3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(1):113-125. doi:10.7150/jca.87259 This issue Cite
Research Paper
Long-Term Survival and Cancer Risk in the Hepatitis C Virus-Infected Patients After Antiviral Treatment: A Nationwide Cohort Study
1. Department of Surgery, Taichung Veterans General Hospital, Taichung 40704, Taiwan.
2. Department of Animal Science and Biotechnology, Tunghai University, Taichung 40704, Taiwan.
3. Center for health data science, Chung Shan Medical University Hospital, Taichung, Taiwan.
4. Institute of Medicine, College of Medicine, Chung Shan Medical University, Taichung, Taiwan.
5. Department of Bioinformatics and Medical Engineering, Asia University, Taichung 413305, Taiwan.
6. Department of Medical Laboratory Sciences, College of Applied Medical Sciences, University of Bisha, Bisha 61922, Saudi Arabia.
7. Department of Hospitality Management, College of Agriculture, Tunghai University, Taichung 407224, Taiwan.
8. R&D Division, Utopia Holiday Hotel Corporation, Taichung, Taiwan.
Abstract
Background: Exposure to the Hepatitis C virus (HCV) has been identified as one of the most critical risk factors for Hepatocellular carcinoma (HCC). Interferons and direct-acting antivirals (DAAs) have been used to treat HCV infection with high rates (95%) of prolonged virological response, a suitable safety profile, and good compliance rates.
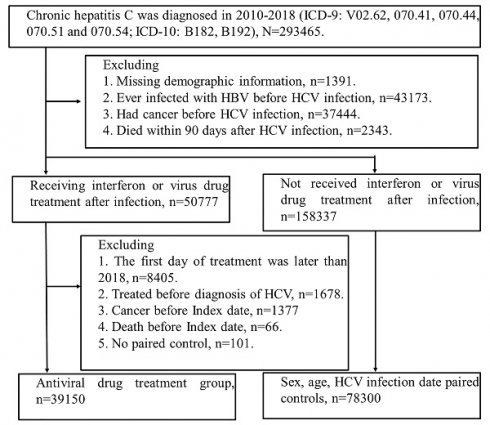
Methods: We obtained information from Taiwan's Health and Welfare Data Science Center. (HWDSC). In this observational cohort research, patients with HCV who received a diagnosis in Taiwan between 2011 and 2018 were included.
Results: 78,300 untreated HCV patients were paired for age, sex, and index date with 39,150 HCV patients who received interferon or DAAs treatment. Compared to the control group, the Interferon or DAAs treatment sample has fewer low-income individuals and more hospitalization requirements. The percentage of kidney illness was reduced in the therapy group compared to the control group, but the treatment group had a greater comorbidity rate of gastric ulcers. Interferon or DAA therapy for HCV-infected patients can substantially lower mortality. All cancer diagnoses after HCV infection with interferon treatment aHR 95% CI = 0.809 (0.774-0.846), Sofosbuvir-based DAA aHR 95% CI = 1.009 (0.737-1.381) and Sofosbuvir free DAA aHR 95% CI = 0.944 (0.584-1.526) showing cancer-protective effects in the INF-treated cohort but not DAA.
Conclusion: Following antiviral therapy, women appear to have a more substantial preventive impact than men against pancreatic, colorectal, and lung cancer. Interferon or DAAs treatment effect was more significant in the cirrhotic group.

 Global reach, higher impact
Global reach, higher impact