediaminetetraacetic acid (EDTA)-containing tubes were used to collect whole-blood specimens from both control and patient groups, which were immediately centrifuged and stored at -80°C.
MACC1 Polymorphism Selection
To investigate the relationship between MACC1 and UCC development, five well-characterized common polymorphisms were selected for this study, based on their association with cancer development and staging [32, 34, 37, 38]. The SNP rs1990172 is located at an intronic region of the MACC1 gene and has been associated with overall survival in colorectal cancer patients [32]. The SNP rs3095007, located at an intron of the MACC1 gene, is one of the most common variants representing the majority of the MACC1 locus [39]. Meanwhile, the SNPs rs975263, rs4721888, and rs3735615, which are located at exons 5, 4, and 7, respectively, were associated with breast cancer susceptibility and clinical outcomes [34, 37].
Genomic DNA extraction
DNA was extracted from whole blood samples using QIAamp DNA blood mini kits, and stored at -20°C in TE buffer until Real-time quantitative PCR analysis was performed [40]. The allelic discrimination of the MACC1 rs1990172, rs975263, rs3095007, rs4721888, and rs3735615 polymorphisms was assessed using the ABI StepOne TM Real-Time polymerase chain reaction (PCR) System (Applied Biosystems, Foster City, CA), and the data were analyzed using SDS v3.0 software (Applied Biosystems, Foster City, CA).
Statistical Analysis
The differences in demographic characteristics between the control and UCC group were compared using the Chi-squared test. Multiple logistic regression models were used to assess the odds ratios (ORs) with 95% confidence intervals (CIs) for the association between genotype frequencies and clinicopathologic characteristics, controlled with other covariates. Disease susceptibility was adjusted by multiple logistic regression models after controlling for age, sex and tobacco consumption. The Kaplan-Meier survival curve and multiple Cox proportional hazards model were used to evaluate disease-specific mortality and all-cause of death. A p value of less than 0.05 was considered statistically significant. The Statistical Analytic System (SAS Institute, Cary, NC, USA) software for Windows was used for all data analysis.
Results
Characteristics of Study Participants
The study cohort comprised 719 patients with UCC and 719 matched healthy controls. Table 1 showed the mean age of patient with UCC was 60.65 ± 7.07 and 68.86 ± 11.53 in control (p<0.001). Male predominance was observed in UCC patients (n=441, 61.3%), with no significant difference in gender distribution between the two groups (p=1.000). At diagnosis, 49.0% (n=352) of UCC patients were diagnosed with stage 1 disease, while 51.0% (n=367) had stage 2-4 disease. Pathological evidence of lymph node metastasis was found in 12.2% (n=88) of patients, while 3.6% (n=26) had confirmed metastatic disease. Histopathological analysis revealed that 89.3% (n=642) of UCC patients had high-grade tumors, and 10.7% (n=77) had low-grade tumors.
The distributions of demographical characteristics in 719 controls and 719 patients with UCC.
| Variable | Controls (N=719)n (%) | Patients (N=719)n (%) | p value |
|---|---|---|---|
| Age (yrs) | |||
| Mean ± S.D. | 60.65 ± 7.07 | 68.86 ± 11.53 | <0.001 |
| Gender | |||
| Female | 278 (38.7%) | 278 (38.7%) | 1.000 |
| Male | 441 (61.3%) | 441 (61.3%) | |
| Stage | |||
| Stage 1 | 352 (49.0%) | ||
| Stage 2-4 | 367 (51.0%) | ||
| Tumor T status | |||
| ≤ T2 | 457 (63.6%) | ||
| > T2 | 262 (36.4%) | ||
| Lymph node status | |||
| N0 | 631 (87.8%) | ||
| N1+N2 | 88 (12.2%) | ||
| Metastasis | |||
| M0 | 693 (96.4%) | ||
| M1 | 26 (3.6%) | ||
| Histopathologic grading | |||
| Low grade | 77 (10.7%) | ||
| High grade | 642 (89.3%) |
Student's t test or Chi-squared test was used between controls and patients with UCC.
Association of MACC1 Gene Polymorphisms with UCC
Table 2 illustrates the genotype distributions of the MACC1 gene, revealing that homozygous CC at rs1990172, homozygous AA at rs975263, homozygous GG at rs3735615, homozygous GG at 4721888, and homozygous CC at rs3095007 exhibit the highest distribution frequencies. However, there is no significant difference observed between MACC1 gene polymorphisms and susceptibility to UCC (Table 2).
Correlation between MACC1 SNPs and Clinical Status of UCC
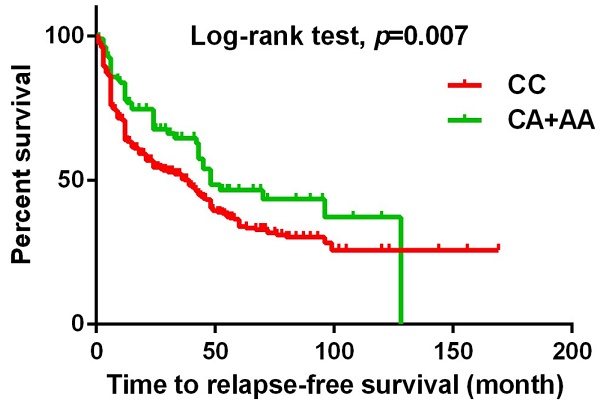
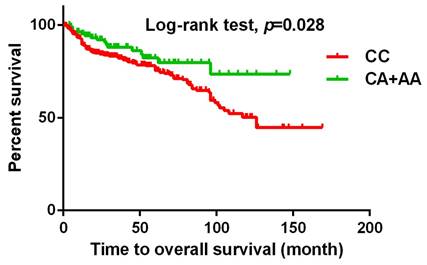
The present study investigated the association between MACC1 SNPs and clinicopathologic characteristics of UCC patients. Table 3 displays patients with at least one A allele at rs3095007 exhibit a lower proportion of advanced lesions (>T2 status) (OR = 0.619, 95% CI = 0.394-0.971, p = 0.036) and less lymph node metastasis disease (OR = 0.448, 95% CI = 0.201-0.998, p = 0.044). Furthermore, Figure 1 demonstrates that the presence of at least one A allele at MACC1 rs3095007 is linked to longer relapse-free survival (p = 0.007), while Figure 2 reveals that this allele is associated with longer overall survival (p = 0.028).
The Kaplan-Meier survival curve was used to analyze the relapse-free survival for 719 patients with UCC. Patients with CA+AA allele at rs3095007 has a longer relapse free survival compared to patients with CC allele (p=0.007).
Kaplan-Meier survival curve was used to analyze the overall survival for 719 patients with UCC. Patients with CA+AA allele at rs3095007 has a longer overall survival compared to patients with CC allele (p=0.028).
Genotype distributions of MACC1 gene polymorphisms in 719 controls and 719 patients with UCC.
| Variable | Controls (N=719) n (%) | Patients (N=719) n (%) | AOR (95% CI) | p value |
|---|---|---|---|---|
| rs1990172 | ||||
| CC | 539 (75.0%) | 543 (75.5%) | 1.000 (reference) | |
| CA | 169 (23.5%) | 158 (22.0%) | 0.969 (0.740-1.271) | 0.822 |
| AA | 11 (1.5%) | 18 (2.5%) | 1.747 (0.747-4.085) | 0.198 |
| CA+AA | 180 (25.0%) | 176 (24.5%) | 1.014 (0.780-1.318) | 0.917 |
| rs975263 | ||||
| AA | 502 (69.8%) | 487 (67.7%) | 1.000 (reference) | |
| AG | 198 (27.5%) | 204 (28.4%) | 1.058 (0.821-1.363) | 0.663 |
| GG | 19 (2.7%) | 28 (3.9%) | 1.848 (0.956-3.573) | 0.068 |
| AG+GG | 217 (30.2%) | 232 (32.3%) | 1.119 (0.877-1.428) | 0.364 |
| rs3735615 | ||||
| GG | 521 (72.5%) | 511 (71.1%) | 1.000 (reference) | |
| GC | 185 (25.7%) | 188 (26.1%) | 1.084 (0.835-1.405) | 0.545 |
| CC | 13 (1.8%) | 20 (2.8%) | 2.027 (0.919-4.471) | 0.080 |
| GC+CC | 198 (27.5%) | 208 (28.9%) | 1.138 (0.884-1.464) | 0.317 |
| rs4721888 | ||||
| GG | 379 (52.7%) | 376 (52.3%) | 1.000 (reference) | |
| GC | 290 (40.3%) | 275 (38.2%) | 0.935 (0.737-1.188) | 0.584 |
| CC | 50 (7.0%) | 68 (9.5%) | 1.432 (0.985-2.659) | 0.075 |
| GC+CC | 340 (47.3%) | 343 (47.7%) | 1.041 (0.830-1.305) | 0.730 |
| rs3095007 | ||||
| CC | 595 (82.8%) | 610 (84.8%) | 1.000 (reference) | |
| CA | 118 (16.4%) | 103 (14.4%) | 0.933 (0.682-1.278) | 0.666 |
| AA | 6 (0.8%) | 6 (0.8%) | 0.952 (0.282-3.217) | 0.937 |
| CA+AA | 124 (17.2%) | 109 (15.2%) | 0.934 (0.687-1.270) | 0.664 |
The adjusted odds ratio (AOR) with their 95% confidence intervals were estimated by multiple logistic regression models after controlling for age, gender and tobacco consumption.
Discussion
The current study represents the initial investigation into the correlation between MACC1 SNPs and clinicopathologic characteristics as well as prognosis of UCC in the Chinese population. Our findings suggest that UCC patients who possess at least one A allele at rs3095007 exhibit less advanced tumor and lymph node metastasis, as well as a prolonged relapse-free survival and overall survival. To our knowledge, this is the first report to document these associations in this patient population.
Distribution frequency of the clinical status and MACC1 rs3095007 genotype frequencies in 719 UCC patients.
| MACC1 (rs3095007) | ||||
|---|---|---|---|---|
| Variable | CC (%) (n=610) | CA + AA (%) (n=109) | OR (95% CI) | p value |
| Stage | ||||
| Stage 1 | 294 (48.2%) | 58 (53.2%) | 1.000 (reference) | |
| Stage 2-4 | 316 (51.8%) | 51 (46.8%) | 0.818 (0.544-1.231) | 0.335 |
| Tumor T status | ||||
| ≤ T2 | 378 (62.0%) | 79 (72.5%) | 1.000 (reference) | |
| > T2 | 232 (38.0%) | 30 (27.5%) | 0.619 (0.394-0.971) | 0.036 |
| Lymph node status | ||||
| N0 | 529 (86.7%) | 102 (93.6%) | 1.000 (reference) | |
| N1+N2 | 81 (13.3%) | 7 (6.4%) | 0.448 (0.201-0.998) | 0.044 |
| Metastasis | ||||
| M0 | 588 (96.4%) | 105 (96.3%) | 1.000 (reference) | |
| M1 | 22 (3.6%) | 4 (3.7%) | 1.018 (0.344-3.014) | 0.974 |
| Histopathologic grading | ||||
| Low grade | 62 (10.2%) | 15 (13.8%) | 1.000 (reference) | |
| High grade | 548 (89.8%) | 94 (86.2%) | 0.709 (0.387-1.298) | 0.263 |
Bold font indicates statistical significance (p < 0.05).
The odds ratio (OR) with their 95% confidence intervals were estimated by logistic regression models.
Overexpression of MACC1 has been demonstrated to upregulate the HGF-MET signaling pathway, which in turn promotes tumor proliferation, invasion, and metastasis in colorectal cancer [15]. Furthermore, elevated MACC1 mRNA expression in lung adenocarcinoma specimens is associated with early recurrence following surgery [21]. In breast cancer specimens, MACC1 protein expression is linked to advanced pathological features, reduced relapse-free survival, and overall survival rates [41]. In UCC tissue, MACC1 is more frequently expressed than in normal bladder mucosa tissue, and its expression is positively associated with tumor stages, grades of differentiation, lymph node metastasis, stages, and overall unfavorable survival rates [26]. RNA interference-mediated knockdown of MACC1 gene expression in T24 cells (human bladder urothelial cell carcinoma cells) resulted in decreased proliferation, expression of apoptosis proteins, and downregulated MET protein levels, thus reducing the invasion abilities of T24 cells [42]. These findings indicate that MACC1 may serve as a promising prognostic indicator or a potential therapeutic target for gene therapy in human UCC.
The promoter activity and gene expression of MACC1 can be influenced by genetic polymorphisms, which may potentially affect tumor growth, invasion, or metastasis [43-46]. Lang et al. conducted the first study to investigate the association between MACC1 SNPs and the survival of colorectal cancer patients. They genotyped 6 tag SNPs located in the intronic region, which represent the majority of common variants at the MACC1 locus. Their findings showed that the rs1990172 SNP was considerably associated with decreased overall survival [32]. Schmid et al. further sequenced the coding exons of MACC1, including three SNPs (rs4721888, rs975263, rs3735615). They found that younger patients with colon cancer with stage I or II and a CT genotype at rs975263 had a shorter metastasis-free survival. However, in cell culture experiments, MACC1 SNPs have no impaction on MACC1-induced cell migration and proliferation [38]. Consistent with Lang et al., Horvat et al. reported that the TT genotype of SNP rs1990172 in the MACC1 gene was associated with worse disease-free survival in resectable colorectal cancer patients [47].
Similar results have been reported in other types of cancer. For instance, Zheng et al. found that patients with hepatocellular carcinoma who were heterozygous for the rs1990172 SNP in the intronic region or for the rs975263 SNP in the exon region had a significantly higher risk of relapse after transplantation [33]. Lin et al. also reported that patients with hepatocellular carcinoma who carried the CA or AA variant at rs1990172 had a lower risk of developing larger tumors, more advanced clinical stages, and vascular invasion [48]. Muendlein et al. found that carriers of the rare G allele at rs1990172 and the rare T allele at rs975263 had an increased risk of disease progression and death in patients with HER2-positive breast cancer [34]. In another cohort study of breast cancer in Han Chinese women, Dai et al. did not find any associations between rs1990172 and breast cancer risk, but they did observe that rs975263 and rs472188 in the exon region were associated with susceptibility to breast cancer in Chinese women [37]. Moreover, Hu et al. revealed that rs975263 may have the potential to be a metastasis marker in oral cancer patient [49]. Similarly, Sun et al. found that Taiwanese women with cervical cancer who had the GG genotype at rs975263 tended to have a higher risk of vaginal invasion than those with AA/AG variants [50].
In our study of UCC patients, we examined two intronic SNPs (rs1990172 and rs3095007) and three exonic SNPs (rs975263, rs4721888 and rs3735615). While rs1990172 is located within an intronic region, previous studies have shown its significant impact on disease prognosis in colorectal cancer, breast cancer, and hepatocellular carcinoma [32-34]. This may be attributed to the fact that introns can affect various aspects of gene expression, including transcription rate, nuclear export, transcript stability, and mRNA translation efficiency [51]. However, we did not observe significant impact of rs1990172 on disease prognosis in our population. Instead, we found that at least one A allele at rs3095007, also located in an intronic region, was associated with a lower risk of advanced tumor stage and lymph node metastasis, as well as longer progression-free survival and overall survival. This is the first report of a positive association between rs3095007 and disease prognosis, although the underlying mechanism is yet to be investigated. Notably, we did not observe any clinical significance of the three exons in UCC patients.
There are several limitations in our study that need to be acknowledged. Firstly, a larger cohort of case-control analysis is necessary to confirm our findings. Although we observed a trend of higher UCC susceptibility in the three exonic SNPs (GG for rs975263, CC for rs3735615, and CC for rs4721888), statistical significance was not reached. Therefore, more cases may be needed to explore the role of these three coding exons in UCC susceptibility. Secondly, the precise mechanism by which these SNPs affect the function of the MACC1 gene requires further investigation. Thirdly, the treatment modality of the patients was not taken into consideration in our study, which may have an impact on the prognosis and outcome of the disease.
In conclusion, this study is the first to report the relationship between MACC1 polymorphism and the clinicopathologic features of UCC. Our findings demonstrate that urothelial cancer patients with MACC1 (rs3095007) CA and AA genotypes have a lower risk of advanced T stage and lymph node metastasis. Additionally, these genotypes were associated with longer relapse-free survival and overall survival, highlighting the potential of these biomarkers as predictors of UCC prognosis.
Acknowledgements
This study was supported by Taichung Veterans General Hospital, Taichung, Taiwan (TCVGH-1085002B).
Abbreviations
BC: Bladder cancer; MACC1: Metastasis-Associated in Colon Cancer 1; SNP: single-nucleotide polymorphism; UCC: Urothelial cell carcinoma.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33
2. Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435-1448
3. Weng WC, Hsieh MH, Chiou HL, Lee CY, Tang CH, Chang LC. et al. Impact of tissue inhibitor of metalloproteinases-3 genetic variants on clinicopathological characteristics of urothelial cell carcinoma. J Cancer. 2023;14:360-366
4. Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P. et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234-241
5. Weng WC, Chen CJ, Chen PN, Wang SS, Hsieh MJ, Yang SF. Impact of Gene Polymorphisms in GAS5 on Urothelial Cell Carcinoma Development and Clinical Characteristics. Diagnostics (Basel). 2020;10:260
6. Teoh JY, Huang J, Ko WY, Lok V, Choi P, Ng CF. et al. Global Trends of Bladder Cancer Incidence and Mortality, and Their Associations with Tobacco Use and Gross Domestic Product Per Capita. Eur Urol. 2020;78:893-906
7. Colt JS, Friesen MC, Stewart PA, Donguk P, Johnson A, Schwenn M. et al. A case-control study of occupational exposure to metalworking fluids and bladder cancer risk among men. Occup Environ Med. 2014;71:667-674
8. Martin C, Leiser CL, O'Neil B, Gupta S, Lowrance WT, Kohlmann W. et al. Familial Cancer Clustering in Urothelial Cancer: A Population-Based Case-Control Study. J Natl Cancer Inst. 2018;110:527-533
9. Huang CH, Chen CJ, Chen PN, Wang SS, Chou YE, Hung SC. et al. Impacts of AURKA Genetic Polymorphism on Urothelial Cell Carcinoma Development. J Cancer. 2019;10:1370-1374
10. Hung SC, Chou YE, Li JR, Chen CS, Lin CY, Chang LW. et al. Functional genetic variant of WW domain containing oxidoreductase gene associated with urothelial cell carcinoma clinicopathologic characteristics and long-term survival. Urol Oncol. 2020;38:41 e41-41 e49
11. Hung SC, Wang SS, Li JR, Chen CS, Lin CY, Chang LW. et al. Impact of RAGE polymorphisms on urothelial cell carcinoma clinicopathologic characteristics and long-term survival. Urol Oncol. 2019;37:573 e579-573 e517
12. Wang SS, Liu YF, Ou YC, Chen CS, Li JR, Yang SF. Impacts of CA9 gene polymorphisms on urothelial cell carcinoma susceptibility and clinicopathologic characteristics in Taiwan. PLoS One. 2013;8:e82804
13. Wu G, Wang F, Li K, Li S, Zhao C, Fan C. et al. Significance of TP53 mutation in bladder cancer disease progression and drug selection. PeerJ. 2019;7:e8261
14. Lenfant L, Cancel-Tassin G, Gazut S, Compérat E, Rouprêt M, Cussenot O. Genetic variability in 13q33 and 9q34 is linked to aggressiveness patterns and a higher risk of progression of non-muscle-invasive bladder cancer at the time of diagnosis. BJU Int. 2021;127:375-383
15. Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I. et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59-67
16. Boissinot M, Vilaine M, Hermouet S. The Hepatocyte Growth Factor (HGF)/Met Axis: A Neglected Target in the Treatment of Chronic Myeloproliferative Neoplasms? Cancers (Basel). 2014;6:1631-1669
17. Sattler M, Salgia R. c-Met and hepatocyte growth factor: potential as novel targets in cancer therapy. Curr Oncol Rep. 2007;9:102-108
18. Galimi F, Torti D, Sassi F, Isella C, Corà D, Gastaldi S. et al. Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer: response to met inhibition in patient xenografts and pathologic correlations. Clin Cancer Res. 2011;17:3146-3156
19. Hohmann T, Hohmann U, Dehghani F. MACC1-induced migration in tumors: Current state and perspective. Front Oncol. 2023;13:1165676
20. Lv M, Jiao Y, Yang B, Ye M, Di W, Su W. et al. MACC1 as a Potential Target for the Treatment and Prevention of Breast Cancer. Biology (Basel). 2023 12
21. Chundong G, Uramoto H, Onitsuka T, Shimokawa H, Iwanami T, Nakagawa M. et al. Molecular diagnosis of MACC1 status in lung adenocarcinoma by immunohistochemical analysis. Anticancer Res. 2011;31:1141-1145
22. Lu G, Zhou L, Zhang X, Zhu B, Wu S, Song W. et al. The expression of metastasis-associated in colon cancer-1 and KAI1 in gastric adenocarcinoma and their clinical significance. World J Surg Oncol. 2016;14:276
23. Yang YP, Qu JH, Chang XJ, Lu YY, Bai WL, Dong Z. et al. High intratumoral metastasis-associated in colon cancer-1 expression predicts poor outcomes of cryoablation therapy for advanced hepatocellular carcinoma. J Transl Med. 2013;11:41
24. Yu L, Zhu B, Wu S, Zhou L, Song W, Gong X. et al. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KiSS-1, and MACC1 in the prediction of metastasis and prognosis in ovarian carcinoma. Diagn Pathol. 2017;12:23
25. Li HF, Liu YQ, Shen ZJ, Gan XF, Han JJ, Liu YY. et al. Downregulation of MACC1 inhibits invasion, migration and proliferation, attenuates cisplatin resistance and induces apoptosis in tongue squamous cell carcinoma. Oncol Rep. 2015;33:651-660
26. Dai C, Liu Y, Yang R, Zhou L. Clinical significance of MACC1, Twist1, and KAI1 expressions in infiltrating urothelial carcinoma of the bladder. Int J Clin Exp Pathol. 2019;12:3877-3885
27. Chou CH, Chang CY, Lu HJ, Hsin MC, Chen MK, Huang HC. et al. IGF2BP2 Polymorphisms Are Associated with Clinical Characteristics and Development of Oral Cancer. Int J Mol Sci. 2020;21:5662
28. Su SC, Lin CW, Ju PC, Chang LC, Chuang CY, Liu YF. et al. Association of LINC00673 Genetic Variants with Progression of Oral Cancer. J Pers Med. 2021;11:468
29. Clemens E, van der Kooi ALF, Broer L, van Dulmen-den Broeder E, Visscher H, Kremer L. et al. The influence of genetic variation on late toxicities in childhood cancer survivors: A review. Crit Rev Oncol Hematol. 2018;126:154-167
30. Chen YT, Lin CW, Chou YE, Su SC, Chang LC, Lee CY. et al. Potential impact of ADAM-10 genetic variants with the clinical features of oral squamous cell carcinoma. J Cell Mol Med. 2023;27:1144-1152
31. Lu HJ, Chuang CY, Su CW, Chen MK, Yang WE, Yeh CM. et al. Role of TNFSF15 variants in oral cancer development and clinicopathologic characteristics. J Cell Mol Med. 2022;26:5452-5462
32. Lang AH, Geller-Rhomberg S, Winder T, Stark N, Gasser K, Hartmann B. et al. A common variant of the MACC1 gene is significantly associated with overall survival in colorectal cancer patients. BMC Cancer. 2012;12:20
33. Zheng Z, Gao S, Yang Z, Xie H, Zhang C, Lin B. et al. Single nucleotide polymorphisms in the metastasis-associated in colon cancer-1 gene predict the recurrence of hepatocellular carcinoma after transplantation. Int J Med Sci. 2014;11:142-150
34. Muendlein A, Hubalek M, Geller-Rhomberg S, Gasser K, Winder T, Drexel H. et al. Significant survival impact of MACC1 polymorphisms in HER2 positive breast cancer patients. Eur J Cancer. 2014;50:2134-2141
35. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474
36. Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. Int J Surg Pathol. 2005;13:143-153
37. Dai ZJ, Liu XH, Kang HF, Wang XJ, Jin TB, Zhang SQ. et al. Genetic Variation in Metastasis-Associated in Colon Cancer-1 and the Risk of Breast Cancer Among the Chinese Han Population: A STROBE-Compliant Observational Study. Medicine (Baltimore). 2016;95:e2801
38. Schmid F, Burock S, Klockmeier K, Schlag PM, Stein U. SNPs in the coding region of the metastasis-inducing gene MACC1 and clinical outcome in colorectal cancer. Mol Cancer. 2012;11:49
39. Li H, Chen YX, Wen JG, Zhou HH. Metastasis-associated in colon cancer 1: A promising biomarker for the metastasis and prognosis of colorectal cancer. Oncol Lett. 2017;14:3899-3908
40. Su SC, Hsieh MJ, Lin CW, Chuang CY, Liu YF, Yeh CM. et al. Impact of HOTAIR Gene Polymorphism and Environmental Risk on Oral Cancer. J Dent Res. 2018;97:717-724
41. Huang Y, Zhang H, Cai J, Fang L, Wu J, Ye C. et al. Overexpression of MACC1 and Its significance in human Breast Cancer Progression. Cell Biosci. 2013;3:16
42. Xu ST, Ding X, Ni QF, Jin SJ. Targeting MACC1 by RNA interference inhibits proliferation and invasion of bladder urothelial carcinoma in T24 cells. Int J Clin Exp Pathol. 2015;8:7937-7944
43. Shastry BS. SNPs: impact on gene function and phenotype. Methods Mol Biol. 2009;578:3-22
44. Chen MK, Chiou HL, Su SC, Chung TT, Tseng HC, Tsai HT. et al. The association between hypoxia inducible factor-1alpha gene polymorphisms and increased susceptibility to oral cancer. Oral Oncol. 2009;45:e222-226
45. Hsiao PC, Chen MK, Su SC, Ueng KC, Chen YC, Hsieh YH. et al. Hypoxia inducible factor-1alpha gene polymorphism G1790A and its interaction with tobacco and alcohol consumptions increase susceptibility to hepatocellular carcinoma. J Surg Oncol. 2010;102:163-169
46. Hua KT, Liu YF, Hsu CL, Cheng TY, Yang CY, Chang JS. et al. 3'UTR polymorphisms of carbonic anhydrase IX determine the miR-34a targeting efficiency and prognosis of hepatocellular carcinoma. Sci Rep. 2017;7:4466
47. Horvat M, Potocnik U, Repnik K, Kavalar R, Zadnik V, Potrc S. et al. Single Nucleotide Polymorphisms in Genes MACC1, RAD18, MMP7 and SDF-1a As Prognostic Factors in Resectable Colorectal Cancer. Radiol Oncol. 2017;51:151-159
48. Lin CH, Hsieh MJ, Lee HL, Yang SF, Su SC, Lee WJ. et al. Effects of MACC1 polymorphisms on hepatocellular carcinoma development and clinical characteristics. J Cancer. 2020;11:1641-1647
49. Hu RH, Chuang CY, Lin CW, Su SC, Chang LC, Wu SW. et al. Effect of MACC1 Genetic Polymorphisms and Environmental Risk Factors in the Occurrence of Oral Squamous Cell Carcinoma. J Pers Med. 2021;11:490
50. Sun YH, Chou YH, Ou CC, Ng SC, Shen HP, Lee YC. et al. Investigation of metastasis-associated in colon cancer-1 genetic variants in the development and clinicopathologcial characteristics of uterine cervical cancer in Taiwanese women. Int J Med Sci. 2020;17:490-497
51. Shaul O. How introns enhance gene expression. Int J Biochem Cell Biol. 2017;91:145-155
Author contact
![]() Corresponding authors: Shun-Fa Yang, Ph.D. or Shian-Shiang Wang, MD., Ph.D., Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); E-mail: sswdoccom.tw (Shian-Shiang Wang)
Corresponding authors: Shun-Fa Yang, Ph.D. or Shian-Shiang Wang, MD., Ph.D., Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); E-mail: sswdoccom.tw (Shian-Shiang Wang)

 Global reach, higher impact
Global reach, higher impact