3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(2):293-308. doi:10.7150/jca.90545 This issue Cite
Review
Dietary Polyphenols against Oxidative Stress in Head and Neck Cancer: What's New, What's Next
1. Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy.
2. Department of Informatics, University of Bari “Aldo Moro”, Bari, Italy.
3. AULSS4 - Veneto Orientale - Portogruaro, Venice, Italy.
4. Department of Precision Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy.
5. CNR Institute of Biomembranes, Bioenergetics and Molecular Biotechnologies (IBIOM), Bari, Italy.
Received 2023-9-27; Accepted 2023-10-24; Published 2024-1-1
Abstract
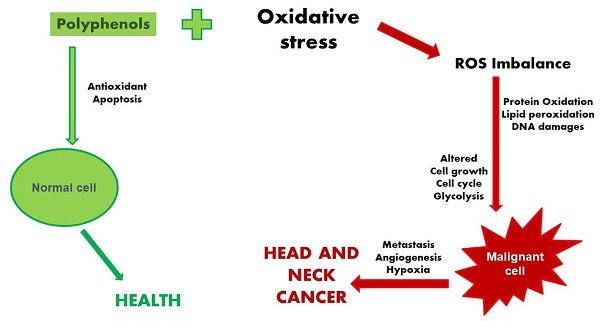
Head and neck cancers (HNC) are a worldwide health problem, accounting for over 5% of all types of cancers. Their varied nature makes it sometimes difficult to find clear explanations for the molecular mechanisms that underline their onset and development. While chemio- and radiotherapy are clearly not to be dismissed, we cannot undervalue the effect that polyphenols - especially dietary polyphenols - can have in helping us to cope with this medical emergency. By influencing several different proteins involved in numerous different metabolic pathways, polyphenols can have a broad spectrum of biological action and can hopefully act synergistically to tackle down head and neck cancer. Moreover, being natural molecules, polyphenols does not present any side effects and can even enhance drugs efficacy, making our clinical therapy against head and neck cancer more and more effective. Certainly, oxidative stress plays an important role, altering several molecular pathways, lowering the body's defenses, and ultimately helping to create a microenvironment conducive to the appearance and development of the tumor. In this regard, the regular and constant intake of foods rich in polyphenols can help counteract the onset of oxidative stress, improving the health of the general population. In this review, we highlight the role of polyphenols in managing oxidative stress, with such positive effects that they can be considered new tools to use in our anti-head and neck cancer strategy.
Keywords: head and neck cancer, polyphenol, oxidative stress, cancer metabolism, reactive oxygen species (ROS), clinical biochemistry, translational medicine, polyphenol therapeutic potential, clinical studies
1. Introduction
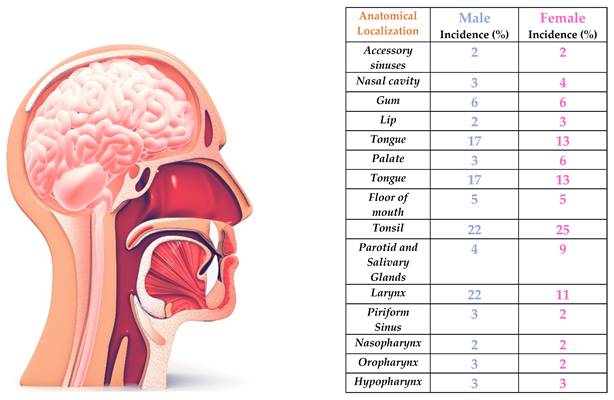
Head and neck cancer (HNC) accounted for more than 66,920 new cases in 2023 (49,190 men and 17,730 women), causing more than estimated 15,400 deaths (11,210 men and 4,190 women), accounts this disease for about 4% of all cancers in the United States alone [1]. Worldwide, the incidence of these heterogeneous types of cancer is equally high, with more than 562,328 people affected in 2020, being the 7th leading cause of deaths for all type of cancer [1,2]. Head and neck cancer usually arise from the mucosal surfaces of head and neck region, but also in salivary glands. HNC includes cancers that develop in the oral cavity, larynx, nasal cavity, and salivary glands (Figure 1); the worldwide 5-year median survival rate at 50% of cases, with the hypopharynx experiencing the worst outcomes [2].
HNC is greatly impacted by environmental (human papilloma virus infection is a known risk condition) and genetic factors, but behavioral habits loom large: alcohol and/or tobacco consumption are present in more than 80% of the total HNC cases. Smoking alone accounts for 42% of cancer incidence [3-4]; if both factors are present, the risk for oral and laryngeal cancer increase by 35-fold [5]. Individual's statistics can only reaffirm the urgency to really challenge HNC: understanding their metabolic pathway and interaction can go a long way in helping general people's health. These data help us to understand the magnitude of the problem, and at the same time make us recognize the need to study in detail the emergence and development of HNC: being able to fully understand the molecular mechanisms will be of great help in devising effective strategies to deal with these pathologies. As usual in cancer patients, the clinical therapy includes surgery, chemotherapy and radiotherapy, and where possible, immunotherapy, also in combination with each other. If HNC is diagnosed in the early developmental stages (I or II), a benign course of the pathology can reasonably be assumed; conversely, if discovered in the late phases (III or IV), the degree of remission of the disease is significantly lowered [6].
Head and neck cancer statistical incidence. HNC can appear in multiple anatomical districts: the most common are tonsils, followed by larynx for men and tongue for women.
HNC cancer etiology is a complex phenomenon, that can arise from different causes and different prognoses in several different districts; hence, it could be difficult to find a common base/perspective for this type of cancer; yet it is possible to find common ground in all these cancers and the internal and external factor that can trigger their development. Particularly intriguing is trying to elucidate the role of oxidative stress (OS) in promoting/facilitating the onset and development of head and neck tumors. As demonstrated by numerous researchers, HNSSC is characterized by high genetic and metabolic heterogeneity, and OS plays a central role in the emergence and development of these tumors. The presence of cellular OS enhances significantly - along with the reactive oxygen species (ROS) overabundance - the possibility of cancer arising.
2. Head and neck cancer metabolism and oxidative stress
As typical know in cancer cells, HNC characteristically shows exponentially, disproportionate, and unlimited proliferation. To support this growth, these cells adopt a peculiar metabolism, which promotes and enhances glucose uptake and anaerobiotic glycolysis, ultimately leading to Adenosine triphosphate (ATP) and lactate production, the latter producing the well-known Warburg effect [7]. This choice has a profound effect on the overall cell metabolism, and conversely, on human body's ability to effectively counteract cancer development. Normally, cells prefer to degrade glucose through aerobic glycolysis, which produces pyruvate that enters the mitochondria in the Tricarboxylic Acid Cycle (TCA) cycle, and subsequently in the electron chain transport, which led to the production of 32 ATP molecules from a single glucose fraction [8]. Conversely, during anaerobic glycolysis, the net ATP production is much lower, with only 2 molecules [8].
By adopting a very simplistic metabolism - i.e., bypassing the mitochondria and its aerobic metabolism - cancer cells can obtain the energy needed for their duplication, and at the same time infringe our ability to inhibit cancer cells specifically. In fact, by abiding by any kind of cellular differentiation - they mostly promote mitosis, i.e., cellular division -, it is difficult for our strategy against cancer to target any kinds of molecular switches, that are just not employed in the cancer cells [7,8].
The OS is an imbalance between the ROS presence and the antioxidants species at cellular level. There are several ways in which OS can impact cancer onset and development; by hypoxia regulation, but the aim of cancer development is to block the body's defense to spread the disease all over the tissue [9]. The ROS are chemically reactive molecules derived from molecular oxygen that play significant roles in cellular signaling and homeostasis. ROS include superoxide anion (O2·-), hydroxyl radical (OH·), hydrogen peroxide (H2O2), and singlet oxygen (·1O2) [10]. Under normal physiological conditions, ROS are produced as byproducts of cellular metabolism through processes such as mitochondrial respiration, enzymatic reactions, and immune responses [7-9]. ROS function as signaling molecules involved in cellular processes such as proliferation, differentiation, and immune responses [9]. An excess of pro-oxidant species production versus antioxidant species provokes OS: in fact, ROS excessive production could be the byproduct of pathological conditions, particularly of OS situations [8]. For the oral cavity, one of the most important ROS sources is i.e., periodontal inflammation [10]. Harmful habits such as cigarette smoke, and drug use, plays a prominent role in ROS production; also, diet - namely, ethanol intake - and high fat and/or high protein diet seems to favor ROS production [10]. Also, dental treatment with laser light, ultraviolet light, ozone, etc., as well the materials used in dental practice, such as dental composites, can contribute to the ROS production [11].
3. Oxidative stress and its importance in HNC tumor development
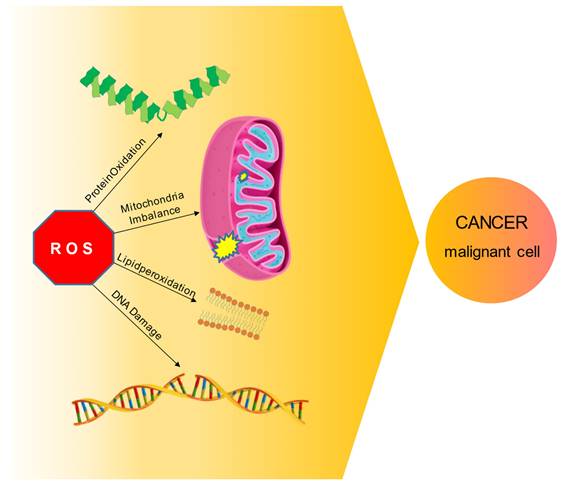
To fully perform their normal biological and metabolic activity, cells need to effectively control ROS production. DNA maintenance, protein regulation, transcription factors activation, immunity system, energy metabolism, pathway control, cell growth, and differentiation, and ultimately cell division (in a controlled manner) or apoptosis, are all biological processes that can be negatively influenced by an excess of ROS [12]. Is well know that ROS presence does not have to be considered only as a harmful effect: experimental research has extensively demonstrated that in physiological conditions ROS signaling as a second messenger in cells is crucial for a variety of biological actions, such as gene expression, signal transduction, and receptors activation [12-13], and also wound healing, tissue regeneration and protection from pathogens [14-15]. However, if ROS levels increase above the usual threshold can lead to serious nefarious consequences for the cell: just the modification in the macromolecules - proteins oxidation, nucleic acids damage, carbohydrates, and lipid peroxidation - could render impossible a healthy cell life; hence, maintaining ROS levels at adequate levels becomes an essential necessity for the cell (Figure 2).
A major part of these molecules is a byproduct of oxidative metabolism, usually generated in the mitochondria. Oxygen is a crucial factor for human metabolism in general: oxidative phosphorylation, the role of multicopper oxidase enzyme (MCO) [16], arachidonic acid pathway - lipoxygenases (LOX), and cyclooxygenases (COX) -, and significantly, inflammatory pathway and particularly endothelial cells require molecular oxygen to be completed [17-19]. Regulating ROS homeostasis is a key factor for cellular well-being: the lipid and carbohydrate peroxidation, protein oxidation, DNA damages and inflammation state due to ROS overproduction could facilitate the development of head and neck cancer [20]. ROS can also cause an imbalance in mitochondrial metabolism, causing change in membrane permeability and halting the ATP production, which in turn cause cell cycle alterations, ultimately leading to the cancer appearance (Figure 2) [21]. Cells normally cope with an excess of ROS through their scavenging capacity; however, in stressful conditions this ability falls short of matching the mitochondrial production of ROS [22-23].
The fight against cancer is truly an uphill battle: so much heterogeneity in the causes, and development and symptoms make it extremely complex and difficult to find a single way to counteract it. However, developing and fortifying our protective mechanisms against cancer insurgence - chemoprevention - can have a very long way in helping us against this powerful enemy. Introduced by Wattenberg [24], the concept of chemoprevention lies on the basis that several natural substances seem to display an ability to prevent cancer development. Interestingly, polyphenols molecules are a class of molecules that can have a positive effect on cancer onset and development, by affecting several distinct metabolic pathways, hence maybe having a synergistic effect: cell cycle, apoptosis, cell division, energy metabolism, DNA maintenance, are just some of the mechanisms of action of polyphenols in our metabolism [25-29]. There is a clear relationship between polyphenol intake and its positive effect on our health: the scientific evidence is stunningly clear, regarding the onset and development of several different pathologies, from cardiovascular diseases, neurogenerative disorders, obesity, diabetes, inflammatory disease, aging, and of course many different types of cancer, including HNC [30-37].
ROS molecular mechanism and HNC cancer. ROS overabundance in cell can have serious nefarious consequences, disrupting macromolecules and altering multiple biological pathways, ultimately leading to malignant cell transformation and cancer development.
4. Polyphenols: structure and function
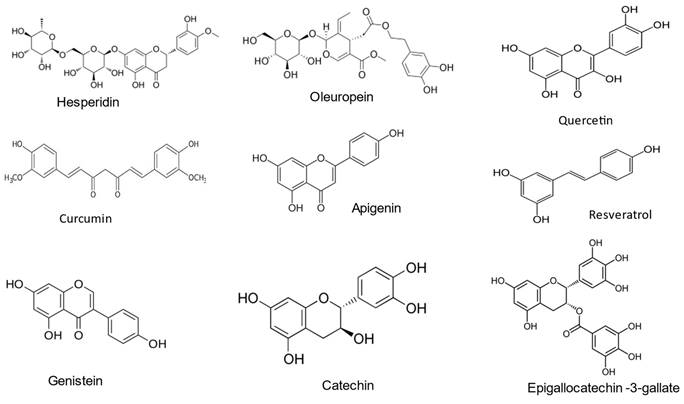
Polyphenols group encompass more than 10,000 molecules: their classification could be operated in several ways, for example by the number of their phenolic groups, or by dividing them into flavonoids and non-flavonoids; in any case, all the molecules classified as polyphenols possess at least one aromatic ring groups with one or more hydroxyl functional groups attached (Figure 3) [38-39]. Polyphenols are classified into different subclasses, including flavonoids, phenolic acids, stilbenes, and lignans, each with unique chemical structures and properties [40].
The therapeutic potential of several different polyphenols molecules has been extensively studied: they are mainly present in the vegetables and fruits groups and seems totally conceivable that are at least partially responsible for the benefit of a plant-based diet. Flavonoids are present mainly in vegetables, cereals, fruits, and legumes. Quercetin is a ubiquitous flavonoid present in a large variety of fruits (apples, grapes, olives, citrus fruits, berries), vegetables (tomatoes, onions, broccoli, capers), beverages (tea and red wine), and herbal extracts; however, its concentration in all species is quite low [41]. Ellagic acid is found in quercus species and particularly in pomegranates [42]. Hesperidin, a flavanone glycoside, is the most abundant polyphenol in citrus fruits [43]. Olive oil, a food often cited as an example as of source of good-for-health unsaturated fatty acids, contains a fair amount of hydroxytyrosol (HT) and oleuropein, polyphenols belonging to the catechol family [44]. For a more complete list of foods with the highest polyphenol content, please refer to Perez-Jimenez et al. [45].
The main representatives of the catechin subfamily are epigallocatechin and epigallocatechin gallate (EGCG), which can be harvested from many types of herbs, fruits, legumes, and algae. Due to their relatively high content in berries, tea - especially green and white tea - [46], is also an important source of catechins [47]. Resveratrol is one of the most studied molecules of the stilbene subfamily, which is usually found in grapes, and consequently in red wine [48]. Therefore, due to their widespread distribution in fruits, cereals, legumes, and vegetables, and ultimately in all plant-based food, polyphenols are the ideal candidate to help the general population cope with oxidative stress situations: by adhering to the recommended guidelines, for 5 portions of fruit and vegetables per day, polyphenols intake ingested could contribute to the individual's health protection [49]. However, it is impossible to correlate the intake of polyphenols present in a peculiar food and correctly evaluate the subsequent amount of polyphenols absorbed: besides environmental factors, such as light, temperature, water availability, nutrient status, and biotic stress, seasoning, cultivar, food processing - cooking, frying, toasting, etc. - can significantly affect polyphenol biosynthesis in plants. These factors modulate the expression of key biosynthetic genes and the accumulation of specific polyphenols, and ultimately affect our ability to estimate their bioavailability, which could render ineffective polyphenol's therapeutic potential in human metabolism. Hence, assessing an individual adequate dietary intake needs to consider polyphenol's bioavailability, which is different for every molecule, and is a very hard parameter to estimate: in fact, it is deeply affected by intestinal absorption, which can range from 3% of chlorogenic acid up to 43% of caffeic acid [50-51].
Usually, polyphenols in foods - except for the flavanols members - are in conjugated form (with carbohydrates), altering their solubility, digestion, and absorption properties, favoring their degradation and greatly limiting their absorption [52]. At this stage, it is extremely important to underline the role played by the intestinal microbiota: the biotransformation reactions of polyphenols in vivo, - i.e., sulfoxide reductase, nitro reductase, glucuronosyltransferase - are all microbial enzymes [53-54] and, consequently, microbiota community composition can greatly influence their function [55].
5. Polyphenols antioxidant activities
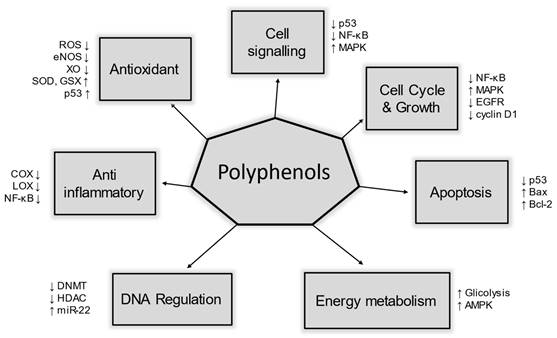
The presence of antioxidant molecules in cells could be defined as a key factor regarding our health: scientific literature has extensively proved that they prevent DNA damage, including the mitochondrial genome, thus positively affecting mitochondria biogenesis [56]. Polyphenols play a role in several molecular pathways that help us to manage oxidative stress. These pathways include enzymatic activity, metabolic regulation, membrane integrity, signal transduction, genetic activation, and epigenetic modifications. All of these are biological mechanisms involving polyphenols. In Figure 4, were illustrated some of the biological actions mediated by polyphenols in our metabolism.
The chemical structure of different food polyphenols.
The role of polyphenols and human health. Dietary polyphenols can impact several proteins involved in many different aspects of our metabolism, including many pathways that are altered in HNC cancer cells.
By interacting with non-polar compounds found in the hydrophobic inner membrane layer, polyphenols support the proper functioning of membranes [57]. This interaction helps preserve the rate of lipid and/or membrane protein oxidation. A study on hyperlipidemic rats demonstrated that the extract of Sempervivum tectorum exhibited antioxidant activity, protecting the organism against lipid peroxidation, and ultimately stabilizing the membranes [58]. These molecular interactions provide insights into the polyphenol's beneficial effects.
The documented evidence shows that polyphenols can interact with endothelial nitric oxide synthases (eNOS). They can modulate nitric oxide production, signaling, and metabolism, thus regulating eNOS expression and activity [59]. Oxidative stress can reduce nitric oxide bioavailability, which in turn contributes to endothelial dysfunction, a characteristic feature of cardiovascular disease. Bacterial infection can further worsen the oxidative injury by stimulating inducible NOS (iNOS) expression. However, polyphenols can improve macrophage function by inhibiting lipopolysaccharide-induced iNOS expression, thereby reducing oxidative stress [60]. Some in vitro studies, demonstrated that the total polyphenols derived from Allium cepa possess the capability to inhibit the activity of xanthine oxidase (XO) [61]. This antioxidant activity of flavanols is significant because XO activity has been associated with oxidative stress-related diseases, particularly ischemia [62], as the stimulation of XO can result in excessive production of free radicals.
Another important target for the antioxidant activity of polyphenols is NADPH oxidase (NOX). The NOX family comprises multiple members present in all human tissues and serves as one of the primary producers of ROS in various cells. In neutrophils and macrophages, NOX plays a crucial role in the oxidative burst, which involves the production of ROS for pathogen elimination [63]. The polyphenol Curcumin, can modulate NOX activity, as observed in supplementation studies using mouse C2C12 myoblasts, where it directly inhibited NADPH oxidase [64]. Additionally, it has been demonstrated that resveratrol could act as an O2*- scavenger, effectively and directly reducing ROS production mediated by NOX [65].
Advanced glycation end products (AGEs) are obviously stimulated by ROS, as well as the protein kinase C (PKC) pathways, leading to an activation of gluconeogenesis and lipogenesis. Experimental studies have demonstrated that polyphenols have the capability to inhibit SGLT1, thereby limiting the intestinal absorption of carbohydrates [66]. Moreover, when PKC is overexpressed, it can exacerbate oxidative stress by stimulating NADPH-oxidases and lipoxygenases, which are enzymes known to generate ROS, as evidenced in human platelet suspensions [67].
The arachidonic acid pathway plays a crucial role in different diseases, including cancer development, arthritis, asthma, and general inflammatory processes [68]. The breakdown of arachidonic acid through multiple enzymes, particularly COX and LOX, leads to the production of prostaglandins and leukotrienes, which are key factors in managing the inflammatory process. Polyphenol supplementation can influence the metabolism and pathway of arachidonic acid. For example, quercetin can modulate the activity of COX, LOX, phospholipase A2s (PLA2s), and cytochrome P450 (CYP) [68]. Similarly, curcumin exhibits potent inhibition of arachidonic acid-induced inflammation in vivo. Therefore, the action of polyphenols ultimately reduces the progression of inflammation, playing a crucial role in preventing adverse health outcomes.
Oxidative stress encompasses many modifications that affect energy metabolism [69] and its regulation, as well as gene expression [70]. It is not surprising that a wide range of genes, including ferritin [71-72], collagen [73], and transcription factors such as CREB [74] and STAT3 [75], along with AMPK [76] and several proto-oncogenes [77], are transcriptional activated in response to increased cellular oxidation. Given their antioxidant capacity, polyphenols can contribute to individual antioxidant and anti-inflammatory defense through different mechanisms: a) by inhibiting the production of ROS and acting as scavengers of free radicals [78-80]; b) by stimulating the production of prostaglandins and leukotrienes, which are anti-inflammatory molecules [81]; c) by reducing levels of pro-inflammatory cytokines [82]. The TNF-α, IL-6, and serum amyloid A, well-known inflammatory marker levels, are significantly reduced by using a blend of green tea polyphenols, comparable to the effects of sulfasalazine, the standard drug for patients with inflammatory bowel disease (IBD) [83].
Animal models with acute or chronic inflammation have been used to test several polyphenols molecules activity: kaempferol, resveratrol, HT, curcumin, and genistein have displayed anti-inflammatory activities in both animal models [84]. Quercetin is beneficial for both chronic and acute inflammatory processes, while curcumin and green tea have been utilized in the treatment of stress-related neurodegenerative diseases [85-86]. Polyphenols can potentially mitigate inflammatory processes through enzymatic and signaling systems, such as tyrosine and serine-threonine protein kinases, which regulate anti-inflammatory cell activation, growth, and differentiation (e.g., T cell proliferation, B lymphocyte activation), as well as cytokine production [87].
Moreover, polyphenols can induce the expression of antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione (GSH) peroxidase (Px) [88]. This effect has been demonstrated in both in vitro and in vivo experiments using resveratrol, specifically in intestinal epithelial cells and porcine enterocytes isolated from the jejunum (IPEC-J2) [89].
6. The Overall polyphenols anticancer activities in HNC
The therapeutic use of polyphenols against cancer is based on their diverse range of biological activities, encompassing antioxidant effects, interactions with cellular receptors, apoptosis induction, modulation of cell signaling, alterations in cell cycle, regulation of cell proliferation, inhibition of angiogenesis, influence on inflammation and the immune system, epigenetic modifications, and modulation of gene expression [90]. Polyphenols can also impact our health by influencing conditions such as diabetes, metabolic syndrome, hypertension, cardiovascular disease, and the production of metabolites by gut microbiota [91].
The scientific literature extensively deals with the potential anticancer activity of polyphenols on various types of cancer cells, including human colon cancer, lung cancer, breast cancer, ovarian cancer, and hepatocellular cell lines [92-96]. Polyphenol extracts have demonstrated effectiveness in preventing skin cancer and have shown utility in the treatment of this highly aggressive form of cutaneous cancer [97]. A study conducted on HNC revealed significant growth inhibition when a blend of polyphenols (quercetin, curcumin, green tea, and resveratrol) was employed [98].
Dietary phytochemicals can display their anticancer potential through different molecular mechanisms; it is evident that polyphenols can interact with and modulate different signaling pathways involved in the onset and development of cancer. Therefore, the development of anti-cancer therapies based on the utilization of polyphenols holds promising potential for cancer treatment [99].
The epidermal growth factor receptor (EGFR), is deeply involved in HNC onset and development: according to the recent statistics, more than 90% of all HNC display an EGFR overexpression; moreover, high EGFR levels are inversely correlated with poor prognosis and cancer's patient survival [100]. EGFR belongs to the ErbB family, which include four members (ErbB1-4): many different polyphenols molecules possess well-documented abilities to exert their influence upon members of the ErbB receptor family in various cancer cell types. Oleuropein and HT emerge as transformative agents capable of degrading EGFR in several cancer cell lines, as well as quercetin, apigenin, EGCG, and resveratrol. Curcumin indeed surpasses the effectiveness of the gefitinib drug in inhibiting colon cancer cell growth, positioning it as a potent agent for suppressing tumor proliferation [101].
Distinguishing between different signaling pathways can be a challenging task as many of them regulate similar cellular processes, such as cell growth, differentiation, and proliferation, albeit with different involved proteins. Thus, attempting to elucidate the role of each pathway could be debatable. Moreover, these pathways can interact and exhibit crosstalk. For instance, the nuclear factor-kappaB (NF-κB), and Hedgehog signaling play crucial roles in determining cellular neoplastic transformation [102-103].
6.1. Polyphenols and HH/GLI pathway
The Hedgehog pathway (HH) serves as a critical regulator of cell differentiation and growth, particularly during embryonic stages. Recent studies have highlighted an overactivation of this pathway in several human cancers [104]. The cascade pathway is governed by the interaction of three components: the sonic hedgehog (Shh) ligand, its pathway repressor Patched 1 (Ptch1), and the pathway activator smoothened (Smo), a transmembrane G-protein. Normally, Smo is negatively regulated by Ptch1. Unlike other signaling pathways, HH typically operates under negative regulation. When Shh binds to Ptch1, it activates Smo, leading to the nuclear translocation of GL1, a zinc finger transcriptionally repressive factor, resulting in cellular proliferation [105]. Dysregulation of the HH signaling pathway has been observed as a clinical hallmark in the development and progression of various cancer types, including gastrointestinal, prostate, lung, breast, and brain tumors [106-110].
Several different polyphenols molecules have individually demonstrated the ability to regulate the HH/GLI pathway. Curcumin induces cell cycle arrest and apoptosis through the HH signaling pathway by inhibiting the transcriptional activity of GL1. Genistein can block MCF-7 breast cancer cells by inhibition of Sonic hedgehog activity [111], and apigenin impact HH/GLI pathway in malignant mesothelioma mouse cell [112]. EGCG halt the growth and metastasis of human chondrosarcoma cells. In liver of cancer-induced mice, oral administration of EGCG significantly reduces the expression of Smo and GL1 [113]. Resveratrol effectively suppresses hypoxia-induced HH stimulation in pancreatic cancer cells [114]. Both EGCG and theaflavin can effectively counteract the carcinogenic effects of N-nitrosodiethylamine (NDEA) in mice by inhibiting the catalytic transformation of PTCH1, thereby preventing the activation of the HH signaling pathway [115].
6.2 NF-κB pathway modulation
The NF-κB plays a role in different processes, including inflammation, immunoregulation, apoptosis, cell growth, and proliferation. NF-κB is a family of transcription factors consisting of several members, such as NF-κB2 p52, NF-κB1 p50, c-Rel, RelA/p65, and RelB [116]. These proteins can form homo/heterodimers and bind to specific DNA sequences known as their target sites. All family members possess a conserved amino acid domain called the Rel Homology Domain (RHD), which is essential for dimerization, inhibitors binding (IkB), nuclear translocation, and DNA binding.
Normally, NF-κB is bound to its inhibitor IkB, thus remaining confined to the cytoplasm: to be activated NF-κB is phosphorylated by the IκB kinase (IKK) complex, which leads to the IkB degradation and allows the transcription factor translocation into the nucleus [117]. Once in the nucleus, NF-κB regulates gene expression, activating different genes depending on its composition, with many involved in inflammation, cell growth, and differentiation. Supplementation with green tea polyphenols can inhibit mitogen-activated protein kinase (MAPK or MAP kinase) signaling in human umbilical vein endothelial cells (HUVECs) that can participate in the regulation of NF-κB transcriptional activity [118]. Catechins were able to suppress NF-κB signaling in a rat model by preventing NF-κB nucleus translocation [119]; also apigenin and genistein blocked NF-κB interaction with DNA targeting sequence in a murine model [120].
6.3 Polyphenol, cell growth arrest and apoptosis
Apoptosis, a programmed cell death, is a defense mechanism activated when cells are damaged beyond repair, preserving the organism from aging, infection, or other degenerative disease [121]. This genetically regulated process is particularly crucial in countering cancer, which is characterized by uncontrolled cell division: indeed, several anticancer drugs induce apoptosis, an essential feature to prevent the development of neoplastic conditions. The apoptotic process involves distinct pathways with different protein, and can be triggered either by intrinsic factors, such as extensive DNA damage, ischemia, oxidative stress, or infections, or by extrinsic factors, which involve the interaction of specific membrane receptors with pro-apoptotic molecules produced elsewhere. Both intrinsic and extrinsic pathways require the activation of the proteolytic caspase cascade, ultimately dismantling and eliminating the dying cell [122].
Olive oil is widely recognized for its antioxidant properties but can also be used against cancer. Several studies have shown that oleuropein decreases cancer cell viability and exhibits pro-apoptotic effects through the p53-dependent pathway and by activating BAX and Bcl-2 genes in breast cancer cells (MCF-7) [123]. HT can also influence cell cycle progression by arresting cancer cells in the G0/G1 phase and reducing cyclin D1 levels.
In ovarian cancer cells curcumin - in a p53-independent way - can induce apoptosis through the activation of p38 kinase, downregulation of Bcl-2 expression, and modulation of Akt signaling [124]. In the MOLT-4 human leukemia cell line, quercetin interacts with the PI3K-dependent/AKT pathway, leading to a decrease in mammalian target of rapamycin (mTOR) activity. Consequently, the anti-apoptotic protein Bcl-2 is downregulated in cancer cells [125].
Synergistic effects of polyphenols have been observed in various biological processes. Curcumin can induce apoptosis in pancreatic cancer cells line, both in vitro and in vivo [126]. Quercetin and ellagic acid demonstrate synergistic effects in p53 phosphorylation, stimulating BAX expression and translocation of p53 protein into mitochondria, ultimately resulting in pro-apoptotic effects. Similar synergistic effects on p53 phosphorylation were observed when treating human lung cancer cells with isoflavones and curcumin, leading to decreased cell growth and proliferation [127]. Resveratrol exhibits potential effectiveness in preventing cancer development in several cancer cell lines, including prostate, breast, stomach, colon, lung, intestinal, thyroid, and pancreatic cancers [125]. Resveratrol induces apoptosis in human leukemic cells by decreasing Akt activation through Ras downregulation [128].
Although resveratrol shows promising anti-cancer potential, its efficacy has been limited to tumors with direct contact, such as skin cancers or gastrointestinal tract cancers, rather than human solid tumor cells, due to its poor bioavailability [128].
6.4 Polyphenols modulation of p53
Approximately half of all human tumors display an altered functioning of p53, a crucial regulator in multiple human metabolic pathways [129]. In fact, p53 plays a vital role by regulating DNA maintenance and repair, halting cell cycle progression to assess DNA damage, and initiates apoptosis. More than 100 genes have been identified as targets of p53, encompassing various aspects of cellular metabolism. Post-translational modifications such as acetylation, methylation, phosphorylation, and ubiquitination can modulate p53 activity in response to a wide range of stresses [130].
Therefore, it is not surprising that p53 can be regulated through various mechanisms, either positively or negatively, involving different pathways and proteins. One common pathway involved in p53 inhibition is the direct interaction between p53 and the Mouse double minute 2 homolog (MDM2). The MDM2 carries out its activity through several mechanisms: 1) facilitating proteasome-mediated degradation of p53; 2) preventing p53 from binding to its DNA target sequence; and 3) promoting the export of p53 out of the nucleus [131]. Experimental evidence suggests that polyphenols can bind to MDM2 through stable hydrophobic interactions, preventing the inactivation of p53 [132].
EGCG, resveratrol, curcumin, genistein, and quercetin can upregulate p53 expression, inhibit cell growth and proliferation in several human cancer cell lines by decreasing cyclins D1 and D2, increasing p21 and BAX synthesis, and triggering apoptosis [133-134]. Specifically, EGCG can directly regulate p53 by stimulating its phosphorylation and acetylation, leading to enhanced stability and activity of p53 [135]. Besides, p53 can be targeted by the theaflavin's biological activity, leading to positively telomerase regulation through inhibition of Telomerase reverse transcriptase (hTERT), a critical factor for cell life expectancy [136]. Moreover, theaflavin - through p53 pathways - can downregulate glycolysis and angiogenesis - suppressing vascular-endothelial growth factor (VEGF) expression -, promoting apoptosis through Bcl-2 inhibition [137].
Numerous studies have suggested that resveratrol can triggers apoptosis in cancer cell in a p53-dependent manner, via MAPK activation [138]. Therefore, polyphenols administration can modulate p53, regulating various aspects of cancer progression, including initiation, proliferation, survival, migration, angiogenesis, and metastasis. Importantly, the combination of polyphenols with chemotherapy or radiotherapy can synergistically upregulate p53 [139].
6.5 Epigenetic and DNA modification
Preventing cancer initiation and progression often could significate preserve the stability and integrity of our genome: in this regard, epigenetic regulation and gene silencing/activation plays a pivotal role for cell development and differentiation. DNA methylation and chromatin modifications, particularly histone acetylation, are crucial for proper development and differentiation, but dysregulation can lead to severe human pathologies, cancer included. The tumor microenvironment acts as an amplifier for epigenetic modifications, facilitating early and frequent remodeling of DNA functionality, and promoting cancerous transformation [140]. Targeting epigenetic modifiers could be a promising strategy for anticancer activity, given the potential reversibility of these changes.
Recent studies have suggested that polyphenols may have the ability to regulate epigenetic processes, which holds significant clinical and therapeutic implications [141]. In ER-positive MCF-7 breast cancer cells, a combination of resveratrol and vitamin D can downregulate DNA methyltransferases (DNMT), leading to reduced promoter methylation of the phosphatase and tensin homolog (PTEN) gene and enabling protein transcription [142]. Resveratrol treatment in breast cancer cells has been associated with DNA hypomethylation based on a genome-wide survey [143]. Interestingly, combining resveratrol with another polyphenol, pterostilbene, reduces methylation at the ERa gene promoter [144].
Undoubtedly, dietary bioactive compounds like polyphenols can act as epigenetic modifiers, establishing a direct link between food and epigenetics, thereby presenting intriguing new therapeutic possibilities.
6.6 miRNAs modulation in HNC
MicroRNAs (miRNAs) are a class of small, single-stranded non-coding RNAs that plays a crucial role in post-transcriptional control: it is estimated that they regulate the expression of at least 30% of mammalian genes [145]. Through binding to target mRNAs, miRNAs can downregulate gene expression, effectively regulating several cellular processes, including cell differentiation, growth, proliferation, and apoptosis; alterations in miRNA expression are considered critical in cancer initiation and development [146].
Curcumin ability to modulate miRNAs in cancer cells has been recently demonstrated: after treating pancreatic cancer cells with curcumin (10 μM), significant changes in the expression of 29 miRNAs were observed, with 11 miRNAs upregulated and 18 miRNAs downregulated [147]. One of the miRNAs affected by curcumin treatment was miR-22, known for its tumor-suppressive function. Curcumin-induced upregulation of miR-22 effectively inhibited its target genes. These findings highlight the modulation of miRNAs by curcumin as an important mechanism underlying its biological effects. Furthermore, curcumin has been shown to induce DNA hypomethylation and inhibit several oncogenes, including histone-modifying proteins, in hepatocellular carcinoma cells [148].
Resveratrol and genistein are also capable of reducing carcinogenesis through the modulation of miRNAs. Additionally, EGCG not only impacts DNA methylation but also histone acetylation, influencing the enzymatic activities of histone deacetylases (HDACs). This EGCG capacity may explain its chemopreventive effect, as it modulates inflammation in cancer cells.
7. Polyphenols Therapeutic Potential in HNC: Epidemiological and Clinical Studies
Many epidemiological studies suggest that diets particularly rich in fruits and vegetables have cancer preventive properties [149-151]. Polyphenols are deemed responsible, at least in part, for these beneficial effects, thanks to their anticancer activity both in animal and human models [152-153].
So far, very few studies have tested polyphenols administration in HNC. Searching the “Clinicaltrials.gov” database (last search: 5 August 2023), using the terms [head and neck cancer] and [polyphenols report], only 5 studies evaluate the use of polyphenols in HNC (Table 1).
Clinical trials with polyphenol supplementation in head and neck cancer patients.
| HNC | NCT | Polyphenol Supplementation | Clinical Relevance |
|---|---|---|---|
| Oral leukoplakia | NCT00176566 | Lozenge intake (green tea preparation) | A Phase II Trial to Assess the Effects of Green Tea in Oral Leukoplakia |
| Oral cancer - gum disease | NCT01514552 | Strawberry gummy and placebo control | Use of Functional Confections in Promoting Oral Health |
| Carcinoma, Squamous Cell | NCT01496521 | Drug: aspirin (100mg 6 months); Dietary Supplement: Tea polyphenols (300mg 6 months) | Chemoprevention of Esophageal Squamous Cell Carcinoma (ESCC) with aspirin and tea polyphenols |
| Gastric and Esophageal Cancer | NCT04027088 | Dietary supplement: arginine, omega 3, olive oil polyphenols, carnitine and antioxidants | Effect of preoperative immunonutrition in upper digestive tract |
| Premalignant lesions of HNC | NCT01116336 | Green tea polyphenon E | Phase I chemoprevention trial with green tea polyphenon e & erlotinib in patients with premalignant lesions of HNC |
Instead, are enlisted 30 clinical studies involving the administration of polyphenols in various types of cancer and 50 clinical studies using polyphenols to counteract oxidative stress (Table 2 and Table 3, respectively).
Selected clinical trials with polyphenol supplementation in cancer disease.
| Conditions | NCT | Polyphenol | Clinical Relevance |
|---|---|---|---|
| Recurrent Prostate Cancer | NCT01912820 | Quercetin | Effect of Quercetin in prostate tissue from patients with prostate cancer |
| Prostate Cancer | NCT00685516 | green tea, decaffeinated black tea | Green Tea, Black Tea in treating patients with prostate cancer undergoing surgery |
| Prostate Cancer | NCT00676780 | Drug: Polyphenon E (EGCG) | Green tea extract and prostate cancer |
| Colorectal Cancer | NCT02439580 | Annona muricata extract | Effect of A. Muricata leaves on colorectal cancer patients and colorectal cancer cells |
| Incident Breast Cancer | NCT00949923 | Dietary Supplement: tea capsula | Green Tea in Breast Cancer Patients |
| Cervical Cancer | NCT03994055 | Omega-3 fatty acids, Probiotics Antioxidants Soluble fiber | Effect of an Anti-inflammatory Diet on Patients with Cervical Cancer |
| Interstitial Pneumonia Neoplasms Malignant | NCT05758571 | Drug: EGCG | Oxygen atomizing inhalation of EGCG in the treatment interstitial pneumonia in cancer patients |
| Advanced Lung Cancer | NCT03751592 | Drug: Chlorogenic acid | Phase Ib/IIa Studies of Chlorogenic acid for injection for safety and efficacy of advanced lung cancer |
| Skin Cancer | NCT01032031 | Dietary Supplement: Green tea + vitamin C high dose | The Effect of Green Tea and Vitamin C on Skin Health |
| Non-small Cell Lung Cancer | NCT01426620 | Dietary Supplement: Blueberry powder | Standard chemotherapy with blueberry powder in nonsmall cell lung cancer |
| Colorectal Serrated Adenomas | NCT01360320 | Dietary Supplement: Green tea extract of Camellia Sinensis | Minimizing the risk of metachronous adenomas of the colorectum with green tea extract -MIRACLE-study |
Selected clinical trials with polyphenol supplementation in oxidative stress conditions.
| Conditions | NCT | Polyphenol | Clinical Relevance |
|---|---|---|---|
| CV disease and oxidative stress | NCT01541826 | Chokeberry extract (250mgx2 for 12 weeks) | Study of Chokeberry to Reduce Cardiovascular Disease Risk in Former Smokers |
| Oxidative stress and inflammation | NCT01780922 | Cranberry extract beverage | Effect of a Dose of Cranberry Beverage on Inflammation and Oxidative Stress |
| Oxidative stress and insulin resistant | NCT02479035 | Red raspberry meal | Raspberries on Insulin Action and Oxidative Stress |
| Cardiomyopathy and oxidative stress | NCT01102140 | POMx pomegranate polyphenol extract | The Impact of Pomegranate Extract on Chronic Cardiomyopathy Complicated by Renal Insufficiency (ImPrOVE): a Pilot Study |
| Metabolic syndrome, oxidative stress, systemic inflammation | NCT03265184 | Green coffee extract | Green Coffee Extract Supplementation and Oxidative Stress, Systemic and Vascular Inflammation |
| Oxidative stress, inflammation | NCT02494739 | Yogurt enriched with polyphenols | Antioxidant and Anti-inflammatory Effects of Yogurt Enriched With Polyphenols |
| Vascular oxidative stress | NCT03053986 | Apple polyphenols | Effect of Apple Polyphenols on Vascular Oxidative Stress and Endothelium Function Study (APP trial_2016) |
| Oxidative stress in diabetic patients | NCT00682149 | PomGT (0.5 g pomegranate extracts, 0.3 g green tea and 60 mg vit. C) | Effects of Polyphenol Containing Antioxidants on Oxidative Stress in Diabetic Patients |
| Oxidative stress | NCT00721643 | Angel's plant - dark green leafy vegetable | Absorption Kinetics of Polyphenols in Angel's Plant (Angelica Keiskei) |
| Oxidative stress, exercise recovery | NCT04959006 | Antioxidant supplement | Investigating a Natural Antioxidant Food Product on Oxidative Stress in Recreationally Active Participants |
| Maternal and fetal oxidative stress | NCT01584323 | Pomegranate pills | Pomegranate to Improve Outcome in Pregnancies Complicated With Preterm Premature Rupture of the Membranes |
| Oxidative stress, gestational diabetes | NCT05393843 | Omega-3 fatty acids, anthocyanins and alpha-cyclodextrins | Prevention of Maternal and Fetal Metabolic Complications With Diet and Nutraceutical Supplementation in Pregnant Women Affected by Gestational Diabetes: a Randomized, Double-blind Placebo Controlled Trial. |
| Oxidative stress | NCT03186573 | Grape juice | Effect of Grape Juice Consumption on the Parameters of Oxidative Stress and Muscle Fatigue in Judo Athletes |
| Oxidative stress, cardiometabolic risk | NCT05771571 | Olive oil, plus orange peel extract | Investigation of the Acute Effect of Novel Olive Oil on Postprandial Oxidative Stress Biomarkers (BioliveCT) |
| Oxidative stress, CV disease, inflammation, | NCT01674231 | Grape (freeze-dried whole grape powder) | The Effects Grapes on Health Indices |
| Chronic obstructive pulmonary disease, oxidative stress | NCT03989271 | Quercetin | Biological Effects of Quercetin in COPD |
| Oxidative Stress, CV Diseases | NCT02295878 | Dietary Supplement: capsule containing seaweed extract | The Effect of Seaweed Derived Polyphenols on Inflammation and Oxidative Stress in Vivo - The SWAFAX Study |
| Oxidative Stress, CV Diseases | NCT04061070 | Supplementation with threalose + polyphenols | Effects of Trehalose & Polyphenols in Vasculopathic Patients |
Given these premises, it seems counterintuitive to not try the use of polyphenols in HNC treatment and prevention. A high intake of polyphenols was linked to a nearly 50% decrease in gastric cancer [154]. The consumption of stilbenes was shown to lower the risk of colorectal adenomas, while anthocyanin and flavanols were associated with a reduced risk of colorectal cancer [155]. In a separate study focused on prostate cancer, the consumption of polyphenols was found to significantly decrease the risk [156], and isoflavones and flavones were inversely associated with the risk of bladder cancer [157].
Polyphenols intake was inversely associated with colon cancer in men, accordingly to the European Prospective Investigation into Cancer and Nutrition (EPIC) study, with a cohort of nearly half a million people from 10 different Countries [158]. A study performed in Hong Kong with people who habitually consume green tea had a reduced risk of cancer of prostate cancer [159].
Although the use of polyphenols to combat oxidative stress or cancer appear extremely promising, the preliminary results of epidemiological studies must be evaluated with caution and extreme care. Ultimately, research of this type can give us some indications, but they do not provide us with any explanation or even a direct link between the use of polyphenols and pathologies. In fact, sometimes, if analyzed carefully, their results can be inconclusive: in the same work cited above, in some types of cancer, therapy with polyphenols did not change the incidence of the disease, or in some cases, it even worsened [158].
However, polyphenols can certainly be useful as nutraceutical coadjuvants: they have already demonstrated great therapeutic potential by increasing the effectiveness of traditional drugs. Furthermore, being molecules normally present in plants, they do not present any toxicity problems; indeed, their use seems to attenuate these side effects, which are present in almost 40% of all HNC patients [160].
8. Discussion
HNC is increasing worldwide, becoming more and more a challenging clinical problem. It is necessary to find viable strategy to contain this type of cancer, and moreover develop alternatives for prevention. As mentioned previously, incorporating an adequate amount of polyphenols into our diet or considering polyphenol supplementation can be highly beneficial for maintaining our health, preventing and helping our body to fight against oxidative stress [161]. The abundant presence of polyphenols in plant-based foods justifies the general recommendation to consume ample amounts of fruits and vegetables for maintaining our well-being. Through in vitro and in vivo experiments, it has been clearly demonstrated that polyphenols can modulate numerous biological pathways. By addressing oxidative stress through diverse molecular mechanisms, their efficacy can be enhanced, opening the door for synergistic interactions among different polyphenol molecules [109].
It is important to recognize that each situation presents its own unique challenges and therefore requires a tailored approach. The ability to utilize different polyphenol molecules based on specific circumstances is a valuable asset. Furthermore, the effectiveness of combining different polyphenols has already been demonstrated in several experiments, highlighting the benefits of synergy. Notably, the use of polyphenols does not entail any side effects, as they are completely non-toxic. This aspect greatly enhances the utility of polyphenols in clinical therapy [37,162].
In the past two decades, significant advancements have been made in the field of food and nutritional sciences. Conventional nutritional recommendations, which primarily focused on the sufficient intake of nutrients to prevent disease development, have been superseded by the concept of personalized nutrition. This approach aims to optimize bodily functions and promote human health through the utilization of bioactive compounds such as polyphenols [148].
Different polyphenols molecules often act in different ways in our metabolism; hence, different polyphenols may act together to produce synergistic effects with enhanced health benefits. Understanding these synergistic interactions and exploring combinatorial approaches can lead to optimized therapeutic strategies. Overcoming the challenge of achieving the biological effectiveness of polyphenol supplementation in our bodies remains a major obstacle. In most cases, these compounds have low bioavailability and solubility, making it extremely challenging to accurately assess their true activity. It is crucial to develop new delivery methods and synthetic analogs that can enhance this critical aspect: although there are already some, further clinical trials are needed to investigate the bioavailability of polyphenols with innovative methods, like enriched foods containing free or encapsulated polyphenols [100].
Many studies on polyphenols primarily consist of observational, in vitro, or in vivo research. While these studies are valuable in elucidating the molecular mechanisms involved, clinical trials participating large cohorts are necessary to truly evaluate the effectiveness of polyphenol supplementation for human health. Without these clinical confirmations, the administration of polyphenols for therapeutic applications becomes challenging, as there is a lack of clear indications regarding their efficacy, optimal intake, and specific pathologies in which they can be utilized [161]. Intriguingly, the results of a recent study investigated the potential anticancer effects of combining two natural dietary compounds green tea EGCG and resveratrol; these compounds were tested both in vitro (in cell cultures) and in vivo (in live animals) in the context of HNC [163]. Overall, the study results suggests that the combination of EGCG and resveratrol, even at low doses where each compound alone has marginal effects on apoptosis, can synergistically enhance apoptosis and inhibit the growth of head and neck tumors. Besides, therapeutic strategy using also depends on the activation of the immune system against tumor [164-166]; intriguingly, a combination of natural polyphenols, containing curcumin (C) with resveratrol (R) and epicatechin gallate (E), termed TriCurin, presenting a unique and synergistic molar ratio, seems to be highly efficient in stimulating the immune system against cancer cells and can be used as a safe immunotherapeutic agent to turn the immune system against HPV+ tumors [167].
However, the polyphenols therapeutic potential is becoming more and more evident, enticing the worldwide researchers with a landscape of therapeutic possibilities to uncover the precise and effective biological role of this molecules in counteract oxidative stress, enabling their implementation in preventive healthcare practices, according to the evidence-based dentistry [168].
9. Conclusion
Cancers of the head and neck are clearly socioeconomically patterned, with those from the poorest backgrounds having the greatest burden, but this socioeconomic risk is not entirely explained by smoking alcohol and dietary behaviors.
Polyphenols have the potential to be a strategic tool in the battle against cancer for several reasons. As a diverse family of molecules, they can combat cancer development through multiple mechanisms, increasing the likelihood of success. The idea of preventing or curing cancer through a polyphenol-rich diet, primarily sourced from plant-based foods, almost feels like a dream come true. Additionally, adopting healthy dietary habits can deter us from consuming comfort foods high in saturated fats and calories, which are detrimental to our health and may contribute to cancer incidence and progression.
Recent studies have demonstrated that dietary polyphenols could regulate several molecular pathways involved in cancer promotion and progression, suggesting chemopreventive and therapeutic capacity of dietary polyphenols against HNC.
However, the concentration, absorption, bioavailability, and pharmacokinetics of polyphenols can pose challenges to their beneficial effects against neoplastic diseases. Further research is necessary to fully understand the cellular mechanisms by which polyphenols operate, enabling the integration of these natural compounds into our cancer-fighting strategies. While overall results appear promising, they remain inconclusive. Randomized controlled clinical trials and meta-analyses are required to test the actual efficacy of polyphenols, providing continuity and perspective to in vitro and cell culture studies. Discovering the optimal combination of polyphenols for HNC, sustained by innovative delivery methods like liposomes and nanoparticles, could significantly benefit patients fighting this type of cancer worldwide.
Acknowledgements
“HEAL ITALIA - health extended alliance for innovative therapies, advanced lab-research, and integraded approaches of precision medicine” (codice identificativo: PE00000019 - CUP D73C22001230006) area tematica 6 “diagnostica e terapie innovative nella medicina di precisione”, presentato in risposta all'Avviso pubblico del MUR adottato con D.D. n. 341 del 15.3.2022; “Progetto Finanziato dall'Unione europea - NextGenerationEU”, Bando PRA-HE, Università degli Sudi di Foggia - Programma “MUR-Fondo Promozione e Sviluppo - DM737/2021.
Author Contributions
“Conceptualization of original idea, A.B., R.A. and K.Z.; methodology K.Z. V.C.A.C. and M.D.; data collection, R.A., A.B., and G.T.; formal analysis K.Z., M.D., and A.B; editing and revision of manuscript K.Z., M.D., and A.B.; resources: F.S., R.P., M.R.: bibliographic research: V.C.A.C. and S.C.; supervision and final approval: G.T., A.B., V.C.A.C. and L.L.M.; critical revision of the manuscript for important intellectual content K.Z. and A.B. Finally, Andrea Ballini and Khrystyna Zhurakivska equally contributed as co-first authors, Roberto Arrigoni and Mario Dioguardi equally contributed as co-last authors. All authors have read and agreed to the published version of the manuscript”.
Competing Interests
The authors have declared that no competing interest exists.
References
1. American Society of Clinical Oncology (ASCO). Head and Neck Cancer - Statistics 2023. https://www.cancer.net/cancer-types/head-and-neck-cancer/statistics/
2. Gormley M, Creaney G, Schache A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br Dent J. 2022;233:780-786
3. Caponio VCA, Troiano G, Adipietro I, Zhurakivska K, Arena C, Mangieri D. et al. Computational analysis of TP53 mutational landscape unveils key prognostic signatures and distinct pathobiological pathways in head and neck squamous cell cancer. Br J Cancer. 2020;123:1302-1314
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249
5. Turati F, Garavello W, Tramacere I, Bagnardi V, Rota M, Scotti L. et al. A meta-analysis of alcohol drinking and oral and pharyngeal cancers. Part 2: results by subsites. Oral Oncol. 2010;46:720-6
6. Silva FFVE, Padín-Iruegas ME, Caponio VCA, Lorenzo-Pouso AI, Saavedra-Nieves P, Chamorro-Petronacci CM. et al. Caspase 3 and Cleaved Caspase 3 Expression in Tumorogenesis and Its Correlations with Prognosis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2022;23:11937
7. Hayyan M, Hashim MA, AlNashef IM. Superoxide Ion: Generation and Chemical Implications. Chem Rev. 2016;116:3029-85
8. Kim G, Lee YE, Kopelman R. Hydrogen peroxide (H₂O₂) detection with nanoprobes for biological applications: a mini-review. Methods Mol Biol. 2013;1028:101-14
9. Nakamura S, Ando N, Sato M, Ishihara M. Ultraviolet Irradiation Enhances the Microbicidal Activity of Silver Nanoparticles by Hydroxyl Radicals. Int J Mol Sci. 2020;21:3204
10. Wójcik P, Gęgotek A, Žarković N, Skrzydlewska E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int J Mol Sci. 2021;22:723
11. Pettini F, Savino M, Corsalini M, Cantore S, Ballini A. Cytogenetic genotoxic investigation in peripheral blood lymphocytes of subjects with dental composite restorative filling materials. J Biol Regul Homeost Agents. 2015;29:229-33
12. D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813-24
13. Korbecki J, Bobiński R, Dutka M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm Res. 2019;68:443-458
14. Onodera Y, Teramura T, Takehara T, Shigi K, Fukuda K. Reactive oxygen species induce Cox-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio. 2015;5:492-501
15. Di Meo S, Reed TT, Venditti P, Victor VM. Harmful and Beneficial Role of ROS. Oxid Med Cell Longev. 2016;2016:7909186
16. Arrigoni R, Arrigoni O. Multicopper oxidases: An innovative approach for oxygen management of aerobic organisms. Rend. Fis. Acc. Lincei. 21: 71-80.
17. Kuang F, Liu J, Tang D, Kang R. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front Cell Dev Biol. 2020;8:586578
18. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126-67
19. Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim Biophys Acta. 2015;1851:414-21
20. Patergnani S, Bouhamida E, Leo S, Pinton P, Rimessi A. Mitochondrial Oxidative Stress and "Mito-Inflammation": Actors in the Diseases. Biomedicines. 2021;9:216
21. Cadenas S. Mitochondrial Uncoupling, ROS Generation and Cardioprotection. Biochim Biophys Acta Bioenerg. 2018;1859:940-950
22. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V. et al. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763
23. Nadarajah KK. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int J Mol Sci. 2020;21:5208
24. Wattenberg LW. Chemoprevention of Cancer. Prev Med. 1996;25:44-45
25. Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 2022;80:1-17
26. Sharma A, Kaur M, Katnoria JK, Nagpal AK. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr Med Chem. 2018;25:4740-4757
27. Stacchiotti A, Corsetti G. Natural Compounds and Autophagy: Allies Against Neurodegeneration. Front Cell Dev Biol. 2020;8:555409
28. Nani A, Murtaza B, Sayed Khan A, Khan NA, Hichami A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules. 2021;26:985
29. Fraga CG, Croft KD, Kennedy DO, Tomás-Barberán FA. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10:514-528
30. Castaldo L, Narváez A, Izzo L, Graziani G, Gaspari A, Minno GD. et al. Red Wine Consumption and Cardiovascular Health. Molecules. 2019;24:3626
31. Yang AJT, Bagit A, MacPherson REK. Resveratrol, Metabolic Dysregulation, and Alzheimer's Disease: Considerations for Neurogenerative Disease. Int J Mol Sci. 2021;22:4628
32. Ohishi T, Fukutomi R, Shoji Y, Goto S, Isemura M. The Beneficial Effects of Principal Polyphenols from Green Tea, Coffee, Wine, and Curry on Obesity. Molecules. 2021;26:453
33. Guasch-Ferré M, Merino J, Sun Q, Fitó M, Salas-Salvadó J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxid Med Cell Longev. 2017;2017:6723931
34. Yahfoufi N, Alsadi N, Jambi M, Matar C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients. 2018;10:1618
35. Queen BL, Tollefsbol TO. Polyphenols and aging. Curr Aging Sci. 2010;3:34-42
36. Maiuolo J, Gliozzi M, Carresi C, Musolino V, Oppedisano F, Scarano F. et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients. 2021;13:3834
37. Arrigoni R, Ballini A, Santacroce L, Cantore S, Inchingolo A, Inchingolo F. et al. Another Look at Dietary Polyphenols: Challenges in Cancer Prevention and Treatment. Curr Med Chem. 2022;29:1061-1082
38. George VC, Dellaire G, Rupasinghe HPV. Plant flavonoids in cancer chemoprevention: role in genome stability. J Nutr Biochem. 2017;45:1-14
39. Zhang J, Sun X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry. 2021;181:112588
40. Tsao R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients. 2010;2:1231-1246
41. Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI. et al. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol Nutr Food Res. 2018 [Epub ahead of print]
42. Sharifi-Rad J, Quispe C, Castillo CMS, Caroca R, Lazo-Vélez MA, Antonyak H. et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid Med Cell Longev. 2022;2022:3848084
43. Li C, Schluesener H. Health-Promoting Effects of the Citrus Flavanone Hesperidin. Crit Rev Food Sci. Nutr. 2017;57:613-631
44. Gorzynik-Debicka M, Przychodzen P, Cappello F, Kuban-Jankowska A, Marino Gammazza A. et al. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int J Mol Sci. 2018;19:686
45. Pérez-Jiménez J, Neveu V, Vos F, Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur J Clin Nutr. 2010;64:S112-20
46. Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial Properties of Green Tea Catechins. Int J Mol Sci. 2020;21:1744
47. Arts IC, van De Putte B, Hollman PC. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J Agric Food Chem. 2000;48:1752-7
48. Roupe KA, Remsberg CM, Yáñez JA, Davies NM. Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol. 2006;1:81-101
49. Williamson G. The Role of Polyphenols in Modern Nutrition. Nutr Bull. 2017;42:226-235
50. Scalbert A, Morand C, Manach C, Rémésy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. 2002;56:276-82
51. Zamora-Ros R, Touillaud M, Rothwell JA, Romieu I, Scalbert A. Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits. Am J Clin Nutr. 2014;100:11-26
52. Clifford MN, van der Hooft JJ, Crozier A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am J Clin Nutr. 2013;98:1619S-1630S
53. Collins SL, Patterson AD. The gut microbiome: an orchestrator of xenobiotic metabolism. Acta Pharm Sin B. 2020;10:19-32
54. Zhurakivska K, Troiano G, Caponio VCA, Dioguardi M, Laino L, Maffione AB. et al. Do Changes in Oral Microbiota Correlate with Plasma Nitrite Response? A Systematic Review. Front Physiol. 2019;10:1029
55. Zhang Y, Yu W, Zhang L, Wang M, Chang W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients. 2022Dec;14:5373
56. Hao J, Shen W, Yu G, Jia H, Li X, Feng Z. et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J Nutr Biochem. 2010;21:634-44
57. Reis A, Perez-Gregorio R, Mateus N, de Freitas V. Interactions of dietary polyphenols with epithelial lipids: advances from membrane and cell models in the study of polyphenol absorption, transport and delivery to the epithelium. Crit Rev Food Sci Nutr. 2021;61:3007-3030
58. Blázovics A, Lugasi A, Kemény T, Hagymási K, Kéry A. Membrane stabilising effects of natural polyphenols and flavonoids from Sempervivum tectorum on hepatic microsomal mixed-function oxidase system in hyperlipidemic rats. J Ethnopharmacol. 2000;73:479-85
59. Forte M, Conti V, Damato A, Ambrosio M, Puca AA, Sciarretta S. et al. Targeting Nitric Oxide with Natural Derived Compounds as a Therapeutic Strategy in Vascular Diseases. Oxid Med Cell Longev. 2016;2016:7364138
60. Plattner C, Hackl H. Modeling therapy resistance via the EGFR signaling pathway. FEBS J. 2019;286:1284-1286
61. Ouyang H, Hou K, Peng W, Liu Z, Deng H. Antioxidant and xanthine oxidase inhibitory activities of total polyphenols from onion. Saudi J Biol Sci. 2018;25:1509-1513
62. Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T. et al. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods. 2019;8:185
63. Franchini AM, Hunt D, Melendez JA, Drake JR. FcγR-driven release of IL-6 by macrophages requires NOX2-dependent production of reactive oxygen species. J Biol Chem. 2013;288:25098-25108
64. Yu T., Dohl J., Wang L., Chen Y., Gasier H. G.; Deuster, P. A. Curcumin Ameliorates Heat-Induced Injury through NADPH Oxidase-Dependent Redox Signaling and Mitochondrial Preservation in C2C12 Myoblasts and Mouse Skeletal Muscle. J Nutr. 2020;150:2257-2267
65. Yu T, Dohl J, Wang L, Chen Y, Gasier HG, Deuster PA. Curcumin Ameliorates Heat-Induced Injury through NADPH Oxidase-Dependent Redox Signaling and Mitochondrial Preservation in C2C12 Myoblasts and Mouse Skeletal Muscle. J Nutr. 2020;150:2257-2267
66. Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H. et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11:1365-402
67. Lu WJ, Li JY, Chen RJ, Huang LT, Lee TY, Lin KH. VAS2870 and VAS3947 attenuate platelet activation and thrombus formation via a NOX-independent pathway downstream of PKC. Sci Rep. 2019;9:18852
68. Elbarbry F, Abdelkawy K, Moshirian N, Abdel-Megied AM. The Antihypertensive Effect of Quercetin in Young Spontaneously Hypertensive Rats; Role of Arachidonic Acid Metabolism. Int J Mol Sci. 2020;21:6554
69. Gorlach S, Fichna J, Lewandowska U. Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett. 2015;366:141-9
70. Klaunig JE. Oxidative Stress and Cancer. Curr Pharm Des. 2018;24:4771-4778
71. Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP. et al. The Role of Resveratrol in Cancer Therapy. Int J Mol Sci. 2017;18:2589
72. Damian MT, Vulturar R, Login CC, Damian L, Chis A, Bojan A. Anemia in Sports: A Narrative Review. Life (Basel). 2021;11:987
73. Roh E, Kim JE, Kwon JY, Park JS, Bode AM, Dong Z, Lee KW. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit Rev Food Sci Nutr. 2017;57:1631-1637
74. Giordano A, Tommonaro G. Curcumin and Cancer. Nutrients. 2019;11:2376. https://doi.org/10.3390/nu11102376
75. Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124
76. McIntyre RL, Liu YJ, Hu M, Morris BJ, Willcox BJ, Donlon TA. et al. Pharmaceutical and nutraceutical activation of FOXO3 for healthy longevity. Ageing Res Rev. 2022;78:101621
77. Jin T, Zhang Y, Botchway BOA, Zhang J, Fan R, Zhang Y, Liu X. Curcumin can improve Parkinson's disease via activating BDNF/PI3k/Akt signaling pathways. Food Chem Toxicol. 2022;164:113091
78. Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as Anticancer Agents. Nutrients. 2020;12:457
79. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev. 2016;2016:7432797
80. Bernatoniene J, Kopustinskiene DM. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules. 2018;23:965
81. Mlcek J, Jurikova T, Skrovankova S, Sochor J. Quercetin and Its Anti-Allergic Immune Response. Molecules. 2016;21:623
82. Haß U, Herpich C, Norman K. Anti-Inflammatory Diets and Fatigue. Nutrients. 2019;11:2315
83. Oz HS, Chen T, de Villiers WJ. Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. Front Immunol. 2013;4:132
84. Oliviero F, Scanu A, Zamudio-Cuevas Y, Punzi L, Spinella P. Anti-inflammatory effects of polyphenols in arthritis. J Sci Food Agric. 2018;98:1653-1659
85. Piccolella S, Crescente G, Candela L, Pacifico S. Nutraceutical polyphenols: New analytical challenges and opportunities. J Pharm Biomed Anal. 2019;175:112774
86. Moradi SZ, Jalili F, Farhadian N, Joshi T, Wang M, Zou L. et al. Polyphenols and neurodegenerative diseases: focus on neuronal regeneration. Crit Rev Food Sci Nutr. 2022;62:3421-3436
87. Cai XZ, Wang J, Li XD, Wang GL, Liu FN, Cheng MS. et al. Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol Ther. 2009;8:1360-8
88. Kapusta-Duch J, Kopeć A, Piatkowska E, Borczak B, Leszczyńska T. The beneficial effects of Brassica vegetables on human health. Rocz Panstw Zakl Hig. 2012;63:389-95
89. Hafeez BB, Siddiqui IA, Asim M, Malik A, Afaq F, Adhami VM. et al. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008;68:8564-72
90. Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA. et al. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33:2221-2243
91. Wan MLY, Co VA, El-Nezami H. Dietary polyphenol impact on gut health and microbiota. Crit Rev Food Sci Nutr. 2021;61:690-711
92. Wang ST, Cui WQ, Pan D, Jiang M, Chang B, Sang LX. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J Gastroenterol. 2020;26:562-597
93. Wang Y, Yuan Y, Wang C, Wang B, Zou W, Zhang N. et al. Theabrownins Produced via Chemical Oxidation of Tea Polyphenols Inhibit Human Lung Cancer Cells in vivo and in vitro by Suppressing the PI3K/AKT/mTOR Pathway Activation and Promoting Autophagy. Front Nutr. 2022;9:858261
94. Dandawate PR, Subramaniam D, Jensen RA, Anant S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin Cancer Biol. 2016;40-41:192-208
95. He Z, Wu S, Lin J, Booth A, Rankin GO, Martinez I. et al. Polyphenols Extracted from Chinese Hickory (Carya cathayensis) Promote Apoptosis and Inhibit Proliferation through the p53-Dependent Intrinsic and HIF-1α-VEGF Pathways in Ovarian Cancer Cells. Appl Sci (Basel). 2020;10:8615
96. Darvesh AS, Bishayee A. Chemopreventive and Therapeutic Potential of Tea Polyphenols in Hepatocellular Cancer. Nutr Cancer. 2013;65:329-344
97. Sajadimajd S, Bahramsoltani R, Iranpanah A, Kumar Patra J, Das G, Gouda S. et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol Res. 2020;151:104584
98. Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients. 2016;8:552
99. Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen YM. et al. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients. 2016;8:515
100. Caponio VCA, Troiano G, Togni L, Zhurakivska K, Santarelli A, Laino L. et al. Pattern and localization of perineural invasion predict poor survival in oral tongue carcinoma. Oral Dis. 2023;29:411-422
101. Shaikh SB, Prabhu A, Bhandary YP. Curcumin Suppresses Epithelial Growth Factor Receptor (EGFR) and Proliferative Protein (Ki 67) in Acute Lung Injury and Lung Fibrosis In vitro and In vivo. Endocr Metab Immune Disord Drug Targets. 2020;20:558-563
102. Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5:209
103. Doheny D, Manore SG, Wong GL, Lo HW. Hedgehog Signaling and Truncated GLI1 in Cancer. Cells. 2020;9:2114
104. Suchors C, Kim J. Canonical Hedgehog Pathway and Noncanonical GLI Transcription Factor Activation in Cancer. Cells. 2022;11:2523
105. Lee DH, Lee SY, Oh SC. Hedgehog signaling pathway as a potential target in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317692266
106. Akyala AI, Peppelenbosch MP. Gastric Cancer and Hedgehog Signaling Pathway: Emerging New Paradigms. Genes Cancer. 2018;9:1-10
107. Hyuga T, Alcantara M, Kajioka D, Haraguchi R, Suzuki K, Miyagawa S. et al. Hedgehog Signaling for Urogenital Organogenesis and Prostate Cancer: An Implication for the Epithelial-Mesenchyme Interaction (EMI). Int J Mol Sci. 2019;21:58
108. Zeng X, Ju D. Hedgehog Signaling Pathway and Autophagy in Cancer. Int J Mol Sci. 2018;19:2279
109. Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn J Basic Med Sci. 2018;18:8-20
110. Jeng KS, Chang CF, Lin SS. Sonic Hedgehog Signaling in Organogenesis, Tumors, and Tumor Microenvironments. Int J Mol Sci. 2020;21:758
111. Fan P, Fan S, Wang H, Mao J, Shi Y, Ibrahim MM. et al. Genistein decreases the breast cancer stem-like cell population through Hedgehog pathway. Stem Cell Res Ther. 2013;4:146
112. Masuelli L, Benvenuto M, Mattera R, Di Stefano E, Zago E, Taffera G. et al. In Vitro and In Vivo Anti-tumoral Effects of the Flavonoid Apigenin in Malignant Mesothelioma. Front Pharmacol. 2017;8:373
113. Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS. et al. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382-90
114. Li W, Cao L, Chen X, Lei J, Ma Q. Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncol Rep. 2016;35:1718-26
115. Sur S, Pal D, Roy R, Barua A, Roy A, Saha P. et al. Tea polyphenols EGCG and TF restrict tongue and liver carcinogenesis simultaneously induced by N-nitrosodiethylamine in mice. Toxicol Appl Pharmacol. 2016;300:34-46
116. Hoesel B, Schmid JA. The Complexity of NF-ΚB Signaling in Inflammation and Cancer. Mol Cancer. 2013;12:86
117. Karin M. Nuclear Factor-KappaB in Cancer Development and Progression. Nature. 2006;441:431-436
118. Schulze-Osthoff K, Ferrari D, Riehemann K, Wesselborg S. Regulation of NF-kappa B activation by MAP kinase cascades. Immunobiology. 1997;198:35-49
119. Bharrhan S, Chopra K, Arora SK, Toor JS, Rishi P. Down-regulation of NF-κB signalling by polyphenolic compounds prevents endotoxin-induced liver injury in a rat model. Innate Immun. 2012;18:70-9
120. Ruiz PA, Haller D. Functional Diversity of Flavonoids in the Inhibition of the Proinflammatory NF-KappaB, IRF, and Akt Signaling Pathways in Murine Intestinal Epithelial Cells. J Nutr. 2006;136:664-671
121. D'Arcy MS. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol Int. 2019;43:582-592
122. Peter ME. Programmed Cell Death: Apoptosis Meets Necrosis. Nature. 2011;471:310-312
123. Papakonstantinou A, Koumarianou P, Diamantakos P, Melliou E, Magiatis P, Boleti H. A Systematic Ex-Vivo Study of the Anti-Proliferative/Cytotoxic Bioactivity of Major Olive Secoiridoids' Double Combinations and of Total Olive Oil Phenolic Extracts on Multiple Cell-Culture Based Cancer Models Highlights Synergistic Effects. Nutrients. 2023;15:2538
124. Casaburi I, Puoci F, Chimento A, Sirianni R, Ruggiero C, Avena P. et al. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: a review of in vitro studies. Mol Nutr Food Res. 2013;57:71-83
125. Mertens-Talcott SU, Bomser JA, Romero C, Talcott ST, Percival SS. Ellagic acid potentiates the effect of quercetin on p21waf1/cip1, p53, and MAP-kinases without affecting intracellular generation of reactive oxygen species in vitro. J Nutr. 2005;135:609-14
126. Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853-61
127. Panji M, Behmard V, Zare Z, Malekpour M, Nejadbiglari H, Yavari S. et al. Synergistic effects of green tea extract and paclitaxel in the induction of mitochondrial apoptosis in ovarian cancer cell lines. Gene. 2021;787:145638
128. Selvakumar P, Badgeley A, Murphy P, Anwar H, Sharma U, Lawrence K. et al. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients. 2020;12:761
129. Wang S, Zhao Y, Aguilar A, Bernard D, Yang CY. Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapy: Progress and Challenges. Cold Spring Harb Perspect Med. 2017;7:a026245
130. Caponio VCA, Zhurakivska K, Mascitti M, Togni L, Spirito F, Cirillo N. et al. High-risk TP53 mutations predict poor primary treatment response of patients with head and neck squamous cell carcinoma. Oral Dis. 2023 [Epub ahead of print]
131. Troiano G, Boldrup L, Ardito F, Gu X, Lo Muzio L, Nylander K. Circulating miRNAs from blood, plasma or serum as promising clinical biomarkers in oral squamous cell carcinoma: A systematic review of current findings. Oral Oncol. 2016;63:30-37
132. Verma S, Grover S, Tyagi C, Goyal S, Jamal S, Singh A. et al. Hydrophobic Interactions Are a Key to MDM2 Inhibition by Polyphenols as Revealed by Molecular Dynamics Simulations and MM/PBSA Free Energy Calculations. PLoS One. 2016;11:e0149014
133. Garrido-Armas M, Corona JC, Escobar ML, Torres L, Ordóñez-Romero F, Hernández-Hernández A. et al. Paraptosis in human glioblastoma cell line induced by curcumin. Toxicol In Vitro. 2018;51:63-73
134. Vitkeviciene A, Baksiene S, Borutinskaite V, Navakauskiene R. Epigallocatechin-3-gallate and BIX-01294 have different impact on epigenetics and senescence modulation in acute and chronic myeloid leukemia cells. Eur J Pharmacol. 2018;838:32-40
135. Thakur VS, Gupta K, Gupta S. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int J Oncol. 2012;41:353-61
136. Leão R, Apolónio JD, Lee D, Figueiredo A, Tabori U, Castelo-Branco P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. J Biomed Sci. 2018;25:22
137. Wang J, Pan Y, Hu J, Ma Q, Xu Y, Zhang Y. et al. Tea polyphenols induce S phase arrest and apoptosis in gallbladder cancer cells. Braz J Med Biol Res. 2018;51:e6891
138. De Amicis F, Giordano F, Vivacqua A, Pellegrino M, Panno ML, Tramontano D. et al. Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation, inhibits estrogen receptor alpha gene expression via p38MAPK/CK2 signaling in human breast cancer cells. FASEB J. 2011;25:3695-707
139. Fesik SW. Promoting Apoptosis as a Strategy for Cancer Drug Discovery. Nat Rev Cancer. 2005;5:876-885
140. Renaude E, Kroemer M, Loyon R, Binda D, Borg C, Guittaut M. et al. The Fate of Th17 Cells is Shaped by Epigenetic Modifications and Remodeled by the Tumor Microenvironment. Int J Mol Sci. 2020;21:1673
141. Vanden Berghe W. Epigenetic Impact of Dietary Polyphenols in Cancer Chemoprevention: Lifelong Remodeling of Our Epigenomes. Pharmacol Res. 2012;65:565-576
142. Stefanska B, Salamé P, Bednarek A, Fabianowska-Majewska K. Comparative effects of retinoic acid, vitamin D and resveratrol alone and in combination with adenosine analogues on methylation and expression of phosphatase and tensin homologue tumour suppressor gene in breast cancer cells. Br J Nutr. 2012;107:781-90
143. Medina-Aguilar R, Pérez-Plasencia C, Marchat LA, Gariglio P, García Mena J, Rodríguez Cuevas S. et al. Methylation Landscape of Human Breast Cancer Cells in Response to Dietary Compound Resveratrol. PLoS One. 2016;11:e0157866
144. Kala R, Tollefsbol TO. A Novel Combinatorial Epigenetic Therapy Using Resveratrol and Pterostilbene for Restoring Estrogen Receptor-α (ERα) Expression in ERα-Negative Breast Cancer Cells. PLoS One. 2016;11:e0155057
145. Dioguardi M, Cantore S, Sovereto D, La Femina L, Caloro GA, Spirito F. et al. Potential Role of miR-196a and miR-196b as Prognostic Biomarkers of Survival in Head and Neck Squamous Cell Carcinoma: A Systematic Review, Meta-Analysis and Trial Sequential Analysis. Life (Basel). 2022;12:1269
146. Dioguardi M, Cantore S, Sovereto D, La Femina L, Spirito F, Caloro GA. et al. Does miR-197 Represent a Valid Prognostic Biomarker in Head and Neck Squamous Cell Carcinoma (HNSCC)? A Systematic Review and Trial Sequential Analysis. J Pers Med. 2022;12:1436
147. Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008;7:464-73
148. Boyanapalli SS, Kong AT. "Curcumin, the King of Spices": Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. Curr Pharmacol Rep. 2015;1:129-139
149. Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78:559S-569S
150. Turati F, Rossi M, Pelucchi C, Levi F, La Vecchia C. Fruit and vegetables and cancer risk: a review of southern European studies. Br J Nutr. 2015;113:S102-10
151. Key TJ. Fruit and vegetables and cancer risk. Br J Cancer. 2011;104:6-11
152. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW. et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218-20
153. Tatullo M, Simone GM, Tarullo F, Irlandese G, Vito D, Marrelli M. et al. Antioxidant and Antitumor Activity of a Bioactive Polyphenolic Fraction Isolated from the Brewing Process. Sci Rep. 2016;6:36042
154. Hernández-Ramírez RU, Galván-Portillo MV, Ward MH, Agudo A, González CA, Oñate-Ocaña LF. et al. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int J Cancer. 2009;125:1424-30
155. Bahrami A, Jafari S, Rafiei P, Beigrezaei S, Sadeghi A, Hekmatdoost A. et al. Dietary intake of polyphenols and risk of colorectal cancer and adenoma-A case-control study from Iran. Complement Ther Med. 2019;45:269-274
156. Ghanavati M, Clark CCT, Bahrami A, Teymoori F, Movahed M, Sohrab G. et al. Dietary intake of polyphenols and total antioxidant capacity and risk of prostate cancer: A case-control study in Iranian men. Eur J Cancer Care (Engl). 2021;30:e13364
157. Rossi M, Strikoudi P, Spei ME, Parpinel M, Serraino D, Montella M. et al. Flavonoids and bladder cancer risk. Cancer Causes Control. 2019;30:527-535
158. Zamora-Ros R, Cayssials V, Jenab M, Rothwell JA, Fedirko V, Aleksandrova K. et al. Dietary intake of total polyphenol and polyphenol classes and the risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Eur J Epidemiol. 2018;33:1063-1075
159. Lee PMY, Ng CF, Liu ZM, Ho WM, Lee MK, Wang F. et al. Reduced prostate cancer risk with green tea and epigallocatechin 3-gallate intake among Hong Kong Chinese men. Prostate Cancer Prostatic Dis. 2017;20:318-322
160. Tsao AS, Garden AS, Kies MS, Morrison W, Feng L, Lee JJ. et al. Phase I/II study of docetaxel, cisplatin, and concomitant boost radiation for locally advanced squamous cell cancer of the head and neck. J Clin Oncol. 2006;24:4163-9
161. Arrigoni R, Cammarota F, Porro R, Cantore S, Dioguardi M, Cazzolla AP. et al. Natural Bioactive Compounds against Oxidative Stress: Dietary Polyphenols Strike Back. Endocr Metab Immune Disord Drug Targets. 2023;23:764-776
162. Dioguardi M, Ballini A, Sovereto D, Spirito F, Cazzolla AP, Aiuto R. et al. Application of the Extracts of Punica granatum in Oral Cancer: Scoping Review. Dent J (Basel). 2022;10:234
163. Amin ARMR, Wang D, Nannapaneni S, Lamichhane R, Chen ZG, Shin DM. Combination of resveratrol and green tea epigallocatechin gallate induces synergistic apoptosis and inhibits tumor growth in vivo in head and neck cancer models. Oncol Rep. 2021;45:87
164. Caponio VCA, Zhurakivska K, Lo Muzio L, Troiano G, Cirillo N. The Immune Cells in the Development of Oral Squamous Cell Carcinoma. Cancers (Basel). 2023;15:3779
165. Russo D, Mariani P, Caponio VCA, Lo Russo L, Fiorillo L, Zhurakivska K. et al. Development and Validation of Prognostic Models for Oral Squamous Cell Carcinoma: A Systematic Review and Appraisal of the Literature. Cancers (Basel). 2021;13:5755
166. Mariani P, Russo D, Maisto M, Troiano G, Caponio VCA, Annunziata M. et al. Pre-treatment neutrophil-to-lymphocyte ratio is an independent prognostic factor in head and neck squamous cell carcinoma: Meta-analysis and trial sequential analysis. J Oral Pathol Med. 2022;51:39-51
167. Mukherjee S, Hussaini R, White R, Atwi D, Fried A, Sampat S. et al. TriCurin, a synergistic formulation of curcumin, resveratrol, and epicatechin gallate, repolarizes tumor-associated macrophages and triggers an immune response to cause suppression of HPV+ tumors. Cancer Immunol Immunother. 2018;67:761-774
168. Ballini A, Capodiferro S, Toia M, Cantore S, Favia G, De Frenza G. et al. Evidence-based dentistry: what's new? Int J Med Sci. 2007;4:174-8
Author contact
![]() Corresponding author: khrystyna.zhurakivskait.
Corresponding author: khrystyna.zhurakivskait.

 Global reach, higher impact
Global reach, higher impact