3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(1):204-217. doi:10.7150/jca.89750 This issue Cite
Review
Periprostatic Adipose Tissue: A New Perspective for Diagnosing and Treating Prostate Cancer
1. Department of Urology II, The First Hospital of Jilin University, Changchun 130021, China.
2. Key Laboratory of Pathobiology, Ministry of Education, Jilin University, Changchun 130021, China.
Received 2023-9-1; Accepted 2023-10-26; Published 2024-1-1
Abstract
Prostate cancer (PCa) is the most common tumor of the male genitourinary system. It will eventually progress to fatal metastatic castration-resistant prostate cancer, for which treatment options are limited. Adipose tissues are distributed in various parts of the body. They have different morphological structures and functional characteristics and are associated with the development of various tumors. Periprostatic adipose tissue (PPAT) is the closest white visceral adipose tissue to the prostate and is part of the PCa tumor microenvironment. Studies have shown that PPAT is involved in PCa development, progression, invasion, and metastasis through the secretion of multiple active molecules. Factors such as obesity, diet, exercise, and organochlorine pesticides can affect the development of PCa indirectly or directly through PPAT. Based on the mechanism of PPAT's involvement in regulating PCa, this review summarized various diagnostic and therapeutic approaches for PCa with potential applications to assess the progression of patients' disease and improve clinical outcomes.
Keywords: prostate cancer, periprostatic adipose tissue, lipids, inflammation, diagnosis, and treatment
1. Introduction
Prostate cancer (PCa) is the most common malignancy of the male genitourinary system. Among men in the United States, the estimated number of new PCa cases in 2023 ranks first among all tumors and second in mortality, second only to lung cancer, with an increasing trend every year [1]. More than 80% of patients with PCa are diagnosed with localized or locally advanced PCa. This stage can be treated using active surveillance, radical surgery, or radiotherapy alone or combined with androgen deprivation therapy (ADT), with satisfactory clinical outcomes [2]. Endocrine therapy is an essential treatment for patients with advanced PCa. However, after a median duration of 18‒24 months of endocrine therapy, PCa progresses to metastatic castration-resistant prostate cancer (mCRPC), defined as PCa that has reached harmful levels of serum testosterone (<50 ng/dl or 1.7 nmol/L) after an initial continuous ADT therapy, persistently elevated prostate-specific antigen (PSA) levels or imaging on progression, reduced patients' quality of life, and shorter survival [3, 4]. The standard first-line treatment chemotherapy drug for treating mCRPC is docetaxel-based drugs [5]. Second-generation androgen receptor (AR) signaling inhibitors (e.g., enzalutamide [6]) and intratumoral androgen synthesis inhibitors (e.g., abiraterone [7]) have been testified for treating mCRPC and have improved survival benefits; however, the prognosis of mCRPC remains poor. Therefore, there is an urgent need for new diagnostic and therapeutic tools for PCa.
Adipose tissues are divided into visceral and subcutaneous adipose tissues according to their anatomical location. Other adipose tissues are in the perivascular, bone marrow cavity, and ectopic storage (e.g., nonalcoholic fatty liver and pancreatic adipose tissue accumulation). Visceral adipose tissues are primarily distributed around the mesentery and omentum [8, 9]. Adipose tissues are classified into white adipose tissues (WAT) and brown adipose tissues (BAT) based on morphological and functional characteristics. WAT is the primary source of physiological fuel and consists of monocular lipid droplets that constitute 95% of adipocytes, a non-thermal energy-storing adipocyte that also provides mechanical protection and resistance to infection and injury [10]. BAT has a limited distribution and is only found in the neck, shoulders, posterior thorax, and some anatomical depots in the abdomen, accounting for 0.2 to 3.0% of total adipose tissue mass [11]. It consists of multicompartmental lipid droplets dispersed in the mitochondria-rich cytoplasm and mediates thermogenesis mainly via uncoupling protein 1 (UCP-1) pathway, and some other thermogenic mechanisms have been shown to exist, based on ATP sinks centered on creatine, lipid, or calcium cycling, along with Fatty acid-mediated UCP1-independent leak pathways driven by the ADP/ATP carrier (AAC) [12]. Adipose tissues consist of many adipocytes, other non-adipocytes, connective tissue matrix, blood vessels, and nerve tissues. The non-adipocyte components include inflammatory cells (macrophages), immune cells, preadipocytes, and fibroblasts [13]. These components, as a whole, affect body lipid metabolism, insulin sensitivity, inflammation, energy homeostasis, angiogenesis, and cell proliferation [14]. Adipose tissue activity is associated with the development of various tumors [15, 16]. Different adipose depots have different morphological and functional characteristics and have different effects on different tumors [17, 18].
Periprostatic adipose tissues (PPAT) are located in the pelvic region and are largely surrounded by a prostatic envelope separated by a layer of fibromuscular sheets of varying thickness, crossed by prostatic vessels and are the closest adipose tissues to PCa. Forty-eight percent of the prostate surface has PPAT, and 44%, 36%, 59%, and 57% of the anterior, posterior, right, and left surfaces have adipose tissue distribution, respectively. Besides, one third of the anterior prostate is in direct contact with PPAT [19, 20]. PPAT is generally considered a white visceral adipose tissue. However, in some cases, Alvarez-Artime, A. et al. speculated that PPAT could be transformed into a beige adipose tissue, possessing white and brown adipose tissue characteristics [21]. Compared with subcutaneous adipose tissues, PPAT has unique morphological and functional characteristics; the adipocytes in PPAT are smaller, have the same basal rate of fatty acid release (lipolysis) but release fewer types of polyunsaturated fatty acids and are more sensitive to isoprenaline-stimulated lipolysis [22]. Therefore, they play a different role than other adipose tissues and their study alone has some significance. PPAT affects various prostate-related diseases, such as prostatitis, benign prostatic hyperplasia (BPH), secondary lower urinary tract symptoms, erectile dysfunction, urethral dysfunction, and PCa [23, 24, 25]. PPAT is an active secretory organ that can affect the PCa lipid microenvironment and inflammatory state, thus promoting PCa progression by secreting lipids, adipokines, and hormones in a paracrine or endocrine manner. PPAT also directly contacts PCa or mediate communication between PPAT and PCa in an exocytic manner [26, 27]. In turn, PCa regulates the biological behavior of adipose tissues, thus promoting its development [26, 28, 29]. Only a few studiies have examined the effects of PCa on PPAT. Hence, to clearly describe the role of PPAT on PCa, this article mainly described the unidirectional effects of PPAT on PCa.
2. PPAT Regulates the Lipid Metabolism of PCa and Changes the Tumor Lipid Microenvironment
Unlike most tumors, early PCa adapts to the energy required for tumor survival and proliferation mainly through lipid metabolic reprogramming of fatty acid β-oxidation for energy supply. As the tumor progresses, glycolysis is gradually enhanced and cancer cells gradually show the Warburg effect with a higher rate of glucose uptake [30]. The process of lipid metabolic reprogramming plays a role in some researchs. Gazi et al. found that lipid-specific translocation between adipocytes and PCa cells by utilizing labeled fatty acids, which appears by direct cellular contact or paracrine [31]. PPAT explants from post-radical PCa co-cultured with PCa cell lines showed decreased expression of lipid metabolism genes (CD36, FASN, PPARG, and CPT1A), indicating a progressive decline in PPAT lipid production and utilization, contrary to that discovered in co-cultured PCa cell lines. Increased lipid absorption and accumulation in PCa cells and increased number of intracellular lipid droplets were associated with increased PCa aggressiveness. They inhibited PCa growth in vivo and in vitro [32, 33]. These studies suggest that PPAT might be a significant source of fatty acids for PCa cells. The metabolic processes and metabolism-related proteins of free fatty acids (FFAs) in PCa correlate with the biological behavior of PCa. PCa cells take up lipids mainly utilizing macrocytic drinking or fatty acid transporter protein CD36 and store them in the cyto-plasm as lipid droplets (LDs). Targeting CD36 reduces FFAs uptake and slow cancer progression [34]. Fatty acid-binding proteins (FABPs) are a family of proteins that serve as intracellular FFAs transporters and are related to the intracellular storage of FFAs, which are translocated to the nucleus in PCa cells to interact with peroxisome proliferation-activated receptor γ (PPARγ) and promote cell proliferation, invasion, and migration [35]. In addition to classical cytoplasmic lipolysis by lipases, lipid droplets also release FFAs through lipophagy, which then provides energy for β-oxidation in the mitochondria. Lipophagy, a selective form of autophagy, is associated with LD degradation. In locally progressive PCa, cancer cells have elevated levels of lipid droplets and autophagy markers in extraprostatic regions in contact with PPAT, and these markers correlate with PCa aggressiveness [36]. These experiments suggest that FFAs secreted by PPAT influence the reprogramming of PCa lipid metabolism and, thus, its progression.
In addition, the amount and type of lipids released by PPAT indicate the risk of PCa progression, and current studies have focused on FFAs. FFAs are classified into saturated fatty acids (SFAs) and unsaturated fatty acids (UFAs) based on the number of double bonds, the latter including monounsaturated fatty acids, polyunsaturated fatty acids (PUFAs), and n-3 and n-6 PUFAs. The fatty acid composition of PPAT as determined using in vitro magnetic resonance (MR) spectroscopy by Iordanescu et al. suggested that the FFA composition of PPAT was changed in patients with aggressive PCa. The unsaturated to saturated fatty acid ratio showed a moderate negative correlation with the Gleason score [37, 38]. Similarly, Altuna-Coy et al.'s study suggested that lipidomic and functional analyses of PPAT indicated lipidomic differences between low and high-risk PCa, with alterations in fatty acid biosynthesis, linoleic acid metabolism, and β-oxidation of very long chain fatty acids having the most significant impact on the PPAT lipidome. When PPAT was grouped according to risk, palmitic, stearic, arachidonic, docosanoic, and linoleic ac-ids (LA) and their metabolites showed a trend toward reduction [33]. Figiel et al. suggested that PUFA composition in PPAT reflects past PUFA absorption, is related to PCa aggres-siveness, and varies according to geographic origin. Low levels of n-6 PUFAs, such as Lin-oleic acid, and high levels of SFAs were associated with PCa aggressiveness in African-Caribbean patients, and n-6 PUFAs were twice as high as in Caucasian patients. Low lev-els of n-3 PUFAs, such as eicosapentaenoic acid (EPA), were associated with PCa invasiveness in Caucasian patients. The in vitro migration potential of PPAT FFA extract-supplemented PCa cell lines was negatively correlated with adipose tissue LA content [39]. Interestingly, a study analyzing the basal secretory FA profile of PPAT exosomes showed no difference between patients with weaker or stronger PCa according to the Gleason score and tumor aggressiveness, and they concluded that there was no relationship between altered biological behavior of PCa and PPAT metabolic reprogramming in obese men [22]. In addition, studies have suggested that cholesterol metabolism in PPAT is also altered, with African-Caribbean patients having lower levels of cholesterol esters in PPAT than Caucasian patients, without any association with markers of PCa aggressiveness. In PCa tissues from African-Caribbean patients, the amount of ABCA1 (aasociated with cholesterol efflux) was reduced and the expression of SREBP-2 (associated with cholesterol uptake) was increased, and the direction of cholesterol accu-mulation in cancer cells correlated with a more frequent epithelial-mesenchymal transition (EMT) status, which may promote PCa aggressiveness in this way [40]. These findings demonstrate that PPAT alters the lipid composition of the PCa microenvironment, which in turn affects PCa progression.
The specific mechanisms for the action of FFAs on PCa in the PPAT microenvironment have been reported. EPA regulates protein kinase C signaling pathway and Akt kinase activity in PCa cells and suppress the growth of PCa xenografts [41, 42]. Figiel et al. found that in the EMT, transcription factor Zeb1 and the Ca2+-activated positive feedback loop between the K+ channel SK3 amplified Ca2+ entry and cell migration. In vitro experiments using human PCa sections and in vitro cultures found that LA and EPA exert anti-cancer effects by regulating Ca2+ entry, which is involved in Zeb1 regulation and cancer cell migration [43]. PPAT co-cultured with PCa or exogenous FFAs induces the expression of NOX5, an isoform of NADPH oxidase, which increases intracellular reactive oxygen species (ROS) and activates the HIF1/MMP14 pathway, which increases tumor cell invasion. In obese patients/samples, adipocytes surrounding the tumor are more likely to activate the described signaling pathway and induce tumor invasion [44]. Adipocytes in PPAT can also directly stimulate PC3 cells to produce MIC-1 (TGF-β family) and prostate mesenchymal fibroblasts to secrete IL-8 by upregulating lipolysis and FFA release. MIC-1 is a TGF-β family molecule, and the enhanced overexpression and secretion of MIC-1 stimulates PCa cell proliferation and invasion and are involved in anticancer therapy resistance [45]. All these mechanisms suggest that PPAT regulates PCa development through the release of FFAs.
3. Factors Secreted by PPAT Impact PCa
PPAT is an active secretory organ that secretes various factors which regulates multiple biological PCa behaviors, including cell proliferation, migration, and invasive capacity [46], which is currently a topic of interest in adipose tissue research. The current factors involved in PPAT secretomes are FFAs, leptin, lipocalin, interleukins, TNFα, chemokines, growth factors, and androgens. These molecules have highly diverse chemical structures and physiological functions, and the effect of PPAT on PCa cells depends on the balance between the pro- and anti-cancer effects of these molecules, which deserves further investigation. We will next discuss pilot studies confirming these molecules. Finley et al. found that interleukin-6 (IL-6) levels in PPAT CM (conditioned medium) was approximately 375-fold higher than that in patient-matched serum, correlated with pathological grade, and IL-6-regulated Stat3 phosphorylation levels were higher in high-grade tumors. This suggests that PPAT may regulate the aggressiveness of PCa by acting as a source of IL-6 [47]. In addition, transgenic expression of IL-6 in the mouse prostate induced autocrine IL-6 and homeostatic activation of STAT3 in prostate tissues, upregulated insulin-like growth factor (IGF) paracrine secretion, reprogrammed prostate oncogene expression, induced PCa production, and amplified inflammation in the prostate and PPAT [48]. Upregulated IL-6 in PPAT may also induce the development of hormone-refractory PCa by promoting neuroendocrine differentiation, inducing androgen production in the prostate, and activating androgen receptors [49]. Zhang et al. also demonstrated that IL-6 was highly expressed in PPAT, and lipocalin was lowly expressed. In addition, IL-6, leptin, and creactive protein levels are significantly elevated with increased PCa aggressiveness, and PPAT quantity increased significantly [50, 51].
In addition to IL-6, matrix metalloproteinases (MMPs) and chemokines play essential roles in the PPAT microenvironment; they promote PCa invasion and metastasis. Extracellular matrix metalloproteinases play significant roles in basement membrane and extracellular matrix degradation, thus promoting tumor invasion and metastasis. Therefore, they are of great interest in cancer research [52]. Sacca et al. demonstrated that PPAT CM secretes more pro-MMP-9 than BPH CM, promoting the invasive ability of PCa [53]. Ribeiro et al. observed increased MMP2 and MMP9 activity in PPAT and increased proliferation and migration capacity when PC-3 cells were stimulated with PPAT CM [54]. The analysis of the stromal vascular fraction (SVF) of PPAT in 6-month-old obese HiMyc mice by Saha et al. suggested that the levels of SVF encoding various chemokines, cytokines, and mRNAs encoding various chemokines, cytokines, growth factors, and angiogenic mediators were significantly increased, CXCL12 gene being one of the most significantly upregulated genes. CXCL12 receptors CXCR4 and CXCR7 were expressed in PCa cell lines and HMVP2 cells, and CXCL12 stimulated the migration and invasion of HMVP2 cells but not control cells. The effect of CXCL12 on HMVP2 cells were inhibited by the CXCR4 antagonist AMD3100 and by the knockdown of CXCR4 or CXCR7. CXCL12 treatment also rapidly activated STAT3, NFkB, and MAPK signaling in HMVP2 cells, which were again attenuated by AMD3100 or CXCR4, or CXCR7 knockdown [55]. Another study showed that PPAT secretes the chemokine CCL7, which diffuses from PPAT into the peripheral zone of the prostate and stimulates the migration of CCR3-expressing tumor cells. When UCB35625 inhibited the CCR3/CCL7 axis, the observed increase in migration associated with obesity completely disappeared [56].
In addition, the role of factors such as TNF-α, VEGF, TGF-β, IGF-1, and androgens in the PPAT microenvironment has been demonstrated. Dahran et al. demonstrated that the expression levels of TNF-α and VEGF on immunostaining in radical prostatectomy (RP) resected PPAT correlated significantly with the aggressiveness of PCa, suggesting the risk of higher-grade PCa [57]. Civita et al. found that PPAT CM culture promoted the migration of two different human androgen non-dependent (AI) PCa cell lines (DU145 and PC3) and upregulated CTGF expression. The well-known TGF-β receptor inhibitor SB431542 counteracted the increased migration and reduced CTGF expression observed in the presence of AdipoCM, suggesting that paracrine secretion of TGF-β by PPAT affects PCa cell motility [58]. Liotta et al. also demonstrated that PPAT upregulates TUBB2Bβ-microtubulin by paracrine. Moreover, IGF-1 isoform promotes resistance to docetaxel in PCa, an effect partially counteracted by the IGF-1 receptor inhibitor AG1024 [59]. Another study investigated all steroid hormones, including active androgens, in human PPAT tissues utilizing liquid chromatography-tandem mass spectrometry (LC-MS/MS). Steroid hormones, including active androgens and androgen synthase CYP17, CYP19, and 5-α-reductase activity, were confirmed in human adipose tissues and may be associated with CRPC through the stimulation of androgen receptor cancer cell development [60, 61]. In addition to the above experimentally confirmed factors, AlZaim, I. et al. also speculated that PPAT may act on PCa through visfatin, omentin,resistin, LCN2, RBP4, osteopontin, chemerin, apelin and other factors, but this needs further verification [62].
Another study using LC-MS/MS-based proteomic analysis revealed the proteomics in PPAT. Compared with CM-BPH, proteins that involved in different biological processes of PCa were expressed diversely. For example, proteins about locomotion, reproduction, immune system functions, catalytic activity, defense activity, transport proteins, metabolism and energy pathways expressed differentially in both groups [63]. These results revealed that multiple differentially expressed proteins in PPAT influence PCa, which warrants further investigation.
In addition to the factors that PPAT can secrete to regulate PCa, PCa can also alter PPAT function, thus promoting its development. The stimulation of PPAT exosomes by PC-3 CM induced the secretion of the bone-bridging protein, TNF-α, and IL-6, which are associated with cancer progression, upregulation of bone-bridging protein expression by 13-fold, and decreased expression of the protective adipokine lipocalin. The stimulation of matrix metalloproteinase-9 activity and higher mitochondrial DNA copy number suggests that PPAT plays a vital role in PCa progression[29]. Vitamin D receptor deficiency in mice with PCa induces fat necrosis and individual cell apoptosis in PPAT, which regulates PCa signaling pathways and affects PCa progression [64].
4. Inflammation of PPAT Influences the Progression of PCa
During weight gain, adipocytes accumulate lipids, become hypertrophic, hypoxic, and eventually their cells die. This cycle increases adipocyte chemokine production and immune cell recruitment, ultimately triggering chronic white adipose tissue (WAT) inflammation associated with carcinogenesis by releasing pro-inflammatory cytokines from adipocytes and immune cells. The pathology of WAT inflammation is characterized by coronal structures (CLS) consisting of dead or dying adipocytes surrounded by macrophages. These macrophages remove lipids and cellular debris and sometimes evolve into a multinucleated giant or foam cells. CLS is associated with a worse prognosis in patients with cancer, and interest in using these structures as prognostic biomarkers is growing [65].
Chronic inflammation and CLS formation also occur in PPAT and are associated with PCa progression. In a prospective study of 169 men with newly diagnosed PCa, periprostatic WAT inflammation was found in 49.7% of patients. It was associated with higher body mass index (BMI), larger adipocyte size, and tumors with a Gleason classification of IV/V. The association between PPAT inflammation and high Gleason grade remained significant after adjustment for BMI [66]. Polymerase chain reaction analysis of PPAT and subcutaneous adipose control tissues (SAT) collected from patients with PCa undergoing radical prostatectomy or BPH control patients undergoing simple prostatectomy showed that many inflammatory genes (e.g., IL8RA, ILRAB, CXCL2, CCL8, and CCL21) in PPAT compared with SAT were associated with high grade (Gleason-9) PCa. Moreover, CCL2, CCL4, and CXCL1-3 were downregulated [67]. The mouse DIO model exhibits marked PPAT inflammation secondary to AT expansion, with increased expression of CD68, MCP1, and TNF-α. They increased CLS formation, consistent with the enrichment of inflammatory response pathways [68, 69]. These studies all suggest an active inflammatory process in PPAT and is closely related to the development of PCa.
PPAT WAT inflammation may result from hypoxia and endoplasmic reticulum stress, and hypertrophic adipocytes that are hypoxic beyond the vascular support may be more sensitive to cell death. Endoplasmic reticulum stress in hypertrophic adipocytes leads to apoptosis, triggering an inflammatory response [70, 71]. Inflammation in PPAT is also associated with higher insulin, triglyceride, and leptin/lipocalin ratios, and lower high-density lipoprotein cholesterol, adipocyte size, and PCa levels compared with pa-tients without PPAT inflammation. In contrast, hyperinsulinemia promotes PCa cell proliferation, inhibits apoptosis, and is associated with adjuvant steroidogenesis. This stimulates the prostate by activating androgen receptors adenoma formation and activating androgen receptors [66]. AlZaim, I. et al. speculated that obesity, metabolic syndrome and diabetes may lead to PPAT inflammation and further affect PCa development by activating Thrombin cascade, while targeting Thrombin, Factor Xa, and protease-activated receptors (PARs) factors in the thrombin system may inhibit this process. In addition, caloric restriction, weight loss, and weight loss surgery, estrogen supplementation, and antidiabetic drugs can improve the efficacy of PCa treatment by improving the inflammatory state of PPAT [62].
5. Some Elements that Indirectly (through PPAT) or Directly Affect PCa
Obesity is a chronic increase in excess adipose tissues [17]. The direct link between obesity and PCa remains controversial. Histological analysis of PCa after transplantation of patient-derived PCa grafts (PDXs) in lean or obese combined immunodeficient (SCID) mice in culture for 10 weeks suggested that systemic obesity did not promote prostate tumorigenesis, neither did the transplantation of PPAT and PDXs together enhance tumor-igenesis [72]. In contrast, several studies have shown that obesity is associated with an increased risk of PCa and poor prognosis, which can be explained by elevated levels of se-rum adipokines such as IL-6, leptin, TNF-α, CCL7, CXCL12, CXCL-1, VEGF, MCP-1, in-creased MMP-9 activity, and altered metabolism of sex hormones in obese individuals [13, 15, 73]. Despite the controversy, several studies have suggested an indirect mechanism of action of obesity through PPAT affecting PCa. Obesity can induce PPAT inflammation, as evidenced by high CLS density and elevated levels of pro-inflammatory mediators. PPAT inflammation is more common in overweight and obese men; however, it is also detected in more than 40% of men with BMI <25, and PPAT volume does not increase during obesity, which may be because of the chronic hypoxic state of PPAT causing inflammation and fibrosis limiting its expansion [71]. Furthermore, during obesity, PPAT is more active in metabolism and secretion in obese men than in lean men, although PPAT volume does not increase. For example, obesity strongly promotes the process by which PPAT-secreting chemokine CCL7 stimulates the migration of CCR3-expressing tumor cells [56]. The expression of NOX5 and MMP14 is upregulated at the front end of PCa invasion, a process that is amplified in patients with obesity [44]. PPAT secretions obtained from patients with obesity stimulate PCa cell proliferation and angiogenesis more effectively than lean patients. The entire epigenomic methylation profile of PPAT was significantly different in obese or overweight patients compared with normal-weight patients with PCa. Epigenetic variants associated with excessive obesity may alter lipid metabolism and immune dysregulation, resulting in an unfavorable PCa microenvironment [74]. PPAT from obese patients with PCa stimulates higher rates of PCa and endothelial cell proliferation compared with subcutaneous adipose tissues from lean or obese patients. In addition, obesity alters the fatty acid (FA) profile in PPA and increases angiogenesis [75]. The function of prostates depends partially on direct hormone receptors on prostate epithelial cells and indirectly on systemic metabolism, including the effects of obesity [76]. Saha, A. et al. reviewed the potential mechanisms by which PPAT promotes PCa in the course of obesity, with particular emphasis on the important role of adipose stromal cells (ASCs) [77].
Scheinberg et al. summarized the relationship between dietary fat intake and the risk of PCa and found contradicting results from various studies. Possible mechanisms by which fat intake increases the occurrence of PCa include the effects on hormone regulation and androgen levels, oxidative stress, inflammation, exposure to toxic pesticides, and the effects of specific fatty acids [78]. Another review suggested that reducing red meat and saturated fat intake could prevent PCa. Consumption of n-3 and n-6 PUFA, phytoestrogens, and different dietary patterns affect the risk of PCa [79]. All these factors can indirectly affect PPAT. The current findings are less uniform; however, a high-fat diet (HFD) can induce obesity to act on PPAT indirectly. Furthermore, a direct effect on PCa progression through blood circulation has been proposed in several studies. Bhardwaj et al. verified that HFD could induce PPAT inflammation in mice. In contrast, the restriction of calorie intake in obese mice resulted in weight loss, reduced PPAT inflammation, and reduced expression of pro-inflammatory genes. Further PPAT transcriptome analysis revealed that excessive calorie intake enriched the inflammatory response pathways, whereas the restriction of calorie intake normalized inflammatory response pathways [68].
Rocha-Rodrigues et al. proposed that exercise training reduces visceral fat volume, increases skeletal muscle mass in patients with PCa, and improves the tumor microenvironment. Exercise training influences PCa progression by modulating the secretion of adipokines from PPAT and other adipose tissues and the secretion of myokines from skeletal muscles [80]. In addition, the participation of patients with PCa in exercise training after diagnosis may improve their survival [81]. Moderate aerobic exercise in young people may decrease the circulating levels of free IGF-1 and reduce the potential to support PCa growth [82]. The mechanisms by which physical activity affects PCa are complex and unknown; however, the potential of physical training to influence PCa progression through PPAT warrants further investigation.
Organochlorine pesticides (OCPs), a series of persistent organic pollutants with endocrine disrupting and bio-accumulative properties [83], are highly lipophilic and tend to accumulate in tissues with high-fat content. Recently, McKinlay et al. suggested that the exposure pattern of OCPs varies according to the ethnic-geographic origin, with most OCPs being present in higher concentrations in Caucasian patients. In addition, pp'-DDE (a kind of OCPs) levels are twice as high in African-Caribbean patients. Chlordecone is only detected in African-Caribbean patients with PPAT. Most OCP concentrations are positively correlated with age and BMI. After adjusting for age, BMI, and PUFA composition of PPAT, no significant correlation was found between OCP levels and the risk of aggressive diseases, except for mirex, which was negatively correlated with aggressive PCa characteristics in Caucasian patients [83].
6. PPAT Provides New Perspectives for Diagnosing and Treating Prostate Cancer
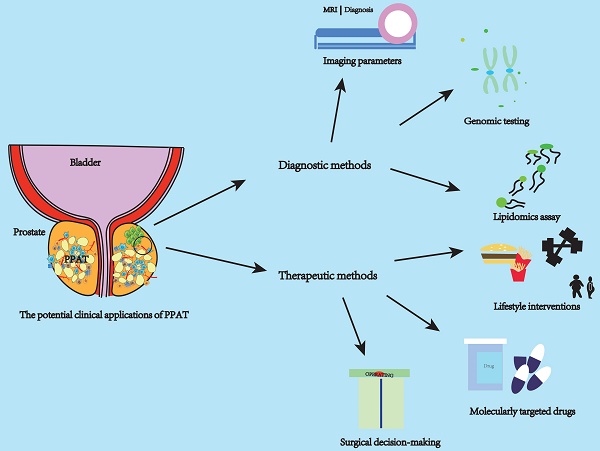
Based on a previous study that strongly suggested a strong association between PPAT and PCa (Figure 1), we explored the use of PPAT in clinical diagnosis and treatment (Figure 2).
6.1 PPAT for Diagnosing PCa
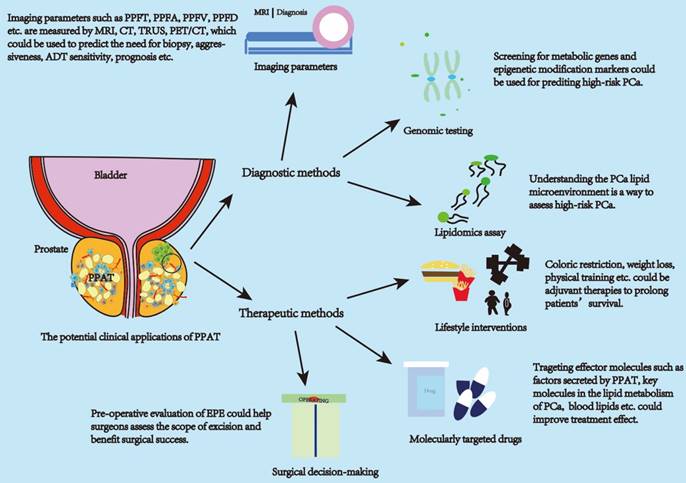
The above studies illustrate the involvement of PPAT in regulating the biological behavior of PCa through the secretion of various lipid molecules and molecules with effective activity and inflammatory status. This affects the clinical progression of the tumor and is closely associated with patient pathological staging, clinical treatment decisions, biochemical recurrence, survival, and other outcomes. Given this close association, it is crucial to translate PPAT features into clinically accessible measurement parameters for diagnosing patients' disease progression status. Current studies show promising results for diagnosing PCa progression status using PPAT imaging parameters, lipid metabolism-related gene assays, and lipid secretomes assays. PPAT-related imaging studies are the main research directions with promising applications.
The measurement of PPAT imaging parameters is currently the most readily available. Clinicians assess PCa aggressiveness and prognosis by imaging parameters such as periprostatic fat (PPF) thickness (PPFT), PPF area (PPFA), and PPF volume (PPFV) measured using rectal ultrasound (TRUS), computed tomography (CT) and magnetic resonance imaging (MRI). Table 1 summarizes the current imaging clinical studies related to PPAT. MRI is the primary modality for measuring PPAT-related parameters and has advantages over TRUS and CT. MRI has a good resolution of soft tissue and presents clear images. MRI is routinely used for the diagnosis, localization, and risk stratification of patients with PCa and is free of ionizing radiation [84]. TRUS is highly operator-dependent, and there may be variability in the choice of the measurement image plane. Moreover, the pressure applied to the prostate during the examination may affect the thickness of the fat surrounding the prostate [85]. CT is an excellent differentiator and quantifier of adipose tissues with good density measurements; however, its resolution is poor with an inherent risk of ionizing radiation [84]. In addition, some studies have used positron emission tomography (PET)/CT measurements, which are not routinely performed in the initial stages of PCa owing to the lack of additional clinical value for early PCa [86, 87].
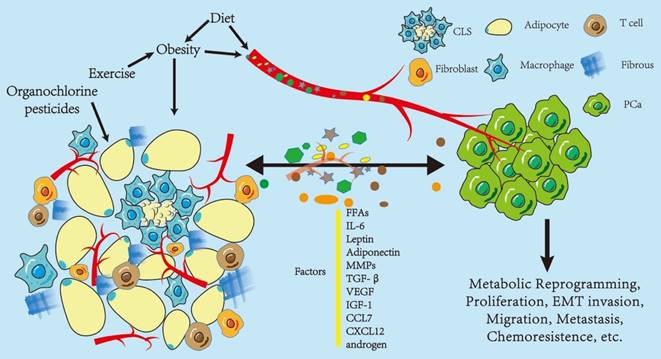
Influence of periprostatic adipose tissue on prostate cancer. Periprostatic adipose tissue (PPAT) consists of many adipocytes, other non-adipocytes, connective tissue matrix, blood vessels, and nerve tissues. The non-adipocyte components include inflammatory cells (macrophages), immune cells, preadipocytes, and fibroblasts. These components, as a whole, are capable of secreting various factors that influence the biological behavior of PCa in a paracrine or endocrine manner, including metabolic reprogramming, proliferation, and epithelial-to-mesenchymal transition (EMT) invasion. Some of these factors promote PCa progression, such as IL-6, leptin, VEGF, and CCL7; however, there are protective factors, such as adiponectin, and the effect on PCa depends on the balance between these two kinds of factors. In turn, PCa regulates the biological behavior of adipose tissues, thus promoting its development. Obesity and diet may enhance the effect of PPAT in an endocrine manner. Diet and exercise may indirectly alter PPAT function by affecting obesity or directly change the function of PPAT, and organochlorine pesticide deposition in PPAT may also affect PPAT function. Abbreviations CLS: crown-like structure; PCa: prostate cancer; IL-6: interleukin 6; MMPs: matrix metalloproteinases; TGF-β: transform growth factor-β; VEGF: vascular endothelial growth factor; IGF-1: insulin-like growth factor; CCL7: C-C motif ligand chemokine 7; CXCL 12: C-X-C motif ligand chemokine 12; MCP-1: monocyte chemoattractant protein 1.
Various PPAT parameters, such as PPFT, PPFV, PPFD, and PPFR, were used in the current study, as shown in Table 1. PPFT is the shortest vertical distance from the pubic symphysis to the prostate [84]. This method is relatively simple and easy to perform. It is based on only one distance in one plane, and can be performed quickly and reproducibly with basic training to identify relevant anatomical structures on MRI, which may be more suitable for clinical use. However, the method does not reflect the general distribution of PPAT, does not consider the differences in PCa location and fat thickness between the left and right sides, and PPFT is susceptible to the influence of prostate volume and pubic symphysis shape. The results obtained should be carefully validated, and further studies are needed to accumulate more reliable data regarding PCa and PPAT [88]. PPFA and PPFV measurements require calculation using advanced imaging software, which is relatively complex and time-consuming in clinical applications, and many hospitals do not have the appropriate equipment [89]. In addition, the heterogeneity of PPAT margins, complex weave structure, minute differences from surrounding tissues, inter-patient variability in shape and size, and heterogeneity in intensity distribution makes it difficult to measure the relevant parameters on MRI accurately. A recent study reported an algorithm that can accurately and automatically segment PCa and PPAT in T2-weighted images, reducing clinicians' workload [90]. However, further simplification of the measurement modality is still needed to facilitate its clinical application.
Several studies have shown a strong correlation between PPAT and PCa; however, some studies have noted that PPAT is unrelated to PCa [91, 92, 93], which the small sample size and heterogeneous differences in study participants may cause. In addition, when considering PPAT imaging parameters, it may be more accurate to combine the inflammatory status of PPAT [62], Gleason score, PSA level, and Prostate Imaging Reporting and Data System (PI-RADS) to predict the subsequent management of patients with PCa.
In addition to measuring the PPAT imaging parameters, some studies have also diagnosed the risk profile of patients with PCa by measuring the genomic expression of genes in PPAT. The expression was significantly different. Those with low CRTC2 expression had high pGS (pathological Gleason Score) values, high seminal vesicle infiltration, significantly poorer pathological outcomes, and significantly lower biochemical recurrence-free survival [94]. Mangiola et al. improved and evaluated the 3-genes (IGHA1, OLFM4, and RERGL) signature in PPAT and obtained discriminatory utility in predicting the presence of high-risk disease [28]. By identifying differentially expressed genes with aberrant methylation patterns on PCa, PPAT can differentiate between localized PCa and locally progressive PCa. These genes will be new diagnostic candidate molecular markers [95]. In addition, previous studies have reported that the lipid composition of PPAT can, to some extent, indicate the risk of PCa progression. Its detection can help us understand the tumor metabolic microenvironment and provide new stratification factors to assess the PCa risk class [33]. Scheinberg et al. also summarized several lipids associated with an increased risk of PCa diagnosis, including 1-stearoyl glycerol, glycerophospholipids, acylcarnitine, and lipids involved in phospholipid metabolism. Furthermore, lipids associated with an increased risk of advanced PCa include phosphatidylcholine and lysophosphatidylcholine, hydroxy sphingosine, or acylcarnitine. Similar trends were observed for aggressive disease and death. However, these studies could not reproduce each other's findings because it is difficult to compare between trials owing to differences in study methods, assays, and metabolites examined [78]. Overall, these results suggested that PPAT-related genomic and lipid metabolomic profiles have critical potential applications for diagnostic applications.
6.2 Improvement of PCa Treatment Outcome by PPAT
Previous studies have suggested that inhibiting the effects of crucial molecules of PPAT secretome may inhibit PCa progression, such as CXCR4 antagonist AMD3100 inhibiting CXCL12 [55], UCB35625 inhibiting the CCR3/CCL7 axis [56], TGF-β receptor inhibiting SB431542 [58], and IGF-1 receptor inhibiting AG1024 [59]. All these inhibitors are molecularly targeted drugs with potential applications requiring further investigation. In addition, several studies have corroborated the potential applications of targeting these molecules. Stoykova et al. suggested that targeting critical metabolic enzymes of PCa lipid uptake, synthesis, and oxidation processes demonstrated anti-PCa effects [79]; for example, the FASN inhibitor IPI-9119 improves cancer metabolomics and induces PCa apoptosis, potentially providing a novel approach for preventing and treating metastatic PCa [52]. The inhibition of the expression of the fatty acid elongase ELOVL7 also leads to the ablation of CRPC xenograft tumors in mice [114]. Carnitine palmitoyltransferase-1 (CPT1A) is an enzyme required for the transport of fatty acyl chains from the cytoplasm to the mito-chondrial membrane gap and subsequent FAO, and the CPT1A inhibitor imodium causes PCa cell growth and decreased AR expression [115]. Statins, a class of lipid-lowering drugs used to treat hypercholesterolemia, reduce the risk of advanced PCa [116] and ameliorate the association between high saturated fat intake and increased PCa invasiveness, thereby reducing PCa invasiveness [117]. In addition to statins, PCSK9 regulates cholesterol metabolism by attaching to the low-density lipoprotein (LDL) receptor and reducing LDL receptor-mediated removal from circulation [117]. Based on the current findings, lipid-targeting agents are unlikely to replace highly effective therapies in metastatic PCa; however, they can be combined to improve patient survival [78].
In addition, other factors secreted by PPAT have been associated with PCa in several studies, and targeting these molecules demonstrated anti-PCa effects. Zhou et al. found that plasma IL-6 and TNF-α levels correlated significantly with graded changes in limited PCa [118] and that the downstream molecule of IL-6, STAT-3 inhibitor Stt, and anti-IL-6R antibody Tcz combined to target tumor cells exhibited anti-cancer effects [119]. In addition, IL-6 causes PCa resistance to radiation therapy by upregulating DNA repair-related mole-cules ATM, ATR, BRCA1, and BRCA2 [120].
Clinical research related to the association between PPAT and PCa
| Study | Country and Year | Patient Number | Method of Measurement | Conclusion |
|---|---|---|---|---|
| Roermund et al. [91] | Netherlands, 2004.04-2008.08 | 902 | SFT/PPFA /PPFD, CT | PPFD is not correlated with PC aggressiveness in patients receving brachytherapy. |
| Roermund et al. [96] | Netherlands, 2003.01-2008.08 | 932 | SFT/PPFA/PPFD, CT | PCa is more aggressive in patients with a higher PPFD. |
| Bhindi et al. [97] | Canada, NA | 931 | PPFT, TRUS | PPFT can predict high-grade PCa at biopsy. |
| Allott et al. [18] | America, 2005-2011 | 308 | SFT/PPFA/PPFD, CT | Visceral fat is related to more aggressive PCa in patients undergoing radiotherapy. |
| Tiberi et al. [92] | Canada, NA | 213 | PPFA/SFT, CT | BMI and body fat distribution influence rectal dose. Periprostatic fat is not associated with rectal dose. |
| Woo et al. [84] | Korea, 2013.01-2013.12 | 190 | SFT/PPFT, MRI | PPFT is correlated with pathological Gleason score and can predict high-grade PCa. |
| Tan et al. [85] | America, 2013.08-2015.02 | 295 | PPFT/PPFR, mpMRI | Higher PPFR is significantly related to a more aggressive PCa. |
| Cao et al. [98] | China, 2013.01-2015.12 | 371 | SFT/PPFT,mpMRI | PPFT can predict PCa and HGPCa, paticulary for PCa with PI-RADS grade 3. |
| Dahran et al. [99] | UK, 2010.01-2015.12 | 162 | SFT/PPFV, MRI | PPFV was associated with prostate cancer aggressiveness in patients undergoing RP. |
| Salji et al. [100] | UK, NA | 224 | PPFV, MRI | The tumor response to ADT is associated with PPFV. |
| Zhai et al. [101] | China, 2013.11-2018.03 | 56 | SFT/PPFR, mpMRI | Periprostatic fat can help predict PCa pathologic upgrading. |
| Huang et al. [89] | China, 2011.06-2017.06 | 150 | PPFT/PPFV, CT or MRI | PPFT predicts the time to CRPC in patients getting ADT. |
| Di Bella et al. [102] | UK, 2005-2011 | 401 | PPFA/PPFD, CT | PPAT increases the risk of recurrence in patients undergoing radiation only but decreases the recurrence risk in patients undergoing radiation and ADT. |
| Iemura et al. [103] | Japan, 2013.03-2017.12 | 220 | SFT/PPFT, mpMRI | PV, Gleason score, and PPFT are independent risk factors for upstaging in men undergoing RP |
| Lee et al. [104] | Korea, 2013.01-2017.12 | 77 | CT-attenuation (HU) and FDG uptake (SUV) of PPAT, PET/CT | PPAT is related to PCa progression. |
| Sasaki et al. [105] | Japan, 2005.03-2014.09 | 85 | SFT/PPFT/PPFR, MRI | PPFT is an independent risk predictor of survival in hormone-naïve patients. |
| Zhai et al. [106] | China, 2016.06-2018.10 | 660 | PPFA /PPFR,MRI | PPFT can help predict PCa or csPCa |
| Gregg et al. [107] | America, NA | 175 | PPFT/PPFV, MRI | Normalized PPFR was related to shorter progression-free survival. |
| Zhai et al. [108] | China, 2016.06-2018.10 | 179 | PPFA /PPFR, MRI | PPFR can predict lymph node metastasis in patients receving RP. |
| Chien et al. [109] | China, 2009.01-2018.12 | 60 | PPFV, MRI | PPFV was associated with prostate cancer aggressiveness. |
| Xiong et al. [110] | China, 2013.03-2022.05 | 901 | PPFT, MRI | PPFT was related to the detection of PCa and csPCa in PCa biopsy. |
| Taussky et al. [111] | Canada, 2009.03-2016.01 | 61 | PPFD/PPFT, CT | 5ARIs appear to affect PPAT volume. |
| Zhang et al. [112] | China, 2006.03-2012.10 | 184 | SFT/PPFT/PPFA, MRI | PPAT can help assess the tumor stage and grade. |
| Laine-Caroff et al. [93] | France,2013.10-2015-03 | 121 | PPFT/PPFV,MRI | PPAT is not associated with PCa aggressiveness. |
| Shahait, M. et al. [113] | America, 2013-2018 | 98 | all surgically resectable visceral adipose tissue anterior to theendopelvic fascia extending from the prostatic base to theapex | PPAT features derived from MRI scans predict patients with clinically significant PCa |
PPFT: periprostatic fat thickness; PPFA: periprostatic fat area; PPFD: periprostatic fat density; PPFR: periprostatic fat ratio; PCa: prostate cancer; RP: radical prostatectomy; csPCa: clinical significance PCa.
PPAT has potentially valuable clinical applications. The clinical applications of PPAT can be divided into diagnostic and therapeutic approaches. Diagnostic approaches focus on applying PPAT imaging parameters, genomics, and lipidomics. Therapeutic approaches can be in molecularly targeted drugs, lifestyle interventions, and surgical approaches to decision-making. Imaging parameters can be used to assess the aggressiveness of PCa, time to CRPC, and patient prognosis. PPAT lipid metabolism genomic and epigenetic assays can be used to predict high-risk PCa. Lipidomic assays can be used to assess the PCa lipid metabolism microenvironment and predict high-risk PCa. Life style intervations and targeted drugs can improve the effect of treatment. The amount and distribution of PPAT can serve as a consideration for the surgeon to predict the surgical plan. Abbreviations: PPAT: periprostatic adipose tissue; PPFT: periprostatic fat thickness; PPFA: periprostatic fat area; PPFD: periprostatic fat density; PPFR: periprostatic fat ratio; PCa: prostate cancer.
Zhang et al. found that the TGF-β receptor I antagonist alisertib significantly inhibited tumor growth and progression in a TRAMP-C1 cell line-derived subcutaneous tumor model [121]. Leptin activation induces PCa cancer proliferation, promote invasion, and inhibit apoptosis [122]. Philp et al. further validated that the leptin receptor antagonist Alloaca inhibited LNCaP xenograft tumor growth, delayed progression to CRPC in mice, and suggested that leptin receptor blockade combined with androgen axis inhibition is a promising new therapeutic strategy for treating advanced PCa [123]. Hu et al. found that patients with PCa had lower serum lipocalin. The use of the peptide lipocalin receptor (ADIPOR) agonist ADP355 in subcutaneous LNCaP xenograft mice slowed tumor growth and retarded the progression of the serum PCa biomarker PSA [124]. This inhibition can be achieved by altering cellular energy, cellular stress, and protein synthesis, ultimately leading to apop-tosis [125]. In addition, GV1001 inhibits CRPC cell activity, induces apoptosis, and sup-presses angiogenesis by inhibiting the AKT/NF-κB/VEGF signaling pathway [126]. All aspects of PCa progression are closely related to androgen levels and androgen receptor (AR) status. Almost all treatments, from desensitized PCa (CSPC) to deadly resistant PCa (CRPC), target androgen metabolic pathways and AR. Altered androgen metabolism and its response are among the leading causes of drug resistance in PCa [127]. These findings further support the potential application of targeting PPAT-secreting molecules to control PCa progression.
Other drugs inhibit PPAT inflammation and may improve the prognosis of male patients with PCa. Pioglitazone, a PPARγ ligand, is used for treating diabetes and has anti-inflammatory properties. Miyazawa et al. found that pioglitazone inhibited PPAT inflammation in obese mice, reduced the density of CLS in periprostatic fat, and inhibited the levels of TNF-α, TGF-β, and chemokine monocyte chemotactic protein-1 (MCP-1), thus improving PCa [69]. In addition, supplementation with 17β-estradiol (E2) suppressed caloric intake, induced weight loss, reduced PPAT inflammation in obese mice, and down-regulated the expression of genes associated with inflammation, including Cd68, Mcp1, and Tnf [66]. Mangiola et al. suggested that ADT is associated with PPAT pro-inflammation and obesity-like adipose tissue microenvironment. The beneficial effects of ADT treatment may be partially offset by the metabolic and inflammatory side effects of PPAT [128].
In addition to developing relevant drugs, lifestyle interventions such as weight loss, calorie intake control, and increased physical activity may improve the prognosis of patients with PCa, as previously described. Calorie restriction (CR) partially inhibits the progression of PCa by modulating the IGF axis, and IGF-1 receptor (IGF-1R) blockade inhibits PCa xenograft growth. Combining CR with IGF-1R blockade would have a superimposed effect on PCa growth [129]. Elliott et al. demonstrated an association between high saturated fat intake and the risk of aggressive PCa [117]. In addition, PPAT influences multiple steps of the surgical procedure for radical PCa, and it is associated with surgical difficulty. Preoperative attention to PPAT-related parameters may benefit surgical success [130], but this prognostic factor requires further validation. When PPAT is invaded by PCa can be defined as extraprostatic extension (EPE), which tends to predict a worse prognosis. EPE also influences the doctor's decision on the surgical approach, which may often require more extensive resection [131, 132].
7. Conclusions and Future Considerations
The effects of adipose tissues on tumors have long been reported. As different adipose depots in the body have unique morphological structures and physiological functions, further differentiation of the effects of each adipose depot on specific tumors may be beneficial for the precise diagnosis and treatment of tumors. PPAT is only one of the adipose tissues that can secrete these substances in vivo; however, it is the closest adipose tissue to PCa, thus it may play a unique role. The adjacent anatomical location makes PPAT more likely to affect the peripheral zone of the prostate, and whether this is related to the higher incidence of PCa in the peripheral zone requires further investigation. In addition, obesity, expansion of other adipose tissues in the body because of diet, and lipid metabolism directly or indirectly affect PCa, thereby enhancing the effect of PPAT. Controlling these factors may improve the prognosis of patients with PCa. Studies have suggested that PPAT contributes to the development of PCa; however, other studies have suggested that PPAT has little or no effect on PCa [23,72,90,92]. Because of the complexity of the mechanisms of action of PPAT on PCa, further studies are needed to explore the mechanisms of PPAT effects on PCa. Furthermore, analysis of the whole genome of PCa PPAT by Ribeiro et al. showed reduced local immune monitoring of PPAT, mainly associated with the down-regulation of complement CFH [133]. This suggests that the local immune microenvironment of PPAT can affect PCa; hence, there is scope for further basic research on PPAT. More research evidence is needed to apply PPAT in the clinical setting using specific mechanisms to develop appropriate therapeutic tools in the future. Overall, the study of PPAT provides a new perspective for diagnosing and treating PCa.
Acknowledgements
Funding
The present study was supported by grants from the following organization: Jilin Provincial Department of Finance (No: JLSWSRCZX2020-058), Jilin Provincial Department of science and technology (No: 20210401154), WU JIEPING Medical Foundation (No: 320.6750.2020-06-37).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author contributions
H.Z. and S.W. conceived the research. H.C. searched the publications and drafted the manuscript. H.C., D.Z., and B.L. edited the tables and figures. Y.W. reviewed the manuscript and polished the grammar. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48
2. Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet. 2021;398:1075-1090
3. Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T. et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol. 2017;71:630-642
4. Sartor O, de Bono JS. Metastatic Prostate Cancer. N Engl J Med. 2018;378:645-657
5. de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147-1154
6. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197
7. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L. et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005
8. Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018;27:68-83
9. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11-18
10. Cinti S. Adipose Organ Development and Remodeling. Compr Physiol. 2018;8:1357-1431
11. Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S. et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci U S A. 2017;114:8649-8654
12. Roesler A, Kazak L. UCP1-independent thermogenesis. Biochem J. 2020;477:709-725
13. Uehara H, Kobayashi T, Matsumoto M, Watanabe S, Yoneda A, Bando Y. Adipose tissue: Critical contributor to the development of prostate cancer. Journal of Medical Investigation. 2018;65:9-17
14. Buti S, Cortellini A, Bersanelli M. Reassessing Human Adipose Tissue. N Engl J Med. 2022;386:e61
15. Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533-1541
16. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer-Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794-798
17. Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1-11
18. Allott EH, Howard LE, Song H-J, Sourbeer KN, Koontz BF, Salama JK. et al. Racial Differences in Adipose Tissue Distribution and Risk of Aggressive Prostate Cancer among Men Undergoing Radiotherapy. Cancer Epidemiology Biomarkers & Prevention. 2014;23:2404-2412
19. Hong H, Koch MO, Foster RS, Bihrle R, Gardner TA, Fyffe J. et al. Anatomic distribution of periprostatic adipose tissue - A mapping study of 100 radical prostatectomy specimens. Cancer. 2003;97:1639-1643
20. Kiyoshima K, Yokomizo A, Yoshida T, Tomita K, Yonemasu H, Nakamura M. et al. Anatomical features of periprostatic tissue and its surroundings: a histological analysis of 79 radical retropubic prostatectomy specimens. Japanese Journal of Clinical Oncology. 2004;34:463-468
21. Alvarez-Artime A, Garcia-Soler B, Sainz RM, Mayo JC. Emerging Roles for Browning of White Adipose Tissue in Prostate Cancer Malignant Behaviour. International Journal of Molecular Sciences. 2021 22
22. Miladinovic D, Cusick T, Mahon KL, Haynes AM, Cortie CH, Meyer BJ. et al. Assessment of Periprostatic and Subcutaneous Adipose Tissue Lipolysis and Adipocyte Size from Men with Localized Prostate Cancer. Cancers (Basel). 2020 12
23. Alexandre EC, Calmasini FB, Sponton A, de Oliveira MG, André DM, Silva FH. et al. Influence of the periprostatic adipose tissue in obesity-associated mouse urethral dysfunction and oxidative stress: Effect of resveratrol treatment. Eur J Pharmacol. 2018;836:25-33
24. Zhang B, Chen X, Liu YH, Gan Y, Liu PH, Chen Z. et al. Periprostatic fat thickness measured on MRI correlates with lower urinary tract symptoms, erectile function, and benign prostatic hyperplasia progression. Asian J Androl. 2021;23:80-84
25. Passos GR, Ghezzi AC, Antunes E, de Oliveira MG, Mónica FZ. The Role of Periprostatic Adipose Tissue on Prostate Function in Vascular-Related Disorders. Front Pharmacol. 2021;12:626155
26. Cancel M, Pouillot W, Maheo K, Fontaine A, Crottes D, Fromont G. Interplay between Prostate Cancer and Adipose Microenvironment: A Complex and Flexible Scenario. International Journal of Molecular Sciences. 2022 23
27. Feng S, Lou K, Luo C, Zou J, Zou X, Zhang G. Obesity-Related Cross-Talk between Prostate Cancer and Peripheral Fat: Potential Role of Exosomes. Cancers (Basel). 2022 14
28. Mangiola S, Stuchbery R, Macintyre G, Clarkson MJ, Peters JS, Costello AJ. et al. Periprostatic fat tissue transcriptome reveals a signature diagnostic for high-risk prostate cancer. Endocrine-Related Cancer. 2018;25:569-581
29. Ribeiro RJT, Monteiro CPD, Cunha VFPM, Azevedo ASM, Oliveira MJ, Monteiro R. et al. Tumor Cell-educated Periprostatic Adipose Tissue Acquires an Aggressive Cancer-promoting Secretory Profile. Cellular Physiology and Biochemistry. 2012;29:233-240
30. Giunchi F, Fiorentino M, Loda M. The Metabolic Landscape of Prostate Cancer. Eur Urol Oncol. 2019;2:28-36
31. Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J Lipid Res. 2007;48:1846-1856
32. Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B. et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393-406
33. Altuna-Coy A, Ruiz-Plazas X, Sanchez-Martin S, Ascaso-Til H, Prados-Saavedra M, Alves-Santiago M. et al. The lipidomic profile of the tumoral periprostatic adipose tissue reveals alterations in tumor cell's metabolic crosstalk. BMC Med. 2022;20:255
34. Watt MJ, Clark AK, Selth LA, Haynes VR, Lister N, Rebello R. et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci Transl Med. 2019 11
35. Guaita-Esteruelas S, Gumà J, Masana L, Borràs J. The peritumoural adipose tissue microenvironment and cancer. The roles of fatty acid binding protein 4 and fatty acid binding protein 5. Mol Cell Endocrinol. 2018;462:107-118
36. Fontaine A, Bellanger D, Guibon R, Bruyere F, Brisson L, Fromont G. Lipophagy and prostate cancer: association with disease aggressiveness and proximity to periprostatic adipose tissue. J Pathol. 2021;255:166-176
37. Venkatasubramanian PN, Brendler CB, Plunkett BA, Crawford SE, Fitchev PS, Morgan G. et al. Periprostatic Adipose Tissue from Obese Prostate Cancer Patients Promotes Tumor and Endothelial Cell Proliferation: A Functional and MR Imaging Pilot Study. Prostate. 2014;74:326-335
38. Iordanescu G, Brendler C, Crawford SE, Wyrwicz AM, Venkatasubramanian PN, Doll JA. MRS measured fatty acid composition of periprostatic adipose tissue correlates with pathological measures of prostate cancer aggressiveness. Journal of Magnetic Resonance Imaging. 2015;42:651-657
39. Figiel S, Pinault M, Domingo I, Guimaraes C, Guibon R, Besson P. et al. Fatty acid profile in peri-prostatic adipose tissue and prostate cancer aggressiveness in African-Caribbean and Caucasian patients. Eur J Cancer. 2018;91:107-115
40. Lethongsavarn V, Pinault M, Diedhiou A, Guimaraes C, Guibon R, Bruyère F. et al. Tissue cholesterol metabolism and prostate cancer aggressiveness: Ethno-geographic variations. Prostate. 2021;81:1365-1373
41. Gu Z, Wu J, Wang S, Suburu J, Chen H, Thomas MJ. et al. Polyunsaturated fatty acids affect the localization and signaling of PIP3/AKT in prostate cancer cells. Carcinogenesis. 2013;34:1968-1975
42. Pandian SS, Sneddon AA, Bestwick CS, McClinton S, Grant I, Wahle KW. et al. Fatty acid regulation of protein kinase C isoforms in prostate cancer cells. Biochem Biophys Res Commun. 2001;283:806-812
43. Figiel S, Bery F, Chantome A, Fontaine D, Pasqualin C, Maupoil V. et al. A Novel Calcium-Mediated EMT Pathway Controlled by Lipids: An Opportunity for Prostate Cancer Adjuvant Therapy. Cancers. 2019 11
44. Laurent V, Toulet A, Attane C, Milhas D, Dauvillier S, Zaidi F. et al. Periprostatic Adipose Tissue Favors Prostate Cancer Cell Invasion in an Obesity-Dependent Manner: Role of Oxidative Stress. Molecular Cancer Research. 2019;17:821-835
45. Huang M, Narita S, Koizumi A, Nara T, Numakura K, Satoh S. et al. Macrophage inhibitory cytokine-1 induced by a high-fat diet promotes prostate cancer progression by stimulating tumor-promoting cytokine production from tumor stromal cells. Cancer Communications. 2021;41:389-403
46. Moreira A, Pereira SS, Costa M, Morais T, Pinto A, Fernandes R. et al. Adipocyte Secreted Factors Enhance Aggressiveness of Prostate Carcinoma Cells. Plos One. 2015 10
47. Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Zaldivar F. et al. Periprostatic Adipose Tissue as a Modulator of Prostate Cancer Aggressiveness. Journal of Urology. 2009;181:49-49
48. Liu G, Zhang J, Frey L, Gang X, Wu K, Liu Q. et al. Prostate-specific IL-6 transgene autonomously induce prostate neoplasm through amplifying inflammation in the prostate and peri-prostatic adipose tissue. J Hematol Oncol. 2017;10:14
49. Stenman UH. Words of wisdom. Re: Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. Eur Urol. 2010;57:541-542
50. Zhang Q, Sun LJ, Yang ZG, Zhang GM, Huo RC. Influence of adipocytokines in periprostatic adipose tissue on prostate cancer aggressiveness. Cytokine. 2016;85:148-156
51. Zhang Q, Sun LJ, Qi J, Yang ZG, Huang T. Influence of adipocytokines and periprostatic adiposity measurement parameters on prostate cancer aggressiveness. Asian Pac J Cancer Prev. 2014;15:1879-1883
52. Liu RZ, Godbout R. An Amplified Fatty Acid-Binding Protein Gene Cluster in Prostate Cancer: Emerging Roles in Lipid Metabolism and Metastasis. Cancers (Basel). 2020 12
53. Sacca PA, Creydt VP, Choi H, Mazza ON, Fletcher SJ, Vallone VB. et al. Human periprostatic adipose tissue: its influence on prostate cancer cells. Cell Physiol Biochem. 2012;30:113-122
54. Ribeiro R, Monteiro C, Cunha V, Oliveira MJ, Freitas M, Fraga A. et al. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. Journal of Experimental & Clinical Cancer Research. 2012 31
55. Saha A, Ahn S, Blando J, Su F, Kolonin MG, DiGiovanni J. Proinflammatory CXCL12-CXCR4/CXCR7 Signaling Axis Drives Myc-Induced Prostate Cancer in Obese Mice. Cancer Research. 2017;77:5158-5168
56. Laurent V, Guerard A, Mazerolles C, Le Gonidec S, Toulet A, Nieto L. et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016 7
57. Dahran N, Szewczyk-Bieda M, Vinnicombe S, Fleming S, Nabi G. Periprostatic fat adipokine expression is correlated with prostate cancer aggressiveness in men undergoing radical prostatectomy for clinically localized disease. Bju Int. 2019;123:985-994
58. La Civita E, Liotti A, Cennamo M, Crocetto F, Ferro M, Liguoro P. et al. Peri-Prostatic Adipocyte-Released TGF beta Enhances Prostate Cancer Cell Motility by Upregulation of Connective Tissue Growth Factor. Biomedicines. 2021 9
59. Liotti A, La Civita E, Cennamo M, Crocetto F, Ferro M, Guadagno E. et al. Periprostatic adipose tissue promotes prostate cancer resistance to docetaxel by paracrine IGF-1 upregulation of TUBB2B beta-tubulin isoform. Prostate. 2021;81:407-417
60. Arai S, Shibata Y, Koike H, Matsui H, Ito K, Honma S. et al. Androgen Production in Periprostatic Adipose Tissue in Prostate Cancer Patients. Journal of Urology. 2012;187:E389-E390
61. Shibata Y, Arai S, Koike H, Matsui H, Ito K, Honma S. et al. Expression of Genes Encoding Enzymes Involved in Androgen Synthesis in Human Adipose Tissue. European Urology Supplements. 2011;10:264-264
62. AlZaim I, Al-Saidi A, Hammoud SH, Darwiche N, Al-Dhaheri Y, Eid AH. et al. Thromboinflammatory Processes at the Nexus of Metabolic Dysfunction and Prostate Cancer: The Emerging Role of Periprostatic Adipose Tissue. Cancers (Basel). 2022 14
63. Sacca PA, Mazza ON, Scorticati C, Vitagliano G, Casas G, Calvo JC. Human Periprostatic Adipose Tissue: Secretome from Patients With Prostate Cancer or Benign Prostate Hyperplasia. Cancer Genomics Proteomics. 2019;16:29-58
64. Guzey M, Jukic D, Arlotti J, Acquafondata M, Dhir R, Getzenberg RH. Increased apoptosis of periprostatic adipose tissue in VDR null mice. Journal of Cellular Biochemistry. 2004;93:133-141
65. Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15:139-154
66. Gucalp A, Iyengar NM, Zhou XK, Giri DD, Falcone DJ, Wang H. et al. Periprostatic adipose inflammation is associated with high-grade prostate cancer. Prostate Cancer and Prostatic Diseases. 2017;20:418-423
67. Finley DS, Galet C, Yamashiro J, Kono E, Chau T, Pantuck A. et al. Pro-Inflammatory and Mitogenic Activity of Periprostatic Adipose Tissue in Prostate Cancer. Journal of Urology. 2011;185:E295-E296
68. Bhardwaj P, Ikeda T, Zhou XK, Wang H, Zheng XE, Giri DD. et al. Supplemental estrogen and caloric restriction reduce obesity-induced periprostatic white adipose inflammation in mice. Carcinogenesis. 2019;40:914-923
69. Miyazawa M, Subbaramaiah K, Bhardwaj P, Zhou XK, Wang H, Falcone DJ. et al. Pioglitazone Inhibits Periprostatic White Adipose Tissue Inflammation in Obese Mice. Cancer Prevention Research. 2018;11:215-226
70. Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799
71. Roumiguie M, Esteve D, Manceau C, Toulet A, Gilleron J, Belles C. et al. Periprostatic Adipose Tissue Displays a Chronic Hypoxic State that Limits Its Expandability. American Journal of Pathology. 2022;192:926-942
72. Lo JC, Clark AK, Ascui N, Frydenberg M, Risbridger GP, Taylor RA. et al. Obesity does not promote tumorigenesis of localized patient-derived prostate cancer xenografts. Oncotarget. 2016;7:47650-47662
73. Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L. et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439-445
74. Cheng Y, Monteiro C, Matos A, You J, Fraga A, Pereira C. et al. Epigenome-wide DNA methylation profiling of periprostatic adipose tissue in prostate cancer patients with excess adiposity-a pilot study. Clinical Epigenetics. 2018 10
75. Venkatasubramanian PN, Mafi M, Brendler CB, Plunkett BA, Doll J. Mouse Periprostatic Adipose (Ppa) Tissue Mimics Human Ppa Activity on Prostate Cancer Cells: A Model System to Study Ppa - Prostate Cancer Interactions. Journal of Urology. 2014;191:E581-E582
76. Xu H, Chen Y, Gu M, Liu C, Chen Q, Zhan M. et al. Fatty Acid Metabolism Reprogramming in Advanced Prostate Cancer. Metabolites. 2021 11
77. Saha A, Kolonin MG, DiGiovanni J. Obesity and prostate cancer - microenvironmental roles of adipose tissue. Nat Rev Urol. 2023
78. Scheinberg T, Mak B, Butler L, Selth L, Horvath LG. Targeting lipid metabolism in metastatic prostate cancer. Ther Adv Med Oncol. 2023;15:17588359231152839
79. Stoykova GE, Schlaepfer IR. Lipid Metabolism and Endocrine Resistance in Prostate Cancer, and New Opportunities for Therapy. Int J Mol Sci. 2019 20
80. Rocha-Rodrigues S, Matos A, Afonso J, Mendes-Ferreira M, Abade E, Teixeira E. et al. Skeletal Muscle-Adipose Tissue-Tumor Axis: Molecular Mechanisms Linking Exercise Training in Prostate Cancer. International Journal of Molecular Sciences. 2021 22
81. Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical Activity and Survival After Prostate Cancer. Eur Urol. 2016;70:576-585
82. Sridhar R, Bond V Jr, Dunmore-Griffith J, Cousins VM, Zhang R, Millis RM. Relationship Between Aerobic Fitness, the Serum IGF-1 Profiles of Healthy Young Adult African American Males, and Growth of Prostate Cancer Cells. Am J Mens Health. 2017;11:92-98
83. McKinlay R, Plant JA, Bell JN, Voulvoulis N. Endocrine disrupting pesticides: implications for risk assessment. Environ Int. 2008;34:168-183
84. Woo S, Cho JY, Kim SY, Kim SH. Periprostatic fat thickness on MRI: correlation with Gleason score in prostate cancer. AJR Am J Roentgenol. 2015;204:W43-47
85. Wei Phin T, Lin C, Chen M, Deane LA. Periprostatic Fat: A Risk Factor for Prostate Cancer? Urology. 2016;98:107-111
86. Bednarova S, Lindenberg ML, Vinsensia M, Zuiani C, Choyke PL, Turkbey B. Positron emission tomography (PET) in primary prostate cancer staging and risk assessment. Transl Androl Urol. 2017;6:413-423
87. Rayn KN, Elnabawi YA, Sheth N. Clinical implications of PET/CT in prostate cancer management. Transl Androl Urol. 2018;7:844-854
88. Shimbo M. Editorial Comment to Periprostatic fat thickness quantified by preoperative magnetic resonance imaging is an independent risk factor for upstaging from cT1/2 to pT3 in robot-assisted radical prostatectomy. Int J Urol. 2020;27:1149
89. Huang H, Chen S, Li W, Bai P, Wu X, Xing J. Periprostatic Fat Thickness on MRI is an Independent Predictor of Time to Castration-resistant Prostate Cancer in Chinese Patients With Newly Diagnosed Prostate Cancer Treated With Androgen Deprivation Therapy. Clinical Genitourinary Cancer. 2019;17:E1036-E1047
90. Li Y, Wu Y, Huang M, Zhang Y, Bai Z. Automatic prostate and peri-prostatic fat segmentation based on pyramid mechanism fusion network for T2-weighted MRI. Comput Methods Programs Biomed. 2022;223:106918
91. van Roermund JGH, Bol GH, Witjes JA, Bosch JLHR, Kiemeney LA, van Vulpen M. Periprostatic fat measured on computed tomography as a marker for prostate cancer aggressiveness. World Journal of Urology. 2010;28:699-704
92. Tiberi D, Gruszczynski N, Meissner A, Delouya G, Taussky D. Influence of body mass index and periprostatic fat on rectal dosimetry in permanent seed prostate brachytherapy. Radiat Oncol. 2014;9:93
93. Laine-Caroff P, Bruyere F, Mathieu R, Monleon L, Brunereau L, Fromont G. et al. The volume and thickness of preprostatic fat on MRIs are not associated with prostate cancer aggressiveness in men undergoing radical prostatectomy. Prog Urol. 2022;32:341-353
94. Lee H, Lee M, Hong SK. CRTC2 as a novel prognostic biomarker for worse pathologic outcomes and biochemical recurrence after radical prostatectomy in patients with prostate cancer. Investigative and Clinical Urology. 2019;60:84-90
95. Liu Q, Reed M, Zhu H, Cheng Y, Almeida J, Fruhbeck G. et al. Epigenome-wide DNA methylation and transcriptome profiling of localized and locally advanced prostate cancer: Uncovering new molecular markers. Genomics. 2022 114
96. van Roermund JGH, Hinnen KA, Tolman CJ, Bol GH, Witjes JA, Bosch JLHR. et al. Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. Bju Int. 2011;107:1775-1779
97. Bhindi B, Trottier G, Elharram M, Fernandes KA, Lockwood G, Toi A. et al. Measurement of peri-prostatic fat thickness using transrectal ultrasonography (TRUS): a new risk factor for prostate cancer. Bju Int. 2012;110:980-986
98. Cao Y, Cao M, Chen Y, Yu W, Fan Y, Liu Q. et al. The combination of prostate imaging reporting and data system version 2 (PI-RADS v2) and periprostatic fat thickness on multi-parametric MRI to predict the presence of prostate cancer. Oncotarget. 2017;8:44040-44049
99. Dahran N, Szewczyk-Bieda M, Wei C, Vinnicombe S, Nabi G. Normalized periprostatic fat MRI measurements can predict prostate cancer aggressiveness in men undergoing radical prostatectomy for clinically localised disease. Scientific Reports. 2017 7
100. Salji M, Hendry J, Patel A, Ahmad I, Nixon C, Leung HY. Peri-prostatic Fat Volume Measurement as a Predictive Tool for Castration Resistance in Advanced Prostate Cancer. European Urology Focus. 2018;4:858-866
101. Zhai L, Fan Y, Sun S, Wang H, Meng Y, Hu S. et al. PI-RADS v2 and periprostatic fat measured on multiparametric magnetic resonance imaging can predict upgrading in radical prostatectomy pathology amongst patients with biopsy Gleason score 3+3 prostate cancer. Scandinavian Journal of Urology. 2018;52:333-339
102. Di Bella CM, Howard LE, Oyekunle T, De Hoedt AM, Salama JK, Song H. et al. Abdominal and pelvic adipose tissue distribution and risk of prostate cancer recurrence after radiation therapy. Prostate. 2020;80:1244-1252
103. Iemura Y, Hori S, Tatsumi Y, Fukui S, Miyake M, Matsumura Y. et al. Periprostatic fat thickness quantified by preoperative magnetic resonance imaging is an independent risk factor for upstaging from cT1/2 to pT3 in robot-assisted radical prostatectomy. International Journal of Urology. 2020;27:1144-1149
104. Lee JW, Jeon YS, Kim KH, Yang HJ, Lee CH, Lee SM. Prognostic Value of CT-Attenuation and (18)F-Fluorodeoxyglucose Uptake of Periprostatic Adipose Tissue in Patients with Prostate Cancer. J Pers Med. 2020 10
105. Sasaki T, Sugino Y, Kato M, Nishikawa K, Kanda H. Pre-treatment ratio of periprostatic to subcutaneous fat thickness on MRI is an independent survival predictor in hormone-naive men with advanced prostate cancer. International Journal of Clinical Oncology. 2020;25:370-376
106. Zhai TS, Jin L, Hu LT, Kadier A, Zhou Z, Liu X. et al. Impact of peri-prostatic fat measurements using MRI on the prediction of prostate cancer with transrectal ultrasound-guided biopsy. Urol Oncol. 2020;38:37.e31-37.e39
107. Gregg JR, Surasi DS, Childs A, Moll N, Ward JF, Kim J. et al. The Association of Periprostatic Fat and Grade Group Progression in Men with Localized Prostate Cancer on Active Surveillance. Journal of Urology. 2021;205:122-128
108. Zhai TS, Hu LT, Ma WG, Chen X, Luo M, Jin L. et al. Peri-prostatic adipose tissue measurements using MRI predict prostate cancer aggressiveness in men undergoing radical prostatectomy. J Endocrinol Invest. 2021;44:287-296
109. Chien YH, Hsieh ML, Sheng TW, Chang YH, Wang LJ, Chuang CK. et al. Body composition and pelvic fat distribution are associated with prostate cancer aggressiveness and can predict biochemical recurrence. Medicine (Baltimore). 2022;101:e31076
110. Xiong T, Cao F, Zhu G, Ye X, Cui Y, Zhang H. et al. MRI-measured adipose features as predictive factors for detection of prostate cancer in males undergoing systematic prostate biopsy: a retrospective study based on a Chinese population. Adipocyte. 2022;11:653-664
111. Taussky D, Barkati M, Campeau S, Zerouali K, Nadiri A, Saad F. et al. Changes in periprostatic adipose tissue induced by 5-reductase inhibitors. Andrology. 2017;5:511-515
112. Zhang Q, Sun LJ, Qi J, Yang ZG, Huang T, Huo RC. Periprostatic adiposity measured on magnetic resonance imaging correlates with prostate cancer aggressiveness. Urol J. 2014;11:1793-1799
113. Shahait M, Usamentiaga R, Tong Y, Sandberg A, Lee DI, Udupa JK. et al. Periprostatic Adipose Tissue MRI Radiomics-Derived Features Associated with Clinically Significant Prostate Cancer. J Endourol. 2023
114. Han W, Gao S, Barrett D, Ahmed M, Han D, Macoska JA. et al. Reactivation of androgen receptor-regulated lipid biosynthesis drives the progression of castration-resistant prostate cancer. Oncogene. 2018;37:710-721
115. Schlaepfer IR, Rider L, Rodrigues LU, Gijón MA, Pac CT, Romero L. et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol Cancer Ther. 2014;13:2361-2371
116. Bansal D, Undela K, D'Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One. 2012;7:e46691
117. Allott EH, Arab L, Su LJ, Farnan L, Fontham ET, Mohler JL. et al. Saturated fat intake and prostate cancer aggressiveness: results from the population-based North Carolina-Louisiana Prostate Cancer Project. Prostate Cancer Prostatic Dis. 2017;20:48-54
118. Zhou J, Chen H, Wu Y, Shi B, Ding J, Qi J. Plasma IL-6 and TNF-alpha levels correlate significantly with grading changes in localized prostate cancer. Prostate. 2022;82:531-539
119. Méndez-Clemente A, Bravo-Cuellar A, González-Ochoa S, Santiago-Mercado M, Palafox-Mariscal L, Jave-Suárez L. et al. Dual STAT3 and IL-6R inhibition with stattic and tocilizumab decreases migration, invasion and proliferation of prostate cancer cells by targeting the IL-6/IL-6R/STAT3 axis. Oncol Rep. 2022 48
120. Chen X, Chen F, Ren Y, Weng G, Xu L, Xue X. et al. IL-6 signaling contributes to radioresistance of prostate cancer through key DNA repair-associated molecules ATM, ATR, and BRCA 1/2. J Cancer Res Clin Oncol. 2019;145:1471-1484
121. Zhang B, Zhu Z, Zhang X, Li F, Ding A. Inhibition of the proliferation, invasion, migration, and epithelial-mesenchymal transition of prostate cancer cells through the action of ATP1A2 on the TGF-β/Smad pathway. Transl Androl Urol. 2022;11:53-66
122. Xu CJ, Dong LL, Kang XL, Li ZM, Zhang HY. Leptin promotes proliferation and inhibits apoptosis of prostate cancer cells by regulating ERK1/2 signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:8341-8348
123. Philp LK, Rockstroh A, Sadowski MC, Taherian Fard A, Lehman M, Tevz G. et al. Leptin antagonism inhibits prostate cancer xenograft growth and progression. Endocr Relat Cancer. 2021;28:353-375
124. Hu X, Hu C, Zhang C, Zhang M, Long S, Cao Z. Role of Adiponectin in prostate cancer. Int Braz J Urol. 2019;45:220-228
125. Philp LK, Rockstroh A, Lehman M, Sadowski MC, Bartonicek N, Wade JD. et al. Adiponectin receptor activation inhibits prostate cancer xenograft growth. Endocr Relat Cancer. 2020;27:711-729
126. Park YH, Jung AR, Kim GE, Kim MY, Sung JW, Shin D. et al. GV1001 inhibits cell viability and induces apoptosis in castration-resistant prostate cancer cells through the AKT/NF-κB/VEGF pathway. J Cancer. 2019;10:6269-6277
127. Zhang H, Zhou Y, Xing Z, Sah RK, Hu J, Hu H. Androgen Metabolism and Response in Prostate Cancer Anti-Androgen Therapy Resistance. Int J Mol Sci. 2022 23
128. Mangiola S, Stuchbery R, McCoy P, Chow K, Kurganovs N, Kerger M. et al. Androgen deprivation therapy promotes an obesity-like microenvironment in periprostatic fat. Endocrine Connections. 2019;8:547-558
129. Galet C, Gray A, Said JW, Castor B, Wan J, Beltran PJ. et al. Effects of calorie restriction and IGF-1 receptor blockade on the progression of 22Rv1 prostate cancer xenografts. Int J Mol Sci. 2013;14:13782-13795
130. Kaneko G, Miyajima A, Yuge K, Hasegawa M, Takeda T, Jinzaki M. et al. Periprostatic Fat Area Is an Independent Factor That Prolonged Operative Time in Laparoscopic Radical Prostatectomy. Urology. 2013;82:1304-1309
131. Alessi S, Pricolo P, Summers P, Femia M, Tagliabue E, Renne G. et al. Low PI-RADS assessment category excludes extraprostatic extension (≥pT3a) of prostate cancer: a histology-validated study including 301 operated patients. Eur Radiol. 2019;29:5478-5487
132. Mehralivand S, Shih JH, Harmon S, Smith C, Bloom J, Czarniecki M. et al. A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI. Radiology. 2019;290:709-719
133. Ribeiro R, Monteiro C, Catalan V, Hu P, Cunha V, Rodriguez A. et al. Obesity and prostate cancer: gene expression signature of human periprostatic adipose tissue. Bmc Medicine. 2012 10
Author contact
![]() Corresponding author: Honglan Zhou, E-mail: walkerzhouhlcom; Song Wang, E-mail: w_sedu.cn.
Corresponding author: Honglan Zhou, E-mail: walkerzhouhlcom; Song Wang, E-mail: w_sedu.cn.

 Global reach, higher impact
Global reach, higher impact