Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(1):166-175. doi:10.7150/jca.87758 This issue Cite
Research Paper
Expression and role of FKBPL in lung adenocarcinoma
1. Department of Pathology, Affiliated Tumour Hospital of Nantong University, Nantong, China.
2. Department of Pathology, Nantong Sixth People's Hospital, Nantong, China.
† Lili She and Xingsong Zhang have contributed equally to this work.
Received 2023-7-4; Accepted 2023-10-29; Published 2024-1-1
Abstract
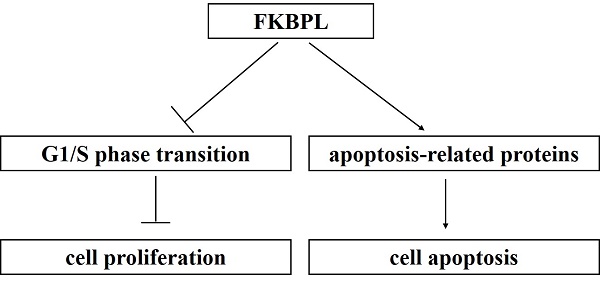
Dysregulated expression of FK506-binding protein like (FKBPL) has been demonstrated to play crucial roles in tumour development. However, the role of FKBPL in lung adenocarcinoma (ADC) remains unclear. Using immunohistochemical staining, we showed that FKBPL expression was significantly lower in lung ADC than the normal tissues (P < 0.0001). Patients with well or moderately differentiated tumours have higher FKBPL expression compared with patients with poor differentiated tumours (P = 0.037). However, no significant associations were found between FKBPL expression and other clinicopathological variables (P > 0.05 for all). Cox univariate analysis showed that high FKBPL expression was correlated with prolonged overall survival (OS) (P = 0.010). Kaplan-Meier analysis further confirmed that the FKBPL-low group showed a significantly shorter OS than the FKBPL-high group (P = 0.0081). FKBPL expression was not shown as an independent prognostic factor for OS in the multivariate analysis (P = 0.063). Moreover, our study demonstrated that FKBPL could suppress the proliferation of lung ADC cells by delaying cell cycle G1/S phase transition. In addition, FKBPL resulted in increased apoptosis in lung ADC cells. Using the Human Apoptosis Array Kit, we observed that overexpression of FKBPL in lung ADC A549 cells significantly decreased the anti-apoptotic proteins, including heat shock protein 32 (HSP32), heat shock protein 27 (HSP27), and paraoxonase-2 (PON2). FKBPL depletion significantly attenuated the pro-apoptotic protein, phospho-p53 (S46), in lung ADC H1975 cells. These new findings provide an experimental basis for further theoretical investigation of lung ADC.
Keywords: lung adenocarcinoma, FKBPL, overall survival, cell proliferation, cell apoptosis
1. Introduction
Lung cancer is the leading cause of cancer mortality in China, and adenocarcinoma (ADC) is the most common histological subtype [1, 2]. Lung ADC is heterogeneous in its clinical presentation, morphology, molecular features, biological behaviour, and treatment options [3]. Treatment options for lung ADC include surgery, radiation therapy, chemotherapy, targeted therapy and immunotherapy [4]. Tyrosine kinase inhibitors (TKIs) have improved the outcome of patients harboring epidermal growth factor receptor (EGFR)-activating mutations. More molecular targets such as anaplastic lymphoma kinase (ALK), ROS proto‐oncogene 1, receptor tyrosine kinase (ROS1), and v-raf murine sarcoma viral oncogene homolog B1 (BRAF) have shown promising anti-tumor efficacy. However, the onset of primary and acquired resistances continue to be the main obstacle to further improve clinical outcomes [5]. Therefore, identifying novel biomarkers and molecular targets is integral for improving patient outcomes.
FK506-binding protein like (FKBPL) belongs to the immunophilin protein family, a group of conserved proteins binding to immunosuppressive drugs, such as FK506, rapamycin, and cyclosporin A [6, 7]. Immunophilins are involved in many cellular processes, such as cell signalling, cell cycle progression, metabolic activity and apoptosis [8]. FKBPL has been shown to play a critical role in regulating estrogen receptor (ER), androgen receptor (AR), glucocorticoid receptor (GR) and inflammatory signalling, cancer stem cell differentiation, and inhibition of angiogenesis [9, 10]. Recently, FKBPL is described as a dual leucine zipper kinase (DLK)-interacting protein that regulates DLK degradation via the lysosome and neuronal responses to axon injury [7]. Lin et al. [11] reported that miR-183-5p promotes the proliferation and migration of vascular smooth muscle cells by reducing FKBPL expression. Substantial evidence indicates that FKBPL functions as a tumour suppressor. FKBPL knockdown in breast cancer cell lines leads to an increase in Nanog/Oct4/Sox2 and an increase in cancer stem cell number [12]. Ectopic expression of FKBPL inhibits the proliferation of breast cancer cells by stabilizing the cyclin-dependent kinase (CDK) inhibitor, p21. In addition, FKBPL overexpression renders breast cancer cells more sensitive to tamoxifen. Furthermore, breast cancer patients with high FKBPL expression are more likely to have increased overall survival (OS) and distant metastasis-free survival [13, 14]. FKBPL expression is significantly decreased in endometrioid endometrial carcinoma in comparison to benign endometrial hyperplasia tissue, with a high predictive value of malignancy. FKBPL expression is positively correlated with ER expression and negatively correlated with VEGF-A expression [15]. A recent study on high-grade serous ovarian cancer shows that patients with high endogenous tumour expression of FKBPL have an increased progression-free survival [16]. However, the prognostic role of FKBPL in patients with lung ADC, and the possible function of FKBPL in the development and progression of lung ADC remains unclear. The aim of this study was to investigate the biological function and clinical significance of FKBPL in lung ADC. Our findings suggest that FKBPL can suppress cell proliferation by delaying cell cycle G1/S phase transition, and promote the apoptosis of lung ADC cells.
2. Materials and methods
2.1 Patients and tissue samples
Two hundred and twenty-two cases of lung ADC, diagnosed between January 2014, and December 2018 at the Affiliated Tumour Hospital of Nantong University, were included in this study. The inclusion criteria were as follows: (1) patients were histopathologically confirmed as lung ADC; (2) treatment naïve; (3) with complete follow-up, clinical and pathological information. The exclusion criteria were as follows: (1) with other serious diseases; (2) received other treatments, including radiation therapy, chemotherapy, molecularly targeted therapy, or immunotherapy before specimen collection. H&E slides of all cases were reviewed according to the 2021 World Health Organization (WHO) diagnostic criteria. This study was approved by the Ethics Committee of the Affiliated Tumour Hospital of Nantong University.
2.2 Immunohistochemistry and evaluation of immunohistochemistry
Tissue microarray (TMA) was constructed as described previously [17]. The TMA was constructed using a manual tissue puncher/arrayer. After reviewing the H&E slides of all cases, the representative slides and formalin-fixed paraffin blocks from each case were selected. For each sample, a 3.0 mm diameter core of tissue was rearranged into recipient blocks. Immunohistochemistry was performed according to standard protocols. Briefly, the 4-μm-thick sections were deparaffinized with xylene and dehydrated using gradient alcohol. Antigen retrieval, elimination of the endogenous peroxidase activity, and blocking were carried out according to standard protocols. The sections were then incubated with FKBPL antibody (1:100 dilution, Proteintech Group, Wuhan, China) at 4 °C overnight. Following washing, slides were incubated with HRP-conjugated secondary antibody (Dako REAL Envision Detection System, Dako, Glostrup, Denmark) for 30 min and subsequently with DAB chromogen (Dako REAL Envision Detection System), and counterstained with hematoxylin. Assessment of immunohistochemical staining was performed semi-quantitatively using the Histo-score (H-score). H-scores were calculated to give a score from 0 to 300 based on staining intensity and proportions of stained cells as previously described [18]. Briefly, staining intensity was evaluated as negative (0), weak (1+), moderate (2+), and strong (3+). The percent of cells within each tissue core stained at each intensity was recorded. The H-score was determined as: H-score = 0× (% at 0) + 1× (% at 1+) + 2× (% at 2+) + 3× (% at 3+). The patients were dichotomized into low (H-score <80) and high (H-score ≥80) FKBPL expression level groups by the optimal cut-off threshold calculated by X-tile software (Yale University).
2.3 Cell culture, and transient transfection
The H1975 human lung ADC cell line was kindly provided by Stem Cell Bank, Chinese Academy of Sciences. H1975 cells were routinely maintained in RPMI 1640 medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, GIBCO). The A549 human lung ADC cell line was purchased from Cobioer Biosciences Company (Nanjing, China). A549 cells were incubated in F-12K medium (GIBCO) supplemented with 10% FBS.
The FKBPL overexpression (OE) plasmid and FKBPL small hairpin RNA (shRNA) expression plasmids were synthesized by GeneChem Co., Ltd. (Shanghai, China). FKBPL targeting sequences were: CAGAACTATTTCGAGCTGGGA (shFKBPL#1), GCTTTGTCAAGAAGATCGTAA (shFKBPL#2). A non-specific shRNA with a sequence of TTCTCCGAACGTGTCACGT served as the negative control. Transient transfection of plasmids was performed using Lipofectamine 2000 (Invitrogen, Shanghai, China) following the manufacturer's protocol.
2.4 Antibodies, and western blot
Equal amounts of total protein were loaded and run on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Roche, Basel, Switzerland). The membranes were blocked with 5% skim milk (in TBS-T) for 2 hours at room temperature and then probed with the primary antibodies overnight at 4°C. The next day the membranes were washed three times with TBS-T for 5 min each and incubated with the relevant secondary antibodies for an hour at room temperature. After washed three times with TBS-T, the membranes were visualized using enhanced chemiluminescence (ECL) reagents (NCM Biotech, Suzhou, China) and ChemiDoc Touch Imaging System (Bio-rad, Hercules, CA, USA). Antibodies and dilutions were as follows: anti-FKBPL antibody (1:2000 dilution, Proteintech Group), anti-Flag-M2 antibody (1:1000 dilution, Sigma-Aldrich, St Louis, MO, USA), anti-Cyclin E1 antibody (1:1000 dilution, Proteintech Group), anti-GAPDH antibody (1:5000 dilution, Proteintech Group), Peroxidase AffiniPure Goat Anti-Rabbit IgG (H + L) (1:10000 dilution, Jackson ImmunoResearch Laboratories, West Grove, PA, USA), Peroxidase AffiniPure Goat Anti-Mouse IgG (H + L) (1:10000 dilution, Jackson ImmunoResearch Laboratories).
2.5 Colony formation assay
Cells were trypsinized, counted, and seeded for colony formation assay in 35-mm culture dishes at a density of 1×103 cells/dish. During colony growth, the culture medium was replaced every 3 days for 15 days. Colonies were fixed in 4% paraformaldehyde (Beyotime Biotech, Shanghai, China), and stained with 0.1% crystal violet (Beyotime Biotech).
2.6 Cell cycle analysis
For the cell cycle analysis, cells were harvested by trypsinization, and fixed with 70% ethanol overnight at -20 °C. The fixed cells were then centrifuged to remove ethanol, washed with PBS and resuspended in 0.5 mL of PI/RNase staining buffer (BD Biosciences, San Jose, CA, USA) for 15 min at room temperature in the dark. Cell cycle distributions were determined on the BD FACSCanto II flow cytometer (BD Biosciences).
2.7 Apoptosis detection assay
For apoptosis analysis, cells were stained with APC Annexin V and 7-AAD (Biolegend, San Diego, CA, USA) according to the manufacturer's protocol. Briefly, cells were washed twice with cold BioLegend's cell staining buffer, and resuspend at 1.0 × 107 cells/mL in Annexin V binding buffer. Cells were then placed into 5 mL test tubes, and stained with 5 µL of APC Annexin V and 5 µL of 7-AAD viability staining solution. After gently vortexing, the cells were incubated 15 min at room temperature in the dark. Thereafter, cells were incubated with 400 µL of Annexin V binding buffer and then subjected to flow cytometric analysis.
2.8 Apoptosis antibody array analysis
The expression profiles of apoptosis-related proteins were analyzed using the Proteome Profiler Human Apoptosis Array Kit (ARY009) according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). Briefly, the cells were lysated using the lysis buffer. Membranes containing immobilized antibodies were blocked with array buffer 1 for 1 h at room temperature and incubated with the cell lysates overnight at 4 °C. The membranes were then washed with wash buffer and incubated with detection antibody cocktail for 1 h and streptavidin-HRP for 30 min at room temperature. Protein expression was visualized using chemi reagents and the ChemiDoc Touch Imaging System.
2.9 Human protein atlas and Kaplan-Meier plotter database analysis
The Human Protein Atlas (https://www.proteinatlas.org/) is a website that involve immunohistochemistry-based expression data for distribution and expression of 20 tumour tissues [19]. We used the Human Protein Atlas database to explore the prognostic value of FKBPL in lung ADC. We also used an online Kaplan-Meier plotter database (http://kmplot.com) to explore the value of FKBPL as a novel prognostic biomarker in lung ADC [20].
2.10 Statistical analysis
The associations between FKBPL expression and clinicopathological parameters were evaluated by Pearson's chi-square test. OS was estimated using the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazards regression models were applied to evaluate the potential independent prognostic factors. Comparison between groups was evaluated by Student's t-test. P values of less than 0.05 were regarded as statistically significant. All statistical analysis was performed using SPSS 23.0 and GraphPad Prism 6.
3. Results
3.1 FKBPL expression in lung ADC tissues
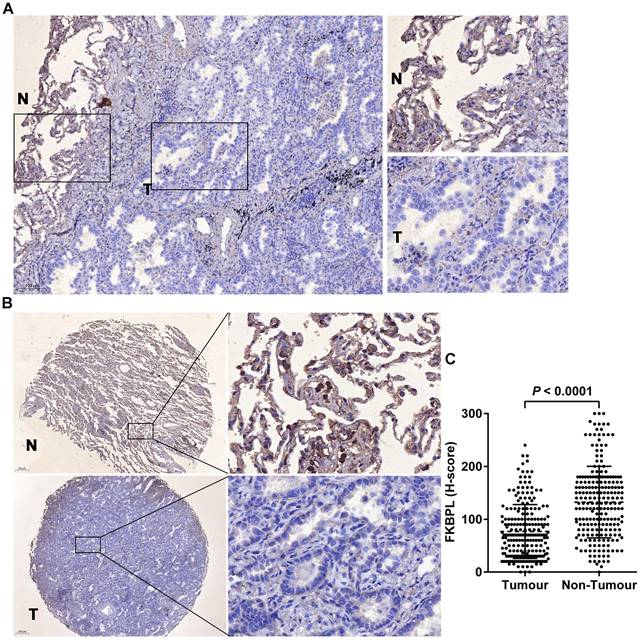
Immunohistochemical staining of the whole tissue sections showed that FKBPL was predominantly localized in the cytoplasm, and its expression levels were decreased in tumours compared with the matching normal tissues (Fig. 1A). The preliminary finding was confirmed in TMA which showed that FKBPL was significantly lower in lung ADC (H-score, 76.71±3.42) than the normal tissues (H-score, 132.40±4.54) (P < 0.0001; Fig. 1B and C). Of the 222 lung ADC patients, 92 (41.4%) cases had high FKBPL expression and 130 (58.6%) cases had low FKBPL expression. FKBPL expression was associated with tumour differentiation (P = 0.037). Patients with well or moderately differentiated tumours had higher FKBPL expression compared with patients with poor differentiated tumours. However, no significant associations were found between FKBPL expression and age, gender, smoking status, T stage, N stage, TNM stage, lymphovascular invasion, and STAS (P > 0.05 for all; Table 1).
FKBPL was decreased in lung ADC compared with the matching normal tissues. (A) Representative image of immunohistochemical staining of FKBPL in whole section. 'T' represents tumour tissue and 'N' represents non-tumour lung tissue. (B) Representative images of immunohistochemical staining of FKBPL in TMAs. 'T' represents tumour tissue and 'N' represents non-tumour lung tissue. (C) Scatter plots showing a statistical analysis of FKBPL expression in tumour tissue and non-tumour lung tissue.
Relationship between expression levels of FKBPL and clinicopathological features of 222 lung ADC specimens
| Variables | n | FKBPL | P value | |
|---|---|---|---|---|
| Low | High | |||
| Age (years) | 0.779 | |||
| <60 | 65 | 39 | 26 | |
| ≥60 | 157 | 91 | 66 | |
| Gender | 0.703 | |||
| Female | 124 | 74 | 50 | |
| Male | 98 | 56 | 42 | |
| Smoking status | 0.310 | |||
| Never smoked | 171 | 97 | 74 | |
| Ex-smoking or currently smoking | 51 | 33 | 18 | |
| Differentiation | 0.037 | |||
| Well/moderate differentiation | 139 | 74 | 65 | |
| Poor differentiation | 83 | 56 | 27 | |
| T stage | 0.653 | |||
| T1/T2 | 208 | 121 | 87 | |
| T3/T4 | 14 | 9 | 5 | |
| N stage | 0.174 | |||
| N0 | 158 | 88 | 70 | |
| N1/N2/N3 | 64 | 42 | 22 | |
| TNM stage | 0.125 | |||
| I/II | 180 | 101 | 79 | |
| III | 42 | 29 | 13 | |
| Lymphovascular invasion | 0.378 | |||
| Negative | 152 | 86 | 66 | |
| Positive | 70 | 44 | 26 | |
| Tumor spread through air spaces (STAS) | 0.441 | |||
| Negative | 109 | 61 | 48 | |
| Positive | 113 | 69 | 44 | |
A P value < 0.05 was considered significant.
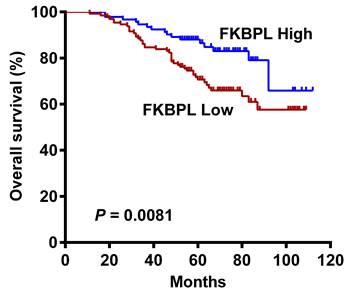
Kaplan-Meier curves for OS in lung ADC patients according to FKBPL expression status. The FKBPL-low group showed a significantly shorter OS than the FKBPL-high group (log-rank P = 0.0081).
3.2 Reduced FKBPL expression predicts poor outcomes
No significant associations were found between FKBPL expression and OS in patients with lung ADC according to the Human Protein Atlas (Supplementary Fig. 1) and Kaplan-Meier plotter (Supplementary Fig. 2) databases. However, our univariate Cox analysis showed that high FKBPL expression was correlated with prolonged OS (HR = 0.471, 95% CI = 0.265 to 0.835, P = 0.010; Table 2). Kaplan-Meier analysis further confirmed that the FKBPL-low group showed a significantly shorter OS than the FKBPL-high group (P = 0.0081; Fig. 2). In univariate analysis, tumour differentiation (P < 0.001), T stage (P = 0.005), N stage (P < 0.001), lymphovascular invasion (P = 0.047), and STAS (P = 0.011) also showed a significant impact on OS. However, no significant associations were found between age, gender, smoking status and OS (P > 0.05 for all). Multivariate analysis demonstrated that T stage (P = 0.026) and N stage (P < 0.001) independently predicted OS. FKBPL expression was not an independent prognostic factor for OS (P = 0.063; Table 2).
3.3 FKBPL suppresses the proliferation of lung ADC cells
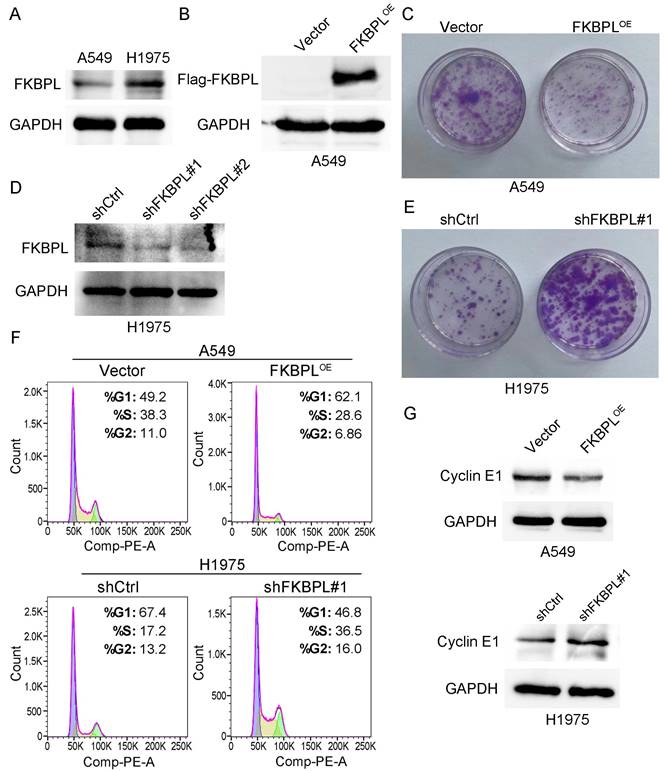
We subsequently explored the effects of FKBPL on the biological behaviors of lung ADC cells. We first performed western blot to examine the expression of FKBPL in two lung ADC cell lines, A549 and H1975. As shown in Fig. 3A, H1975 cells expressed FKBPL at a higher level compared with A549 cells. Previous study demonstrated that FKBPL inhibited proliferation of breast cancer and lymphoma cells [21]. We next investigated whether increased FKBPL expression results in inhibition of proliferation of lung ADC cells. We overexpressed FKBPL by ectopically transfecting FKBPL-overexpression (FKBPLOE) plasmid into A549 cells. The overexpression of FKBPL was confirmed by western blot analysis (Fig. 3B). Colony formation assay showed that ectopic expression of FKBPL significantly decreased the number of colonies compared with control cells (Fig. 3C). We next tested whether knockdown of FKBPL can promote the proliferation of lung ADC cells. We ablated FKBPL expression by transfecting FKBPL-shRNAs (shFKBPL#1 and shFKBPL#2) into H1975 cells. As shown in Fig. 3D, the two shRNAs were highly effective in depleting FKBPL in H1975 cells. We randomly selected shFKBPL#1 for the subsequent experiments. As anticipated, knockdown of FKBPL markedly increased the number of colonies compared with control cells (Fig. 3E). We further tested the effect of FKBPL on cell cycle distribution. As revealed by Fig. 3F, overexpression of FKBPL increased the proportion of cells in the G1 phase, and decreased the proportion of cells in the S phase in A549 cells. In contrast, inhibition of FKBPL decreased the proportion of cells in the G1 phase, and increased the proportion of cells in the S phase in H1975 cells. We next examined the effect of FKBPL on the protein levels of cell-cycle regulator, Cyclin E1. Western blot analysis showed that overexpression of FKBPL decreased Cyclin E1 expression in A549 cells. In contrast, silencing of FKBPL increased Cyclin E1 expression in H1975 cells (Fig. 3G). Collectively, these data suggest that FKBPL suppresses the proliferation of lung ADC cells by delaying G1/S phase transition.
FKBPL suppresses the proliferation of lung ADC cells. (A) Protein levels of FKBPL in two lung ADC cell lines, A549 and H1975, were determined by western blot assays. GAPDH was used as a loading control. (B) Overexpression of FKBPL using Flag tagged FKBPL overexpression plasmid. A549 cells were transiently transfected with either Flag tagged FKBPL overexpression plasmid (FKBPLOE) or empty vector control plasmid (Vector), and Flag tagged FKBPL was confirmed by western blot assays. GAPDH was used as a loading control. (C) Ectopic expression of FKBPL decreases the number of colonies in A549 cells. A549 cells were transfected with FKBPLOE or Vector, and colony formation assay was used to demonstrate the inhibitory effect of FKBPL on cell colony formation. (D) Knockdown of FKBPL using control shRNA (shCtrl) or FKBPL shRNAs (shFKBPL#1 and shFKBPL#2). H1975 cells were transiently transfected with either shCtrl plasmid or FKBPL shRNA plasmid, and efficiency of FKBPL knockdown was confirmed by western blot assays. GAPDH was used as a loading control. (E) Knockdown of FKBPL increases the number of colonies in H1975 cells. H1975 cells were transfected with shCtrl or shFKBPL#1, and colony formation assay was used to demonstrate the enhancement effect of FKBPL knockdown on cell colony formation. (F) FKBPL upregulation delays G1/S phase transition, while FKBPL knockdown promotes G1/S phase transition. A549 cells were transfected with FKBPLOE or Vector, H1975 cells were transfected with shCtrl or shFKBPL#1, and cell cycle distribution was determined by flow cytometry. (G) FKBPL upregulation decreased Cyclin E1 expression, while FKBPL silencing enhances Cyclin E1 expression. A549 cells were transfected with FKBPLOE or Vector, H1975 cells were transfected with shCtrl or shFKBPL#1, and protein levels of Cyclin E1 in A549 and H1975 were determined by western blot assays. GAPDH was used as a loading control.
Univariate and multivariate analysis of prognostic factors in 222 lung ADC specimens
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (≥60 vs. <60) | 0.925 (0.537 to 1.595) | 0.780 | - | - | |
| Gender (male vs. female) | 0.610 (0.367 to 1.015) | 0.057 | - | - | |
| Smoking status (ex-smoking or currently smoking vs. never smoked) | 1.156 (0.644 to 2.074) | 0.628 | - | - | |
| Differentiation (poor differentiation vs. well/moderate differentiation) | 2.477 (1.488 to 4.124) | <0.001 | 1.295 (0.703 to 2.384) | 0.407 | |
| T stage (T3/T4 vs. T1/T2) | 1.715 (1.181 to 2.489) | 0.005 | 1.562 (1.054 to 2.315) | 0.026 | |
| N stage (N1/N2/N3 vs. N0) | 5.040 (2.997 to 8.475) | <0.001 | 4.397 (2.465 to 7.843) | <0.001 | |
| Lymphovascular invasion (positive vs. negative) | 1.709 (1.008 to 2.895) | 0.047 | 0.889 (0.495 to 1.594) | 0.692 | |
| STAS (positive vs. negative) | 1.985 (1.167 to 3.375) | 0.011 | 1.099 (0.592 to 2.040) | 0.764 | |
| FKBPL (high vs. low) | 0.471 (0.265 to 0.835) | 0.010 | 0.575 (0.321 to 1.030) | 0.063 | |
3.4 FKBPL promotes the apoptosis of lung ADC cells
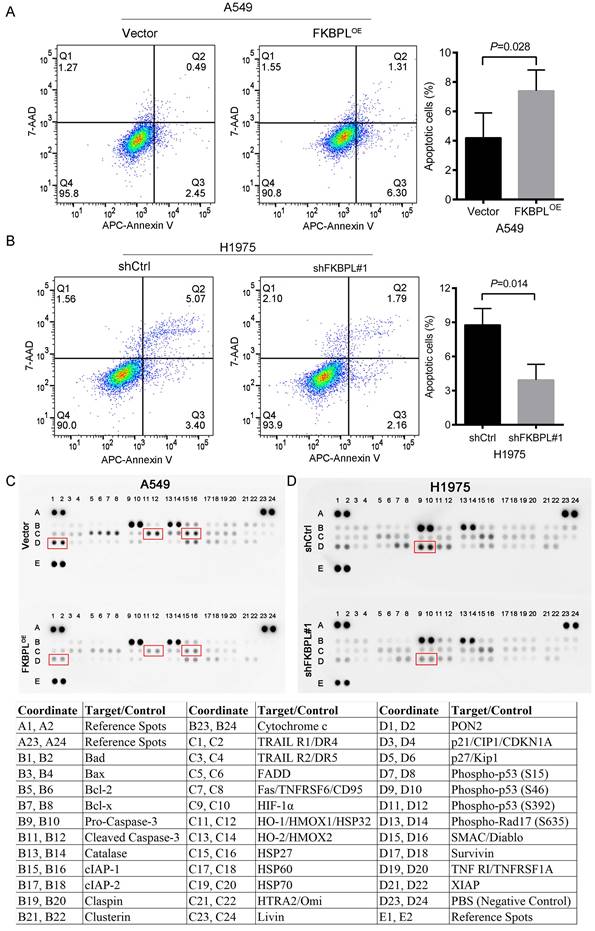
We further tested whether FKBPL could regulate the apoptosis of lung ADC cells. As shown in Fig. 4A, ectopic expression of FKBPL resulted in increased apoptosis in A549 cells. In contrast, inhibition of FKBPL led to decreased apoptosis in H1975 cells (Fig. 4B). To further identify proteins contributing to the apoptotic phenotype, the Proteome Profiler Human Apoptosis Array Kit was used to analyze the expression profiles of apoptosis-related proteins. We observed that overexpression of FKBPL in A549 cells significantly decreased the anti-apoptotic proteins, including heat shock protein 32 (HSP32), heat shock protein 27 (HSP27), and paraoxonase-2 (PON2) (Fig. 4C). FKBPL depletion significantly attenuated the pro-apoptotic protein, phospho-p53 (S46) in H1975 cells (Fig. 4D). Together, our data indicate that FKBPL promotes the apoptosis of lung ADC cells.
4. Discussion
FKBPL, also known as WAF-1/CIP1 stabilizing protein 39, is a divergent member of the FK506-binding protein (FKBP) family. It shares homology with the FKBP family mostly in the C-terminal tetra-trico-peptide repeat (TPR) domain, but lacks the crucial residues within a weakly homologous peptidyl prolyl cis/trans isomerase (PPIase) domain that are required for enzymatic activity [21].
FKBPL has been demonstrated to regulate steroid receptor and inflammatory signalling via CD44, heat shock protein 90 (HSP90) and signal transducer and activator of transcription-3 (STAT-3) [10]. Upon binding to HSP90, FKBPL regulates the stability of p21 and forms co-chaperone complexes with GR, AR and ERα [22]. Heat shock proteins are a large family of evolutionarily preserved molecular chaperones, and key regulators of post-translational protein folding. Dysregulated expression of heat shock protein has been demonstrated to play crucial roles in tumour development. Heat shock proteins are reported to be involved in hallmarks of cancer, such as mitosis, and apoptosis [23]. Aberrant expression of HSP27 is associated with tumorigenesis and metastasis in various cancers. It has been identified as a regulator of the Hippo signalling pathway, which controls cancer initiation/progression, organ development, and stem cell maintenance and regeneration [24, 25]. HSP27 prevents apoptosis by associating with caspase-3, cytochrome c, Bax, IκB kinase (IKK), and others [26]. HSP32, also known as heme oxygenase 1 (HO-1), is closely related to participation in diminishing oxidative stress damage and exerting anti-apoptotic effects [27]. In this study, we demonstrate that, overexpression of FKBPL in A549 cells significantly decreased the anti-apoptotic proteins, including HSP32, and HSP27. The tumour suppressor p53 regulates various pathways, including those involved in DNA damage, and cytostatic/cytotoxic responses. P53 exerts pro-apoptotic activity in transcription-dependent and independent manners. As a transcription factor, p53 directly regulates the transcription of hundreds of genes, leading to cell-cycle arrest and apoptosis. P53 also enhances apoptosis in a transcription independent manner by activating the mitochondria death pathway [28, 29]. It has been reported that p53 phosphorylation at Ser46 is critical for apoptosis induction. Mdm2-dependent homeodomain-interacting protein kinase 2 (HIPK2) degradation prevents phosphorylation of p53 at Ser46, which ultimately leads to cell survival [30]. In the present study, FKBPL depletion significantly attenuated phospho-p53 (S46) in H1975 cells. It was reported that FKBPL overexpression decreases proliferation and clonogenicity of breast cancer cells via stabilizing newly synthesized p21. Moreover, FKBPL overexpression inhibits proliferation of leukemic U937 cells by delaying cells in the G0-G1 phase of the cell cycle [14]. Our study suggests that FKBPL can suppress the proliferation of lung ADC cells by delaying G1/S phase transition.
FKBPL promotes the apoptosis of lung ADC cells. (A) FKBPL upregulation promotes the apoptosis of A549 cells. A549 cells were transfected with FKBPLOE or Vector, and cell apoptosis was analyzed by flow cytometry. (B) FKBPL knockdown decreases the apoptosis of H1975 cells. H1975 cells were transfected with shCtrl or shFKBPL#1, and cell apoptosis was analyzed by flow cytometry. (C) Overexpression of FKBPL in A549 cells decreased the anti-apoptotic proteins, including heat shock protein 32 (HSP32), heat shock protein 27 (HSP27), and paraoxonase-2 (PON2). A549 cells were transfected with FKBPLOE or Vector, and the expression levels of apoptosis-related proteins were determined by the proteome profiler human apoptosis array analysis. (D) FKBPL depletion attenuated the pro-apoptotic protein, phospho-p53 (S46) in H1975 cells. H1975 cells were transfected with shCtrl or shFKBPL#1, and the expression levels of apoptosis-related proteins were determined by the proteome profiler human apoptosis array analysis.
FKBPL has been shown to function as a tumour suppressor in various types of human malignancies. In the present study, we showed that FKBPL was significantly lower in lung ADC than the normal tissues. Patients with well or moderately differentiated tumours have higher FKBPL expression compared with patients with poor differentiated tumours. However, no significant associations were found between FKBPL expression and other clinicopathological variables. Previous studies demonstrated that high FKBPL expression was significantly correlated with prolonged OS and distant metastasis-free survival in patients with breast cancer [13, 14]. Moreover, high endogenous tumour expression of FKBPL was significantly associated with increased progression-free survival in patients with high-grade serous ovarian cancer [16]. Our immunohistochemistry study showed that high FKBPL expression was correlated with prolonged OS in patients with lung ADC. However, no significant associations were found between FKBPL expression and OS according to the Human Protein Atlas and Kaplan-Meier plotter databases. The Human Protein Atlas is an online database containing large sets of protein expression data from immunohistochemically stained TMAs [31]. The Kaplan-Meier plotter is an online survival analysis database for 54,000 genes in 21 malignant tumours [32]. The observed discrepancy between our immunohistochemistry study and the Human Protein Atlas and Kaplan-Meier plotter database analysis could be explained by the fact that our immunohistochemistry data are from a single center, which might have led to inherent biases and requires further external validation.
Taken together, our study revealed that high FKBPL expression was correlated with prolonged OS in patients with lung ADC. Moreover, FKBPL could suppress the proliferation of lung ADC cells by delaying G1/S phase transition. Our study also demonstrated that FKBPL overexpression could promote the apoptosis of lung ADC cells. These new findings provide an experimental basis for further theoretical investigation of lung ADC.
Supplementary Material
Supplementary figures.
Acknowledgements
This work was supported by grants from the Nantong Science and Technology Project (MS12019017).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Xia C, Dong X, Li H, Cao M, Sun D, He S. et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135(5):584-90
2. Wang F, Wang CL, Yi YQ, Zhang T, Zhong Y, Zhu JJ. et al. Comparison and fusion prediction model for lung adenocarcinoma with micropapillary and solid pattern using clinicoradiographic, radiomics and deep learning features. Sci Rep. 2023;13(1):9302
3. Jhala H, Harling L, Rodrigo A, Nonaka D, McLean E, Ng W. et al. Clinicopathological predictors of survival in resected primary lung adenocarcinoma. J Clin Pathol. 2022;75(5):310-15
4. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B. et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):40
5. Wang W, Wang S, Wang M, Ma Y, Hu W, Wu B. et al. Effects of TRAF3 on the proliferation and migration of lung adenocarcinoma depend partly on pyroptosis. BMC Cancer. 2023;23(1):942
6. Todd N, McNally R, Alqudah A, Jerotic D, Suvakov S, Obradovic D. et al. Role of A Novel Angiogenesis FKBPL-CD44 Pathway in Preeclampsia Risk Stratification and Mesenchymal Stem Cell Treatment. J Clin Endocrinol Metab. 2021;106(1):26-41
7. Lee B, Oh Y, Cho E, DiAntonio A, Cavalli V, Shin JE. et al. FK506-binding protein-like and FK506-binding protein 8 regulate dual leucine zipper kinase degradation and neuronal responses to axon injury. J Biol Chem. 2022;298(3):101647
8. McClements L, Annett S, Yakkundi A, O'Rourke M, Valentine A, Moustafa N. et al. FKBPL and its peptide derivatives inhibit endocrine therapy resistant cancer stem cells and breast cancer metastasis by downregulating DLL4 and Notch4. BMC Cancer. 2019;19(1):351
9. Ghorbanpour SM, Richards C, Pienaar D, Sesperez K, Aboulkheyr Es H, Nikolic VN. et al. A placenta-on-a-chip model to determine the regulation of FKBPL and galectin-3 in preeclampsia. Cell Mol Life Sci. 2023;80(2):44
10. Chhor M, Chen H, Jerotic D, Tesic M, Nikolic VN, Pavlovic M. et al. FK506-Binding Protein like (FKBPL) Has an Important Role in Heart Failure with Preserved Ejection Fraction Pathogenesis with Potential Diagnostic Utility. Biomolecules. 2023;13(2):395
11. Lin JJ, Chen R, Yang LY, Gong M, Du MY, Mu SQ. et al. Hsa_circ_0001402 alleviates vascular neointimal hyperplasia through a miR-183-5p-dependent regulation of vascular smooth muscle cell proliferation, migration, and autophagy. J Adv Res. 2023 S2090-1232(23)00201-1
12. McClements L, Yakkundi A, Papaspyropoulos A, Harrison H, Ablett MP, Jithesh PV. et al. Targeting treatment-resistant breast cancer stem cells with FKBPL and its peptide derivative, AD-01, via the CD44 pathway. Clin Cancer Res. 2013;19(14):3881-93
13. Nelson L, McKeen HD, Marshall A, Mulrane L, Starczynski J, Storr SJ. et al. FKBPL: a marker of good prognosis in breast cancer. Oncotarget. 2015;6(14):12209-23
14. McKeen HD, Byrne C, Jithesh PV, Donley C, Valentine A, Yakkundi A. et al. FKBPL regulates estrogen receptor signaling and determines response to endocrine therapy. Cancer Res. 2010;70(3):1090-100
15. Obradovic DD, Milic NM, Miladinovic N, McClements L, Opric DM. Loss of Expression of Antiangiogenic Protein FKBPL in Endometrioid Endometrial Carcinoma: Implications for Clinical Practice. Medicina (Kaunas). 2022;58(10):1330
16. Annett S, Moore G, Short A, Marshall A, McCrudden C, Yakkundi A. et al. FKBPL-based peptide, ALM201, targets angiogenesis and cancer stem cells in ovarian cancer. Br J Cancer. 2020;122(3):361-71
17. Song L, Liu D, Zhang X, Zhu X, Lu X, Huang J. et al. Low expression of PDHA1 predicts poor prognosis in gastric cancer. Pathol Res Pract. 2019;215(3):478-82
18. Wu Y, Zhang X, Shen R, Huang J, Lu X, Zheng G. et al. Expression and significance of ETFDH in hepatocellular carcinoma. Pathol Res Pract. 2019;215(12):152702
19. Wu ZH, Cai F, Zhong Y. Comprehensive Analysis of the Expression and Prognosis for GBPs in Head and neck squamous cell carcinoma. Sci Rep. 2020;10(1):6085
20. Lanczky A, Gyorffy B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J Med Internet Res. 2021;23(7):e27633
21. Robson T, James IF. The therapeutic and diagnostic potential of FKBPL; a novel anticancer protein. Drug Discov Today. 2012;17(11-12):544-48
22. Donley C, McClelland K, McKeen HD, Nelson L, Yakkundi A, Jithesh PV. et al. Identification of RBCK1 as a novel regulator of FKBPL: implications for tumor growth and response to tamoxifen. Oncogene. 2014;33(26):3441-50
23. Magyar CTJ, Vashist YK, Stroka D, Kim-Fuchs C, Berger MD, Banz VM. Heat shock protein 90 (HSP90) inhibitors in gastrointestinal cancer: where do we currently stand?-A systematic review. J Cancer Res Clin Oncol. 2023;149(10):8039-50
24. Yun CW, Kim HJ, Lim JH, Lee SH. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells. 2019;9(1):60
25. Pavel M, Renna M, Park SJ, Menzies FM, Ricketts T, Fullgrabe J. et al. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat Commun. 2018;9(1):2961
26. Kuramitsu Y, Wang Y, Taba K, Suenaga S, Ryozawa S, Kaino S. et al. Heat-shock protein 27 plays the key role in gemcitabine-resistance of pancreatic cancer cells. Anticancer Res. 2012;32(6):2295-99
27. Lu JJ, Abudukeyoumu A, Zhang X, Liu LB, Li MQ, Xie F. Heme oxygenase 1: a novel oncogene in multiple gynecological cancers. Int J Biol Sci. 2021;17(9):2252-61
28. Li L, Su Z, Zou Z, Tan H, Cai D, Su L. et al. Ser46 phosphorylation of p53 is an essential event in prolyl-isomerase Pin1-mediated p53-independent apoptosis in response to heat stress. Cell Death Dis. 2019;10(2):96
29. Gaidarenko O, Xu Y. Transcription activity is required for p53-dependent tumor suppression. Oncogene. 2009;28(49):4397-401
30. Zhang XP, Liu F, Wang W. Interplay between Mdm2 and HIPK2 in the DNA damage response. J R Soc Interface. 2014;11(96):20140319
31. Bozoky B, Szekely L, Ernberg I, Savchenko A. AtlasGrabber: a software facilitating the high throughput analysis of the human protein atlas online database. BMC Bioinformatics. 2022;23(1):546
32. Xu Z, Wang S, Ren Z, Gao X, Xu L, Zhang S. et al. An integrated analysis of prognostic and immune infiltrates for hub genes as potential survival indicators in patients with lung adenocarcinoma. World J Surg Oncol. 2022;20(1):99
Author contact
![]() Corresponding author: Xiaobing Miao. E-mail: miaoxiaobingedu.cn. Song He. E-mail: ntzlyyhesong515edu.cn.
Corresponding author: Xiaobing Miao. E-mail: miaoxiaobingedu.cn. Song He. E-mail: ntzlyyhesong515edu.cn.

 Global reach, higher impact
Global reach, higher impact