3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(1):90-102. doi:10.7150/jca.90090 This issue Cite
Research Paper
Incidence of HER2-targeted antibody-drug conjugates-related cardiac events: a meta-analysis
1. Department of Pharmacy, Hunan Cancer Hospital, the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha 410011, Hunan, China.
2. Department of Pharmacy, the Second Xiangya Hospital, Central South University, Changsha 410011, Hunan, China.
3. Department of Pharmacy, Yantai Hospital of Traditional Chinese Medicine, Yantai 264000, Shandong, China.
Received 2023-9-12; Accepted 2023-10-29; Published 2024-1-1
Abstract
Background: Human epidermal growth factor receptor 2 (HER2)-targeted antibody-drug conjugate (ADC) has emerged as a hotspot for research and brought breakthroughs in the treatment of breast cancer and other solid tumors. While the occurrence of cardiac events (CEs) has yet not been systematically reported.
Methods: The prospective clinical trials of marketed HER2-targeted ADCs were systematically searched in PubMed, Embase, Cochrane Library, and ClinicalTrials.gov from inception to May 2023. Two investigators independently extracted data with priority given to ClinicalTrials.gov, followed by peer-reviewed articles. Stata 15.0 software was used to perform the meta-analysis. The effect statistics were estimated as pooled incidence with 95% confidence intervals (CI). The primary objectives were to assess the incidence of all-grade and ≥3 /serious grades CEs related to HER2-targeted ADC. Our study strictly adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and has been registered on PROSPERO (NO. CRD42023440448).
Results: After conducting a comprehensive literature search, initially 7000 relevant studies were identified, and eventually a total of 47 trials involving 10594 patients were included for analysis. The pooled incidence of all-grade and ≥3/serious grades CEs respectively were 4.7% [95% CI, 3.7-5.8%] and 0.6% (95% CI, 0.5-0.8%). The pooled incidence of CEs leading to dosage discontinuation was 0.8% (95% CI, 0.4-1.3%). Subgroup analysis revealed a significantly higher incidence of all-grade CEs in T-DXd treatment compared to T-DM1 treatment (7.7% versus 3.6%; p=0.017), as well as in phase I/II trials compared to phase III trials (6.9% versus 3.2%; p=0.002) and combination therapy compared to monotherapy (7.6% versus 3.9%; p=0.013). The electrocardiogram QT corrected interval prolonged was identified as the CE with the highest pooled incidence, occurring at a rate of 5.9% (95% CI, 3.3-8.5%).
Conclusions: The incidence of CEs associated with HER2-targeted ADC is relatively low. However, it is crucial to enhance surveillance measures, particularly for T-DXd treatment and combination therapy.
Keywords: human epidermal growth factor receptor 2, antibody-drug conjugate, cardiac events, trastuzumab emtansine, trastuzumab deruxtecan
Introduction
Antibody-drug conjugate (ADC) is a new class of antineoplastic drugs with a special structure different from conventional chemotherapy drugs, containing monoclonal antibodies, cytotoxic drugs and chemical linkers. It has emerged as a hotspot for research and development of antineoplastic drugs. Fifteen ADCs have been approved for the market so far worldwide[1], of which three were human epidermal growth factor receptor 2 (HER2)-targeted ADC. The first HER2-targeted ADC, trastuzumab emtansine (T-DM1), was initially approved in 2013 for HER2-positive metastatic breast cancer[2]. Its indication was later expanded in 2019 to the adjuvant therapy for early-stage HER2-positive breast cancer[3]. The second HER2-targeted ADC, trastuzumab deruxtecan (T-DXd, formerly DS-8201a), received FDA approval in 2019 as a late-line treatment for unresectable or metastatic HER2-positive breast cancer[4]. Subsequently, its usage has been extended to HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer[5], HER2-positive gastric or gastroesophageal (GEJ) adenocarcinoma[6], and unresectable or metastatic non-small cell lung cancer with HER2 mutation[7]. The third HER2-targeted ADC, disitamab vedotin (RC48), received approval in 2021 for locally advanced or metastatic gastric or gastroesophageal junction cancer and urothelial carcinoma from the National Medical Products Administration (NMPA) of China[8]. HER2-targeted ADCs are changing the destiny of HER2-expressing solid tumors[9].
The imbalance of cell signaling in the HER family, including HER1 (also known as EGFR), HER2, HER3 and HER4, has been associated with the occurrence and development of multiple tumor types[10]. The advent of trastuzumab, the first humanized monoclonal antibody targeting HER2, has revolutionized the treatment landscape and brought a significant breakthrough for HER2-positive breast cancer. Trastuzumab has firmly established itself as a cornerstone of adjuvant, neoadjuvant therapy and the systemic treatment for HER2-positive breast cancer for over two decades. With the progressive advancement of HER2-targeted therapeutics, the cardiotoxicity associated with these agents has garnered significant attention. A meta-analysis[11], which included ten randomized controlled trials (RCTs) with a total of 11,882 patients, revealed that trastuzumab significantly increased the risk of Left Ventricular Ejection Fractions (LVEF) decrease (RR = 2.13, 95% CI, 1.31-3.49; p = 0.003), as well as the risk of congestive heart failure (CHF) (RR = 4.19, 95% CI 2.73-6.42; p <0.001). Cardiac events (CEs) associated with other HER2-targeted agents have also been sporadically documented[12]. The EMILIA research indicated that the incidence of ≥3 grades cardiac dysfunction associated with T-DM1 was less than 1%, which was significantly lower than trastuzumab[13], and T-DXd exhibited similar findings[14]. The low incidence of such events makes small-scale studies inadequate for fully capturing the characteristics of HER2-targeted ADC-induced CEs. The impact of factors, including different drugs and drug concentrations, monotherapy or combination therapy, tumor types and stages, on the incidence of CEs associated with HER2-targeted ADC remains uncertain. Therefore, we thoroughly conducted a comprehensive systematic review and literature analysis of all prospective clinical studies on HER2-targeted ADCs to investigate the incidence of CEs at all grades and ≥3/serious grades, and identify the factors influencing these events.
Methods
Search strategy and selection criteria
The study protocol for our meta-analysis had been registered on PROSPERO (NO. CRD42023440448) and was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[15]. The prospective clinical trials on HER2-targeted ADCs were retrieved from PubMed, Embase, Cochrane Library and ClinicalTrials.gov, with a search period ranging from the database's inception to May 2023, and only English-language publications were included. Detailed search strategies were provided in Table S1. The clinical trials meeting the following criteria were included: (1) prospective clinical trials; (2) participants who received monotherapy or combination therapy of the approved HER2-targeted ADC; (3) available count data regarding treatment-related cardiotoxicity. Exclusion criteria: (1) studies with a sample size of less than ten participants; (2) trial in progress or no available date of CEs; (3) duplicate studies.
Data extraction
The trials were independently searched and screened by two researchers (Fen Liu and Huamin Li) according to inclusion and exclusion criteria. Any disagreements were resolved by a third researcher, Guisen Yin. The existence of reporting discrepancies between the results on ClinicalTrials.gov and those in peer-reviewed publications is widely acknowledged[16]. Given that ClinicalTrals.gov provides comprehensive reporting of all adverse events (AEs) and regularly updates them even after publication. Moreover, limited data are available in peer-reviewed publications, so we prioritized extracting data from ClinicalTrials.gov followed by articles. If multiple identical articles exist for the same sample, only the one with the most comprehensive documentation for CEs was chosen. Additionally, we also considered contacting the authors for data if necessary. The study was excluded if data could not be obtained. The following information was extracted: first author, publication year, study name, study types, study phase, NCT numbers, HER2-targeted ADCs used and their dosage, cancer type and cancer status, number of patients in the safety analysis, and number of all-grade and ≥3/serious grades CEs related to HER2-targeted ADC. The high-grade CEs reported in publication and serious CEs reported in ClinicalTrials.gov were pooled for a meta-analysis.
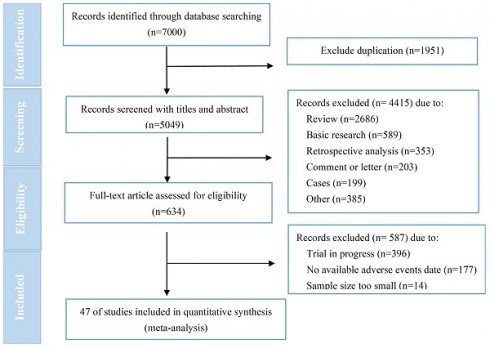
Flow diagram of included studies.
Study objective
The primary objectives were to assess the incidence of all-grade and ≥3/serious grades CEs related to HER2-targeted ADC and the occurrence of CEs leading to dosage discontinuation. The secondary objectives aimed to analyze the incidence of all-grade and ≥3 or serious grades CEs based on different drugs and drug concentrations, monotherapy or combination therapy, study phase, tumor types and tumor status.
Statistical analysis
Stata 15.0 software was used to perform the meta-analysis. The effect statistics were estimated as pooled incidence with 95% confidence intervals (CI). A combination of Chi-square test (α=0.10) and I2 value was employed to assess heterogeneity among the trials. If I2>50% or p<0.10, it indicated substantial heterogeneity across studies, necessitating the adoption of a random effects model. Subgroup analysis was conducted to compare the differences between subgroups, p<0.05 meant statistical difference. The methodological quality of literature was independently assessed by two researchers (Fen Liu and Huamin Li) using the Cochrane risk-of-bias tool (ROB) for randomized controlled trials (RCTs) and the Methodological index for non-randomized studies (MINORS) for non-RCTs[17]. Any inconsistencies were resolved through discussion with a third researcher (Guisen Yin). The stability of the pooled incidence estimate was evaluated through a sensitivity analysis, in which each literature was systematically omitted one by one. Additionally, publication bias in the included studies was assessed using a funnel plot and Egger's test (α=0.05).
Results
Study selection and characteristics
7000 relevant studies were identified after conducting a comprehensive literature search. Following a stepwise screening procedure outlined in Figure 1, a total of 47 trials[7, 13, 14, 18-65] were selected for analysis. No one was for RC48 due to a lack of available data. The quality assessment showed that all RCTs (n=16) were classified as high risk, primarily due to the absence of blinding among participants, personnels and assessors (Figure S1). The MINORS scores for non-RCTs (n=31) ranged from 8 to 21, with a mean score of 11.5 (Table S2). Failure to implement blinded evaluation was frequently identified as a common deficiency for non-RCTs. According to established principles of data extraction, we extracted data from ClinicalTrials.gov for 27 trials and published articles or abstracts for 20 trials. The general characteristics of included trials were presented in Table 1. T-DM1 was assessed in 37 trials (n=8664), T-DXd was assessed in 11 trials (n=1930), T-DXd and T-DM1 evaluated within the same study[19]. The 37 trials related to T-DM1 consisted of 19 monotherapy trials, 14 combination therapy trials, and 4 trials that involved both monotherapy and combination therapy. It was predominantly administered in advanced/metastatic tumors (30/37). Breast cancer emerged as the most prevalent tumor type for T-DM1 application (32 /37), followed by lung cancer (3 /37), gastric cancer (2 /37), and other solid tumors (1/37). Among these, 13 were randomized controlled trials (RCT) and 24 were non-RCT. The 11 trials on T-DXd consisted of 4 RCTs and 7 non-RCTs. These trials were all conducted in patients with advanced/metastatic disease (11/11), primarily focusing on monotherapy (10/11). As well, breast cancer emerged as the most prevalent tumor type for T-DXd application (8/11), followed by gastric cancer (3 /11), other solid tumors (2/11), and lung cancer (1 /11).
General characteristics of included studies in this meta-analysis.
| First author | Year | Study name | NCT NO. | Study types | Phase | ADC Drug | Dosage | Cancer | Cancer status | No. of patients | No. of CEs | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All-grade | ≥3/serious grades | Discontinuation | |||||||||||
| R. Barroso-Sousa[18] | 2022 | ATEMPT | NCT01853748 | RCT | 2 | T-DM1 | 3.6 mg/kg q3w | breast | early | 383 | 27 | 1 | 3 |
| J. Cortés[19] | 2022 | DESTINY-Breast03 | NCT03529110* | RCT | 3 | T-DM1 | 3.6 mg/kg q3w | breast | metastatic | 263 | 1 | 0 | NA |
| Iwama E[20] | 2022 | NA | Iwama E, 2022* | single-arm | 2 | T-DM1 | 3.6 mg/kg q3w | lung | locally advanced/metastatic | 22 | 1 | 1 | 0 |
| Peters, S[21] | 2019 | NA | NCT02289833 | two-arm | 2 | T-DM1 | 3.6 mg/kg q3w | lung | locally advanced/metastatic | 49 | 2 | 0 | 0 |
| Montemurro, F[22] | 2019 | KAMILLA | NCT01702571 | single-arm | 3 | T-DM1 | 3.6 mg/kg q3w | breast | advanced | 2002 | 13 | 13 | NA |
| Watanabe, J[23] | 2017 | JO29317 | JapicCTI-132395* | single-arm | 2 | T-DM1 | 3.6 mg/kg q3w | breast | advanced | 232 | 2 | 2 | 2 |
| Krop, I. E[24] | 2017 | TH3RESA | NCT01419197 | RCT | 3 | T-DM1 | 3.6 mg/kg q3w | breast | advanced | 403 | 12 | 1 | NA |
| Thuss-Patience PC[25] | 2017 | GATSBY | NCT01641939 | RCT | 2/3 | T-DM1 | 2.4mg/kg qw 3.6 mg/kg q3w | gastric | advanced | 293 | 2 | 2 | NA |
| Krop, I. E[26] | 2015 | TDM4874g | NCT01196052* | single-arm | 2 | T-DM1 | 3.6 mg/kg q3w | breast | early | 148 | 9 | 1 | 1 |
| Hurvitz, S. A[27] | 2013 | TDM4450g | NCT00679341 | RCT | 2 | T-DM1 | 3.6 mg/kg q3w | breast | metastatic | 69 | 6 | 2 | NA |
| Dieras, V[13] | 2017 | EMILIA | NCT00829166 | RCT | 3 | T-DM1 | 3.6 mg/kg q3w | breast | advanced | 490 | 5 | 5 | NA |
| Burris, H. A., 3rd[28] | 2011 | TDM4258g | NCT00509769* | single-arm | 2 | T-DM1 | 3.6 mg/kg q3w | breast | metastatic | 112 | 2 | 0 | 0 |
| Beeram, M[29] | 2012 | TDM3569g | NCT00932373 | single-arm | 1 | T-DM1 | 0.3-4.8 mg/kg q3w 1.2 -2.9mg/kg qw | breast | advanced | 52 | 1 | NA | NA |
| Krop, I. E[30] | 2012 | TDM4374g | NCT00679211 | single-arm | 2 | T-DM1 | 3.6 mg/kg q3w | breast | metastatic | 110 | 1 | 1 | 0 |
| de Vries, E. G. E[31] | 2023 | KAMELEON | NCT02999672 | single-arm | 2 | T-DM1 | 2.4 mg/kg qw 3.6 mg/kg q3w | UC/PC/CAA | advanced | 20 | 1 | 0 | 0 |
| Yardley, D. A[32] | 2015 | TDM4884g | NCT01120561* | single-arm | NA | T-DM1 | 3.6 mg/kg q3w | breast | metastatic | 215 | 14 | 4 | 4 |
| Kashiwaba, M[33] | 2016 | JO22997 study | JAPIC CTI-101277* | single-arm | 2 | T-DM1 | 3.6 mg/kg q3w | breast | locally advanced/recurrent /metastatic | 73 | 2 | 0 | 1 |
| Yamamoto, H[34] | 2015 | NA | Yamamoto H,2015* | single-arm | 1 | T-DM1 | 3.6 mg/kg q3w | breast | metastatic | 10 | 2 | 0 | 0 |
| Huang, C. S[35] | 2021 | KATHERINE | NCT01772472* | RCT | 3 | T-DM1 | 3.6 mg/kg q3w | breast | residual invasive disease after neoadjuvant | 740 | 23 | 4 | NA |
| Cortes, J[36] | 2020 | TRAXHER2 | NCT01702558 | RCT | 1/2 | T-DM1 + /-capecitabine | 2.4/ mg/kg qw 3.6 mg/kg q3w | gastric/breast | locally advanced/metastatic | 178 | 10 | 1 | NA |
| Martin, M[37] | 2016 | BP22572 | NCT00934856 | non-RCT | 1/2 | T-DM1 + docetaxel+/-P | 2.4mg/kg qw 3.6 mg/kg q3w | breast | locally advanced/metastatic | 98 | 3 | 0 | 0 |
| Perez, E. A[38] | 2019 | MARIANNE | NCT01120184 | RCT | 3 | T-DM1 +/-P | 3.6 mg/kg q3w | breast | advanced | 727 | 28 | 5 | NA |
| Abraham, J[39] | 2019 | NA | NCT02236000 | single-arm | 1/2 | T-DM1+ neratinib | 3.6 mg/kg q3w | breast | metastatic | 76 | 4 | 0 | NA |
| Jebbink, M[40] | 2023 | TRAEMOS | NCT03784599* | single-arm | 1/2 | T-DM1+ osimertinib | 3.6 mg/kg q3w | lung | metastatic | 27 | 1 | 0 | 1 |
| Emens, L. A[41] | 2020 | KATE2 | NCT02924883* | RCT | 2 | T-DM1+/-atezolizumab | 3.6 mg/kg q3w | breast | metastatic | 200 | 2 | 2 | NA |
| Gupta, M[42] | 2013 | TDM4688g | NCT00943670 | single-arm | 2 | T-DM1+/-P | 3.6 mg/kg q3w | breast | metastatic | 71 | 8 | 1 | 1 |
| Jain, S[43] | 2018 | NA | NCT02038010 | single-arm | 1 | T-DM1+alpelisib | 3.6 mg/kg q3w | breast | metastatic | 17 | 15 | 0 | NA |
| Patel, T. A[44] | 2019 | TEAL | NCT02073487 | RCT | 2 | T-DM1+lapatinib+nab-paclitaxel | 3.0 mg/kg q3w | breast | stage II to III | 14 | 1 | 0 | NA |
| Lopez-Miranda, E[45] | 2020 | THELMA | NCT02562378 | single-arm | 1 | T-DM1+non-pegylated liposomal doxorubicin | 3.6 mg/kg q3w | breast | metastatic | 15 | 13 | 0 | 0 |
| Krop, I. E[46] | 2022 | KAITLIN | NCT01966471 | RCT | 3 | TDM-1+P | 3.6 mg/kg q3w | breast | early | 912 | 36 | 7 | NA |
| Hurvitz, S. A[47] | 2019 | KRISTINE | NCT02131064 | RCT | 3 | T-DM1+P | 3.6 mg/kg q3w | breast | stage II to III | 223 | 1 | 1 | NA |
| Miller, K. D[48] | 2014 | NA | NCT00875979* | single-arm | 1/2 | T-DM1+P | 3.6 mg/kg q3w | breast | locally advanced/metastatic | 64 | 2 | 2 | 1 |
| Filho, O. M[49] | 2021 | NA | Filho OM, 2021* | single-arm | 2 | T-DM1+P | 3.6 mg/kg q3w | breast | early | 163 | 5 | 0 | NA |
| Krop, I. E[50] | 2016 | TDM4652g | NCT00951665 | single-arm | 1/2 | T-DM1+paclitaxel ±P | 1.2-2.4 mg/kg qw 2.0-3.6mg/kg q3w | breast | metastatic | 104 | 2 | 2 | 0 |
| Waks, A. G[51] | 2022 | NA | NCT03032107* | single-arm | 1 | T-DM1+pembrolizumab | 3.6 mg/kg q3w | breast | metastatic | 20 | 1 | 0 | NA |
| Spring, L. M[52] | 2021 | NA | NCT02657343* | single-arm | 1 | T-DM1+ribociclib | 3.6 mg/kg q3w | breast | advanced/metastatic | 12 | 5 | 0 | NA |
| Borges, V. F[53] | 2018 | NA | NCT01983501* | single-arm | 1 | T-DM1+tucatinib | 3.6 mg/kg q3w | breast | advanced | 57 | 2 | 0 | 2 |
| Cortes, J[19] | 2022 | DESTINY-Breast03 | NCT03529110* | RCT | 3 | T-DXd | 5.4 mg/kg q3w | breast | metastatic | 261 | 7 | 0 | NA |
| Modi, S[54] | 2020 | DESTINY-Breast01 | NCT03248492 | single-arm | 2 | T-DXd | 5.4 mg/kg q3w | breast | metastatic | 184 | 14 | 1 | 0 |
| Andre, F[55] | 2023 | DESTINY-Breast02 | NCT03523585* | RCT | 3 | T-DXd | 5.4 mg/kg q3w | breast | metastatic | 406 | 18 | 2 | 2 |
| Modi, S[14] | 2022 | DESTINY-Breast04 | NCT03734029* | RCT | 3 | T-DXd | 5.4 mg/kg q3w | breast | metastatic | 371 | 63 | 7 | NA |
| Bartsch, R[56] | 2022 | TUXEDO-1 trial | NCT04752059* | single-arm | 2 | T-DXd | 5.4 mg/kg q3w | breast | metastases | 15 | 1 | 1 | 1 |
| Shimomura, A[57] | 2023 | DS8201-A-J102 | NCT03366428 | single-arm | 1 | T-DXd | 6.4 mg/kg q3w | breast | metastatic | 51 | 7 | 0 | 1 |
| Chang D.Y[58] | 2019 | NA | NCT03368196 | single-arm | 1 | T-DXd | 6.4 mg/kg q3w | breast/gastric | advanced | 12 | 1 | 0 | 0 |
| Tamura K[59], Modi, S[60], Shitara, K[61], Tsurutani, J[62], Doi, T[63] | 2019 | J101 | NCT02564900 | single-arm | 1 | T-DXd | 5·4/ 6·4 mg/kg q3w 0.8-8.0mg/kg q3w | breast/gastric/other solid tumors | advanced/metastatic | 299 | 42 | 0 | 0 |
| Yamaguchi, K[64] | 2023 | DESTINY-Gastric01 | NCT03329690* | RCT | 2 | T-DXd | 6.4 mg/kg q3w | gastric | advanced | 169 | 4 | 3 | NA |
| Li, B. T[7] | 2022 | DESTINY-Lung01 | NCT03505710 | single-arm | 2 | T-DXd | 5.4/6.4 mg/kg q3w | lung | relapsed/refractory | 181 | 10 | 2 | NA |
| Takahashi, S[65] | 2021 | NA | NCT03383692 | single-arm | 1 | T-DXd +Ritonavir/Itraconazole | 5.4 mg/kg q3w | solid tumor | unresectable/metastatic | 40 | 2 | 0 | NA |
Abbreviations: CE: cardiac event, NA: not available;*data extracted from published articles or abstracts.
The incidence of ADC-related cardiac events. (A) The incidence of all-grade ADC-related cardiac events in 47 trials. (B) The incidence of ≥3/serious grades ADC-related cardiac events in 26 trials. (C) The incidence of ADC-related cardiac events resulting in the dosage discontinuation in 12 trials
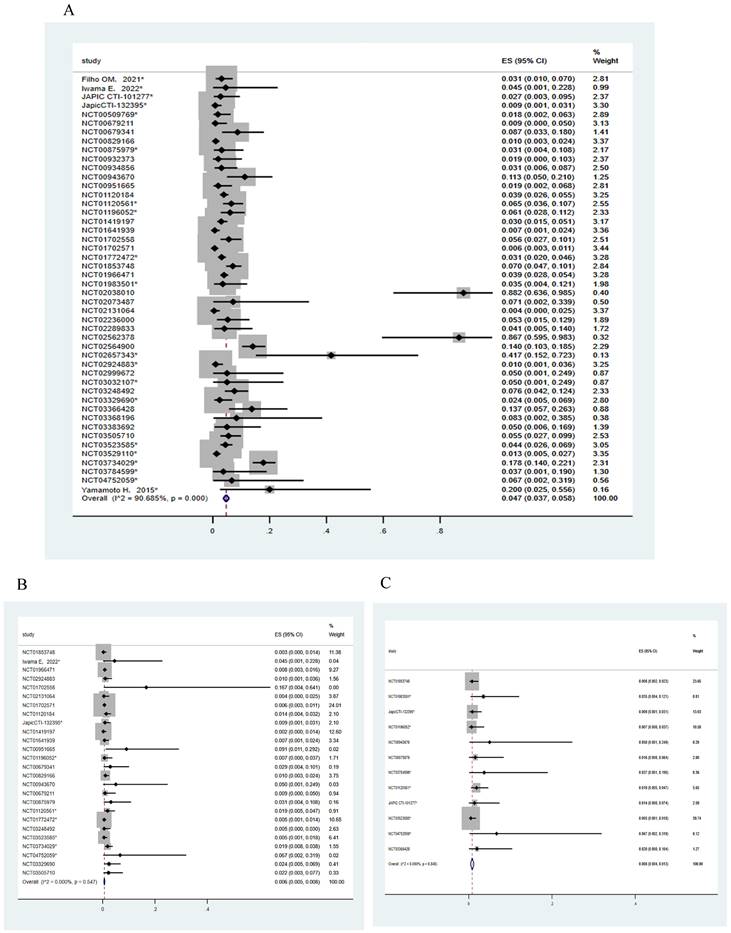
The incidence of HER2-targeted ADC-related CEs
A total of 47 trials involving 10594 patients and 26 trials involving 8112 patients were included for analyzing the incidence of all-grade and ≥3/serious grades HER2-targeted ADC-related CEs, respectively. The incidence of CEs leading to dosage discontinuation was analyzed in 12 trials involving 1691 patients. The random effects model was employed for meta-analysis due to the substantial heterogeneity. As shown in Figure 2, the incidence of CEs at all-grade and ≥3/serious grades respectively were 4.7% (95% CI, 3.7-5.8%) and 0.6% (95% CI, 0.5-0.8%). The incidence of CEs leading to dosage discontinuation was 0.8% (95% CI, 0.4-1.3%).
The incidence of all-grade CEs across different subgroups
The incidence of all-grade CEs across different drugs
The incidence of all-grade CEs associated with T-DM1 and T-DXd were respectively assessed in 37 trials and 11 trials. The incidence of all-grade CEs associated with T-DXd was 7.7% (95% CI, 4.5-10.9%), which was significantly higher than that of T-DM1[3.6% (95% CI, 2.6-4.6%)](p=0. 017)(Table 2).
The incidence of all-grade CEs between phase I/II trials and phase III trials
A total of 35 phase I/II trials and 10 phase III trials were included for analysis. Two trials were excluded, one with the unknown study phase (NCT01120561) and another with phase II/III (NCT01641939). The incidence of all-grade CEs in phase III trials was 3.2% (95% CI, 1.9-4.5%), which was significantly lower than that of phase I/II trials[6.9% (95% CI, 4.9-8.9%)] (p=0.002) (Table 2). Further, a subgroup analysis of various drugs was performed. Similarly, the incidence of all-grade CEs associated with T-DM1 in phase III trials was 1.9% (95% CI, 1.0-2.8%), which was significantly lower than that of phase I/II trials[6.6% (95% CI, 4.4-8.9%)] (p<0.001) (Figure S2A). However, the incidence of all-grade CEs associated with T-DXd between phase I/II and III trials were similar, with rates of 7.6 (95% CI, 3.9-11.2%) and 8.1% (95 %CI, 1.2-14.9%) (p=0.906) (Figure S2B ).
The incidence of all-grade CEs between monotherapy and combination therapy
47 trials were included in the analysis, with 4 of them (NCT02924883, NCT01702558, NCT01120184, NCT00943670) examining both monotherapy and combination therapy. The data from these four trials were further divided, resulting in 32 studies assessing monotherapy and 19 studies evaluating combination therapy. As shown in Table 2, the incidence of all-grade CEs in combination therapy was 7.6% (95% CI, 4.9-10.4%), which was significantly higher than that of monotherapy at the rate of 3.9% (95% CI, 3.0-4.9%) (p=0.013). Data on monotherapy were further analyzed according to drug subgroup. As presented in Figure S3A, the incidence of all-grade CE in T-DXd monotherapy treatment was 8.8% (95%CI, 4.9-12.7%), which was significantly higher than that of T-DM1 monotherapy treatment at the rate of 2.3% (95% CI, 1.5-3.0%) (p=0.001). As presented in Figure S4A, the incidence of all-grade CEs in T-DM1 combination therapy was 7.8% (95 %CI, 5.0-10.7%), which was significantly higher than that of monotherapy at a rate of 2.3% (95 %CI, 1.5-3.0%) (p<0.001).
The incidence of all-grade CEs in different solid tumor
Further, the CEs of various tumor types were analyzed. The two trials (NCT01702558, NCT02564900) were analyzed separately for breast and gastric cancer. The trial (NCT03368196) was excluded from this analysis due to the inability to separate data from breast and gastric cancer. The final analysis results were shown in Table 2. The incidence of all-grade CEs was 5.5% (95 %CI, 3.7-7.6%) for breast cancer, 4.5% (95% CI, 2.1-7.5%) for lung cancer, 3.0% (95% CI, 0.0-9.1%) for gastric cancer, and 4.8% (95% CI, 0.3-12.5%) for other solid tumors.
Breast cancer emerged as the most prevalent tumor type in our meta-analysis. Subsequently, we conducted a subgroup analysis based on the tumor status of breast cancer. As shown in Table 2, the incidence of all-grade CEs was 3.7% (95% CI, 1.8-5.6%) for early-stage breast cancer and 5.8% (95% CI, 4.3-7.3%) for advanced/metastatic breast cancer, with no statistical differences (p=0.093).
The incidence of all-grade CEs between different dosages for T-DM1 and T-DXd
The incidence of CEs of various dose subgroups for T-DM1 and T-DXd was analyzed. As shown in Table 2, the incidence of all-grade CEs of T-DM1 was 4.3% (95% CI, 2.7-6.2 %) for 3.6mg/kg treatment and 3.8% (95 %CI, 0.0-19.1%) for less than 3.6mg/kg treatment, with no statistical differences (p=0.933). Likewise, the incidence of all-grade CEs of T-DXd was 7.2% (95% CI, 2.8-13.0%) for 5.4mg/kg treatment and 7.1% (95% CI, 1.4-15.9%) for 6.4 mg/kg treatment, with no statistical differences (p=0.933).
The incidence of ≥3/serious grades CEs across different subgroups
Table 3 showed no statistically significant difference in the incidence of ≥3/serious grades CEs between T-DXd and T-DM1, phase I/II and III trials, monotherapy and combination therapy, different tumor types and tumor status. However, a trend towards a higher incidence of ≥3/serious grades CEs was observed in T-DXd compared to T-DM1 (1.0% versus 0.6%; p=0.307), as well as in advanced/metastatic breast cancer compared to early-stage breast cancer (0.7% versus 0.5%; p=0.379) (Table 3).
Types of CEs associated with HER2-targeted ADC
The CEs documented in the 47 literatures underwent analysis. As presented in Table 4, a total of 440 cases and 73 cases of CEs at all grades and ≥3/serious grades CEs were respectively documented. The incidence of special CEs reported in more than three trials was pooled and analyzed, while only descriptive analysis was performed for those reported in three or fewer trials. The analysis revealed that electrocardiogram QT corrected interval prolonged was identified as the CE with the highest pooled incidence, at a rate of 5.9% (95% CI, 3.3-8.5%).
Subgroup analysis for the incidence of all-grade HER2-targeted ADC-related cardiac events.
| Subgroup variables | No. of trials | No. of patients | Pooled ES (95% CI) | Measure of heterogeneity | |
|---|---|---|---|---|---|
| p | I2 | ||||
| ADC Drug | 0.017* | / | |||
| T-DM1 | 37 | 8664 | 0.036 (0.026-0.046) | <0.001 | 90.003% |
| T-DXd | 11 | 1945 | 0.077 (0.045-0.109) | <0.001 | 86.798% |
| Phase of trials | 0.002* | / | |||
| Ⅰ/Ⅱ | 35 | 3303 | 0.069 (0.049,0.089) | <0.001 | 89.244% |
| III | 10 | 6798 | 0.032 (0.019,0.045) | <0.001 | 93.437% |
| Combination therapy | 0.013* | / | |||
| No | 32 | 7888 | 0.039(0.030,0.049) | <0.001 | 86.641% |
| Yes | 19 | 2460 | 0.076 (0.049,0.104) | <0.001 | 93.201% |
| Cancer type | 0.787* | / | |||
| Breast cancer | 38 | 9534 | 0.055 (0.037,0.076) | <0.001 | 91.727% |
| Lung cancer | 4 | 279 | 0.045(0.021,0.075) | 0.994 | 0.000% |
| Gastric cancer | 3 | 462 | 0.030(0.000,0.091) | / | / |
| Other solid tumor | 2 | 60 | 0.048(0.003,0.125) | / | / |
| Cancer status | 0.093* | / | |||
| Early-stage breast cancer | 7 | 2583 | 0.037(0.018,0.056) | <0.001 | 86.008% |
| advanced/metastatic stage breast cancer | 32 | 6963 | 0.058(0.043,0.073) | <0.001 | 92.527% |
| Dosage for T-DM1 | 0.933* | / | |||
| 3.6 mg/kg q3w | 34 | 8178 | 0.043(0.027,0.062) | <0.001 | 89.187% |
| Less than 3.6 mg/kg q3w | 3 | 253 | 0.038(0.000,0.191) | / | / |
| Dosage for T-DXd | 0.933* | / | |||
| 5.4 mg/kg q3w | 7 | 1386 | 0.072(0.028,0.130) | <0.001 | 89.935% |
| 6.4 mg/kg q3w | 4 | 232 | 0.071(0.014,0.159) | 0.011 | 73.305% |
Abbreviations: ES: Effect Size, CI: confidence interval, *test for subgroup differences.Statistically significant values are marked in boldface.
Subgroup analysis for the incidence of ≥3/serious grades HER2-targeted ADC-related cardiac events.
| Subgroup variables | No. of trials | No. of patients | Pooled ES (95% CI) | Measure of heterogeneity | |
|---|---|---|---|---|---|
| p | I2 | ||||
| ADC Drug | 0.307* | / | |||
| T-DM1 | 20 | 6920 | 0.006 (0.004,0.008 ) | 0.621 | 0.000% |
| T-DXd | 6 | 1192 | 0.010 (0.003,0.017) | 0.270 | 21.735% |
| Phase of trials | 0.969* | / | |||
| Ⅰ/Ⅱ | 35 | 3303 | 0.006 (0.002,0.010) | 0.502 | 0.000% |
| III | 10 | 6798 | 0.006 (0.004,0.008) | 0.394 | 4.910% |
| Combination therapy | 0.239* | / | |||
| No | 19 | 6367 | 0.005 (0.003,0.009) | 0.127 | 27.834% |
| Yes | 8 | 1745 | 0.004 (0.000,0.015) | 0.046 | 51.013% |
| Cancer type | 0.197* | / | |||
| Breast cancer | 21 | 7575 | 0.005 (0.002,0.008) | 0.081 | 31.863% |
| Lung cancer | 2 | 113 | 0.021(0.000,0.062) | / | / |
| Gastric cancer | 2 | 418 | 0.010(0.002,0.023) | / | / |
| Cancer status | 0.379* | / | |||
| Early-stage breast | 4 | 2583 | 0.005(0.002,0.008) | 0.620 | 0.000% |
| advanced/metastatic stage breast | 19 | 6963 | 0.007(0.005,0.009) | 0.450 | 0.112% |
Abbreviations: ES: Effect Size, CI: confidence interval. *test for subgroup differences.
Types of cardiac events associated with HER2-targeted ADC
| Types | No. of trials | No. of patients | No. of All- grade CEs | No. of ≥3/serious grades CEs | Pooled analysis of all-grade CEs | ||
|---|---|---|---|---|---|---|---|
| ES (95% CI) | p | I2 | |||||
| Ejection fraction decrease | 17 | 4443 | 173 | 11 | 0.033(0.022,0.044) | <0.001 | 75.991% |
| Electrocardiogram QT corrected interval prolonged | 11 | 639 | 50 | 0 | 0.059(0.033,0.085) | 0.015 | 54.414% |
| Palpitations | 10 | 3296 | 40 | 2 | 0.027(0.010,0.044) | <0.001 | 75.623% |
| Left ventricular systolic dysfunction | 6 | 1207 | 23 | 4 | 0.014(0.001,0.028) | 0.003 | 72.114% |
| Tachycardia | 7 | 1046 | 22 | 2 | 0.025(0.005,0.044) | 0.005 | 67.708% |
| Sinus tachycardia | 7 | 1933 | 19 | 2 | 0.016(0.000,0.052) | <0.001 | 88.174% |
| Cardiac failure(congestive) | 14 | 4867 | 18 | 14 | 0.001(0.000,0.002) | 0.432 | 1.546% |
| Atrial fibrillation | 6 | 1407 | 6 | 6 | 0.003(0.000,0.006) | 0.884 | 0.000% |
| Pericardial effusion | 6 | 2625 | 8 | 5 | 0.002(0.000,0.016) | 0.003 | 71.745% |
| Troponin increased | 2 | 106 | 7 | 1 | / | / | / |
| Brain natriuretic peptide increased | 1 | 15 | 6 | 0 | / | / | / |
| Acute coronary syndrome | 2 | 2308 | 5 | 5 | / | / | / |
| Cardiomyopathy | 3 | 1527 | 3 | 3 | / | / | / |
| Supraventricular tachycardia | 3 | 2751 | 4 | 3 | / | / | / |
| Cardic arrest | 2 | 2022 | 3 | 2 | / | / | / |
| Sinus bradycardia | 3 | 1312 | 3 | 1 | / | / | / |
| Atrial thrombosis | 2 | 223 | 2 | 2 | / | / | / |
| Ventricular tachycardia | 2 | 749 | 2 | 1 | / | / | / |
| Supraventricular extrasystoles | 2 | 217 | 2 | 1 | / | / | / |
| Nonspecific T Wave Abnormality on ECG | 2 | 58 | 2 | 1 | / | / | / |
| Myocardial infarction | 2 | 20 | 2 | 1 | / | / | / |
| Other* | 13 | 4485 | 40 | 6 | / | / | / |
*include ventricular fibrillation, extrasystoles, angina pectoris , ventricular dysfunction, cardiac tamponade,pericarditis1, Chest pain-cardiac, cardio-respiratory arrest , pulmonary edema , right bundle block, tricuspid valve incompetence, mitral valve incompetence etc.
Abbreviations: CE: cardiac event, ES: Effect Size, CI: confidence interval
Additionally, the ejection fraction decreased emerged as the highest proportion of all grades CEs, accounting for 39.3% (173/440), and cardiac failure (congestive) emerged as the most proportion of ≥3/serious grades CEs with a ratio of 19.2% (14/73).
Sensitivity analysis and publication bias
After excluding single literature, the incidence of all-grade CEs remained consistent with the incidence prior to literature exclusion, indicating the stability and reliability of the meta-analysis results. Funnel plots were utilized to visually assess the publication bias in the literature included within this study. As depicted in Figure S5, the meta-analyses examining the incidence of all grades and ≥3/serious grades CEs exhibited significant publication bias, which were further confirmed by the Egger's test (p<0.001).
Discussion
To our knowledge, this meta-analysis comprehensively evaluated the incidence of CEs associated with commercially available HER2-targeted ADCs first. Our research indicated that the pooled incidence of all grades and ≥3/serious grades CEs associated with HER2-targeted ADC were 4.7% and 0.6%. The pooled incidence of CEs leading to dosage discontinuation was 0.8%. A significantly higher incidence of all-grade CEs was revealed in T-DXd treatment compared to T-DM1 treatment, as well as in phase I/II trials compared to phase III trials and combination therapy compared to monotherapy. No statistical difference was observed in the incidence of CEs across different solid tumors, various dosages of T-DM1 and T-DXd, and tumor status at all grades. The occurrence of ≥3/serious grades CEs did not show notable disparity in any subgroup. Further analysis revealed that electrocardiogram QT corrected interval prolonged emerged as the CE with the highest pooled incidence, occurring at a rate of 5.9%.
Although the three HER2-targeted ADCs share the same targeting antibody-trastuzumab, the incidence of cardiotoxicity may also be influenced by factors such as linker stability, intensity of HER2 signal blocking, and pharmacokinetic parameters, etc., which have not been elucidated by studies. The approval of RC48 was based on objective response rates (ORR) observed in single-arm clinical trials, and currently there is a lack of available studies reporting any clinical data regarding cardiac toxicity. A pooled analysis revealed a total rate of 3.37% (95% CI, 2.6-4.3%) for T-DM1-associated cardiotoxicity in advanced HER2-positive breast cancer[66]. Our findings align with this data, indicating a pooled occurrence of 3.6% (95% CI, 2.6-4.6%) for T-DM1-associated cardiotoxicity across different solid tumors, varying dosages, and tumor statuses. The total incidence of T-DXd-associated CEs has not yet been reported. Our research indicated that the total incidence of CEs associated with T-DXd was 7.7% (95% CI, 4.5-10.9%), significantly higher than that of T-DM1. Notably, the incidence of HER2-targeted ADCs is significantly lower than that of trastuzumab[12], despite the vast majority of patients included in this research were previously exposed to trastuzumab with/without anthracycline. Although we have not yet provided a pharmacological explanation for this difference, the innovative structural design of ADC is believed to be a significant contributing factor.
The combination therapy of T-DM1 or T-DXd in clinical practice is uncommon. Both the American Society of Clinical Oncology (ASCO)[67] and the National Comprehensive Cancer Network (NCCN)[68] recommend T-DM1 or T-DXd as a monotherapy. However, the safety and efficacy of T-DM1 combination therapy have been extensively explored in multiple clinical studies included in our paper. The combination drugs include chemotherapeutic agents (such as docetaxel, taxane, nab-paclitaxel, capecitabine, non-pegylated liposomal doxorubicin), HER2 targeting agents (including tucatinib, neratinib, lapatinib, pertuzumab), EGFR inhibitors (such as osimertinib), PI3K inhibitors (such as alpelisib), immune checkpoint inhibitors (atezolizumab and pembrolizumab), as well as cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor (ribociclib). It is well known that the incidence of CEs was found to be higher when trastuzumab combination therapy, particularly in combination with anthracycline or paclitaxel, at rates of 27% and 13%, respectively[69]. Similarly, our study demonstrated a higher occurrence rate of T-DM1-induced CEs in combination therapy compared to monotherapy (7.8% versus 2.3%; p<0.001). There was limited available data on the combination therapy for T-DXd in this paper, and further investigation is necessary to determine whether there will be an increase in the incidence of CEs with combination treatment. The findings of our research served as a reminder for healthcare professionals to exercise caution when considering the combination administration of T-DM1.
The KATHERINE trial[35]established the role of T-DM1 in HER2-positive early breast cancer patients with the residual invasive disease following neoadjuvant therapy. Our research findings indicated a slightly lower incidence of all-grade CEs in early-stage breast cancer compared to advanced/metastatic cases (3.7% versus 5.8%), although this difference did not reach statistical significance (p=0.093). Possible factors contributing to this phenomenon include poor performance in advanced/metastatic patients, cumulative administration of anthracyclines, the impact of prior treatments, or potential biases arising from early-stage clinical trials with limited data, etc. Although a higher prevalence of interstitial pneumonia was observed in the high-dose group of T-DXd [70], it has been indicated that varying doses had no impact on treatment-related CEs associated with T-DM1 and T-DXd. Given the small sample size of the phase Ⅰ/Ⅱ trial and the lack of extensive research for tumors other than breast cancer, the comparative results of differences in related subgroups should be interpreted cautiously.
Our findings suggested a low all-grade incidence of CEs associated with HER2-targeted ADC and a lower incidence of severe CE or those leading to treatment discontinuation, which is consistent with current studies[66, 70]. Unlike anthracyclines, HER2 targeting agents induce reversible cardiotoxicity characterized by cellular dysfunction and asymptomatic changes in LVEF without inducing cardiomyocyte injury or even death[12]. The most frequently observed CEs associated with HER2-targeted ADC were prolonged corrected QT intervals, with a prevalence rate of 5.9%, which closely aligns with the findings reported by Soares L.R[70]. Prolongation of corrected QT interval is known to be associated with fatal torsade de pointes arrhythmias. However, studies have revealed that both T-DM1[42] and T-DXd[57] did not have a clinically significant impact for the prolonged QTc interval remained below the safety threshold of 10 milliseconds. Our research revealed that ejection fraction decrease and congestive cardiac failure were the highest proportion of all-grade and serious CEs, respectively. The instructions of T-DM1 and T-DXd emphasize the significance of regular monitoring of LVEF before and during treatment. Once symptomatic congestive heart failure occurs, permanent discontinuation is recommended.
Limitations
Our study had several limitations. Firstly, the patient characteristics could not be extracted, including underlying heart disease, baseline LVEF value, cumulative doses of anthracycline, cumulative treatment cycles of previous HER2-targeted agents, etc. Furthermore, the timing, duration, and prognosis of CEs were also unavailable. Consequently, this paper could not discuss the risk factors and characteristics associated with HER2-targeted ADC-induced CEs. Secondly, the limited scope of research on tumors other than breast cancer may introduce a potential bias in subgroup analysis across different tumor types. Last but not least, trials that failed to report CEs were excluded, potentially introducing publication bias and inflating the risk of HER2-targeted ADC-related CEs. On the contrary, the inclusion of all these trials in the study would significantly mitigate the occurrence of CEs, thereby potentially misleading physicians' clinical judgment.
Conclusions
A meta-analysis of 47 trials involving 10594 patients found that HER2-targeted ADC did not result in a high incidence of CEs at all grades and ≥3/serious grades. Further analysis indicated a significantly higher incidence of all-grade CEs in T-DXd treatment, phase I/II trials and combination therapy. Ejection fraction decreased and cardiac failure (congestive) emerged as the highest proportion of all-grade and ≥3/serious grades CEs, respectively. Our study provide a valuable reference for managing cardiotoxicity. Future real-world data are expected to elucidate further the pathogenesis and population characteristics of HER2-targeted ADC-induced cardiotoxicity.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This study was funded by the Hunan Cancer Hospital Climb Plan (No: YF2021004), and Hunan Provincial Health Commission Project (D202313017815).
Author contributions
Fen Liu and Huamin Li made significant contributions to the development of research concepts, the compilation of data, and the composition of articles. Guisen Yin was responsible for the data verification and resolution of disputes. Yong Pan provided constructive discussions during the analysis.
Availability of data and materials
Data will be available upon request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther. 2022;7:93
2. Amiri-Kordestani L, Blumenthal GM, Xu QC, Zhang L, Tang SW, Ha L. et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res. 2014;20:4436-41
3. Wedam S, Fashoyin-Aje L, Gao X, Bloomquist E, Tang S, Sridhara R. et al. FDA Approval Summary: Ado-Trastuzumab Emtansine for the Adjuvant Treatment of HER2-positive Early Breast Cancer. Clin Cancer Res. 2020;26:4180-5
4. Narayan P, Osgood CL, Singh H, Chiu HJ, Ricks TK, Chiu Yuen Chow E. et al. FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast Cancer. Clin Cancer Res. 2021;27:4478-85
5. Narayan P, Dilawari A, Osgood C, Feng Z, Bloomquist E, Pierce WF. et al. US Food and Drug Administration Approval Summary: Fam-Trastuzumab Deruxtecan-nxki for Human Epidermal Growth Factor Receptor 2-Low Unresectable or Metastatic Breast Cancer. J Clin Oncol. 2023;41:2108-16
6. Duval J, Zaanan A. [New drug approval: Trastuzumab-deruxtecan in HER2 positive advanced gastric or gastroesophageal junction cancer after previous treatment with trastuzumab]. Bull Cancer. 2023;110:739-40
7. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazieres J. et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med. 2022;386:241-51
8. Deeks ED. Disitamab Vedotin: First Approval. Drugs. 2021;81:1929-35
9. Indini A, Rijavec E, Grossi F. Trastuzumab Deruxtecan: Changing the Destiny of HER2 Expressing Solid Tumors. Int J Mol Sci. 2021;22:4774
10. Wang Z. ErbB Receptors and Cancer. Methods Mol Biol. 2017;1652:3-35
11. Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y. et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev. 2011;37:312-20
12. Jerusalem G, Lancellotti P, Kim SB. HER2+ breast cancer treatment and cardiotoxicity: monitoring and management. Breast Cancer Res Treat. 2019;177:237-50
13. Dieras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J. et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732-42
14. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E. et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387:9-20
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906
16. Becker JE, Ross JS. Reporting discrepancies between the clinicaltrials.gov results database and peer-reviewed publications. Ann Intern Med. 2014;161:760
17. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-6
18. Barroso-Sousa R, Tarantino P, Tayob N, Dang C, Yardley DA, Isakoff SJ. et al. Cardiac outcomes of subjects on adjuvant trastuzumab emtansine vs paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT) study (TBCRC033): a randomized controlled trial. NPJ Breast Cancer. 2022;8:18
19. Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R. et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med. 2022;386:1143-54
20. Iwama E, Zenke Y, Sugawara S, Daga H, Morise M, Yanagitani N. et al. Trastuzumab emtansine for patients with non-small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations. Eur J Cancer. 2022;162:99-106
21. Peters S, Stahel R, Bubendorf L, Bonomi P, Villegas A, Kowalski DM. et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin Cancer Res. 2019;25:64-72
22. Montemurro F, Ellis P, Anton A, Wuerstlein R, Delaloge S, Bonneterre J. et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: Primary results from the KAMILLA study cohort 1. Eur J Cancer. 2019;109:92-102
23. Watanabe J, Ito Y, Saeki T, Masuda N, Takano T, Takao S. et al. Safety Evaluation of Trastuzumab Emtansine in Japanese Patients with HER2-Positive Advanced Breast Cancer. In vivo. 2017;31:493-500
24. Krop IE, Kim SB, Martin AG, LoRusso PM, Ferrero JM, Badovinac-Crnjevic T. et al. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18:743-54
25. Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H. et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640-53
26. Krop IE, Suter TM, Dang CT, Dirix L, Romieu G, Zamagni C. et al. Feasibility and cardiac safety of trastuzumab emtansine after anthracycline-based chemotherapy as (neo)adjuvant therapy for human epidermal growth factor receptor 2-positive early-stage breast cancer. J Clin Oncol. 2015;33:1136-42
27. Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J. et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157-63
28. Burris HA 3rd, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S. et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398-405
29. Beeram M, Krop IE, Burris HA, Girish SR, Yu W, Lu MW. et al. A phase 1 study of weekly dosing of trastuzumab emtansine (T-DM1) in patients with advanced human epidermal growth factor 2-positive breast cancer. Cancer. 2012;118:5733-40
30. Krop IE, LoRusso P, Miller KD, Modi S, Yardley D, Rodriguez G. et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2012;30:3234-41
31. de Vries EGE, Ruschoff J, Lolkema M, Tabernero J, Gianni L, Voest E. et al. Phase II study (KAMELEON) of single-agent T-DM1 in patients with HER2-positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma. Cancer Med. 2023;12:12071-83
32. Yardley DA, Krop IE, LoRusso PM, Mayer M, Barnett B, Yoo B. et al. Trastuzumab Emtansine (T-DM1) in Patients With HER2-Positive Metastatic Breast Cancer Previously Treated With Chemotherapy and 2 or More HER2-Targeted Agents: Results From the T-PAS Expanded Access Study. Cancer J. 2015;21:357-64
33. Kashiwaba M, Ito Y, Takao S, Doihara H, Rai Y, Kanatani K. et al. A multicenter Phase II study evaluating the efficacy, safety and pharmacokinetics of trastuzumab emtansine in Japanese patients with heavily pretreated HER2-positive locally recurrent or metastatic breast cancer. Jpn J Clin Oncol. 2016;46:407-14
34. Yamamoto H, Ando M, Aogi K, Iwata H, Tamura K, Yonemori K. et al. Phase I and pharmacokinetic study of trastuzumab emtansine in Japanese patients with HER2-positive metastatic breast cancer. Jpn J Clin Oncol. 2015;45:12-8
35. Huang CS, Yang Y, Kwong A, Chen SC, Tseng LM, Liu MC. et al. Trastuzumab emtansine (T-DM1) versus trastuzumab in Chinese patients with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy for HER2-positive breast cancer in the phase 3 KATHERINE study. Breast Cancer Res Treat. 2021;187:759-68
36. Cortes J, Dieras V, Lorenzen S, Montemurro F, Riera-Knorrenschild J, Thuss-Patience P. et al. Efficacy and Safety of Trastuzumab Emtansine Plus Capecitabine vs Trastuzumab Emtansine Alone in Patients With Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer: A Phase 1 and Randomized Phase 2 Trial. JAMA Oncol. 2020;6:1203-9
37. Martin M, Fumoleau P, Dewar JA, Albanell J, Limentani SA, Campone M. et al. Trastuzumab emtansine (T-DM1) plus docetaxel with or without pertuzumab in patients with HER2-positive locally advanced or metastatic breast cancer: results from a phase Ib/IIa study. Ann Oncol. 2016;27:1249-56
38. Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P. et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab with taxane for human epidermal growth factor receptor 2-positive advanced breast cancer: Final results from MARIANNE. Cancer. 2019;125:3974-84
39. Abraham J, Montero AJ, Jankowitz RC, Salkeni MA, Beumer JH, Kiesel BF. et al. Safety and Efficacy of T-DM1 Plus Neratinib in Patients With Metastatic HER2-Positive Breast Cancer: NSABP Foundation Trial FB-10. J Clin Oncol. 2019;37:2601-9
40. Jebbink M, de Langen AJ, Monkhorst K, Boelens MC, van den Broek D, van der Noort V. et al. Trastuzumab-Emtansine and Osimertinib Combination Therapy to Target HER2 Bypass Track Resistance in EGFR Mutation-Positive NSCLC. JTO Clin Res Rep. 2023;4:100481
41. Emens LA, Esteva FJ, Beresford M, Saura C, De Laurentiis M, Kim SB. et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21:1283-95
42. Gupta M, Wang B, Carrothers TJ, LoRusso PM, Chu YW, Shih T. et al. Effects of Trastuzumab Emtansine (T-DM1) on QT Interval and Safety of Pertuzumab Plus T-DM1 in Patients With Previously Treated Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. Clin Pharmacol Drug Dev. 2013;2:11-24
43. Jain S, Shah AN, Santa-Maria CA, Siziopikou K, Rademaker A, Helenowski I. et al. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat. 2018;171:371-81
44. Patel TA, Ensor JE, Creamer SL, Boone T, Rodriguez AA, Niravath PA. et al. A randomized, controlled phase II trial of neoadjuvant ado-trastuzumab emtansine, lapatinib, and nab-paclitaxel versus trastuzumab, pertuzumab, and paclitaxel in HER2-positive breast cancer (TEAL study). Breast Cancer Res. 2019;21:100
45. Lopez-Miranda E, Perez-Garcia JM, Di Cosimo S, Brain E, Ravnik M, Escriva-de-Romani S. et al. Trastuzumab Emtansine Plus Non-Pegylated Liposomal Doxorubicin in HER2-Positive Metastatic Breast Cancer (Thelma): A Single-Arm, Multicenter, Phase Ib Trial. Cancers (Basel). 2020;12:3509
46. Krop IE, Im SA, Barrios C, Bonnefoi H, Gralow J, Toi M. et al. Trastuzumab Emtansine Plus Pertuzumab Versus Taxane Plus Trastuzumab Plus Pertuzumab After Anthracycline for High-Risk Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer: The Phase III KAITLIN Study. J Clin Oncol. 2022;40:438-48
47. Hurvitz SA, Martin M, Jung KH, Huang CS, Harbeck N, Valero V. et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. J Clin Oncol. 2019;37:2206-16
48. Miller KD, Dieras V, Harbeck N, Andre F, Mahtani RL, Gianni L. et al. Phase IIa trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast cancer. J Clin Oncol. 2014;32:1437-44
49. Filho OM, Viale G, Stein S, Trippa L, Yardley DA, Mayer IA. et al. Impact of HER2 Heterogeneity on Treatment Response of Early-Stage HER2-Positive Breast Cancer: Phase II Neoadjuvant Clinical Trial of T-DM1 Combined with Pertuzumab. Cancer Discov. 2021;11:2474-87
50. Krop IE, Modi S, LoRusso PM, Pegram M, Guardino E, Althaus B. et al. Phase 1b/2a study of trastuzumab emtansine (T-DM1), paclitaxel, and pertuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res. 2016;18:34
51. Waks AG, Keenan TE, Li T, Tayob N, Wulf GM, Richardson ET 3rd. et al. Phase Ib study of pembrolizumab in combination with trastuzumab emtansine for metastatic HER2-positive breast cancer. J Immunother Cancer. 2022;10:e005119
52. Spring LM, Clark SL, Li T, Goel S, Tayob N, Viscosi E. et al. Phase 1b clinical trial of ado-trastuzumab emtansine and ribociclib for HER2-positive metastatic breast cancer. NPJ Breast Cancer. 2021;7:103
53. Borges VF, Ferrario C, Aucoin N, Falkson C, Khan Q, Krop I. et al. Tucatinib Combined With Ado-Trastuzumab Emtansine in Advanced ERBB2/HER2-Positive Metastatic Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2018;4:1214-20
54. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K. et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med. 2020;382:610-21
55. Andre F, Hee Park Y, Kim SB, Takano T, Im SA, Borges G. et al. Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;401:1773-85
56. Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S. et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28:1840-7
57. Shimomura A, Takano T, Takahashi S, Sagara Y, Watanabe J, Tokunaga E. et al. Effect of Trastuzumab Deruxtecan on QT/QTc Interval and Pharmacokinetics in HER2-Positive or HER2-Low Metastatic/Unresectable Breast Cancer. Clin Pharmacol Ther. 2023;113:160-9
58. Chang D-Y, Lin C-C, Chen W-W, Lin C-H. Abstract C041: Safety and pharmacokinetic results from a phase 1, multicenter, open-label study of [fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) in subjects with advanced HER2-positive breast cancer. Molecular Cancer Therapeutics. 2019;18:C041
59. Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:816-26
60. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J. et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol. 2020;38:1887-96
61. Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:827-36
62. Tsurutani J, Iwata H, Krop I, Janne PA, Doi T, Takahashi S. et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 2020;10:688-701
63. Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K. et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512-22
64. Yamaguchi K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D. et al. Trastuzumab Deruxtecan in Anti-Human Epidermal Growth Factor Receptor 2 Treatment-Naive Patients With Human Epidermal Growth Factor Receptor 2-Low Gastric or Gastroesophageal Junction Adenocarcinoma: Exploratory Cohort Results in a Phase II Trial. J Clin Oncol. 2023;41:816-25
65. Takahashi S, Karayama M, Takahashi M, Watanabe J, Minami H, Yamamoto N. et al. Pharmacokinetics, Safety, and Efficacy of Trastuzumab Deruxtecan with Concomitant Ritonavir or Itraconazole in Patients with HER2-Expressing Advanced Solid Tumors. Clin Cancer Res. 2021;27:5771-80
66. Ponde N, Ameye L, Lambertini M, Paesmans M, Piccart M, de Azambuja E. Trastuzumab emtansine (T-DM1)-associated cardiotoxicity: Pooled analysis in advanced HER2-positive breast cancer. Eur J Cancer. 2020;126:65-73
67. Giordano SH, Franzoi MAB, Temin S, Anders CK, Chandarlapaty S, Crews JR. et al. Systemic Therapy for Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Guideline Update. J Clin Oncol. 2022;40:2612-35
68. Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D. et al. NCCN Guidelines(R) Insights: Breast Cancer, Version 4.2023. J Natl Compr Canc Netw. 2023;21:594-608
69. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M. et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215-21
70. Soares LR, Vilbert M, Rosa VDL, Oliveira JL, Deus MM, Freitas-Junior R. Incidence of interstitial lung disease and cardiotoxicity with trastuzumab deruxtecan in breast cancer patients: a systematic review and single-arm meta-analysis. ESMO Open. 2023;8:101613
Author contact
![]() Corresponding author: Yong Pan, E-mail: panyongorg.cn.
Corresponding author: Yong Pan, E-mail: panyongorg.cn.

 Global reach, higher impact
Global reach, higher impact