Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(2):309-316. doi:10.7150/jca.89059 This issue Cite
Research Paper
Polyporus ulleungus mycelia cultured in MEB medium produce metabolites with anticancer property
1. Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, Incheon, Korea.
2. Convergence Research Center for Insect Vectors, Incheon National University, Incheon 22012, Korea.
Received 2023-8-11; Accepted 2023-11-13; Published 2024-1-1
Abstract
Cancer cells are characterized by apoptosis evasion and uncontrolled cell cycle progression. To combat these characteristics, efforts have been made to find novel natural-source anticancer compounds. The aim of this work is to find new anticancer compounds in Polyporus ulleungus (P. ulleungus) mycelial culture extracts. P. ulleungus mycelium was cultured on four individual media (DYB, MEB, MYB, and PDB) and four extracts were generated from the mycelium culture media. Extracts of P. ulleungus mycelium cultured in MEB medium (pu-MEB) significantly reduced cancer cell growth by triggering apoptosis and S phase arrest. Furthermore, the anticancer effects of pu-MEB were not confined to one type of cancer. Taken together, our results confirmed that P. ulleungus mycelia cultured in MEB medium produce metabolites that exhibit anticancer properties. Development of an optimal medium for P. ulleungus mycelium through optimization of medium components will enable P. ulleungus mycelium to produce metabolites with more anticancer efficacy.
Keywords: Polyporus ulleungus, mycelium culture extract, drug screening
Introduction
Mushrooms are not only a source of nutrients but are also recognized as health-promoting foods as they produce physiologically active substances [1]. Mushrooms synthesize primary metabolites that are essential for mushroom growth and secondary metabolites that are produced in response to various environmental stimuli [2]. Secondary metabolites exhibit immunomodulatory, anticancer, antibacterial, anti-HIV, and antidiabetic properties [2, 3]. Among them, the anticancer properties of mushrooms are receiving considerable attention, and clinical trials to evaluate the effectiveness of mushrooms are increasing [4]. The 5-year survival rate of non-small cell lung cancer patients treated with reishi mushrooms increased, proving the efficacy of reishi mushrooms [5]. Additionally, Agaricus bisporus has proven efficacy in treating patients with recurrent prostate cancer by inhibiting prostate-specific antigen [6]. Therefore, identification of new mushrooms with anticancer property and clinical application of their efficacy will become a new treatment method.
Mushrooms are organisms characterized by a life cycle consisting of two distinct stages: mycelium and fruiting body [7]. Mycelium is a thread-like organism that grows in soil, wood, or other substrates and absorbs nutrients [7]. The fruiting body is the part of the mushroom that can be found above ground and consists of a cap, stem, gills or stomata, depending on the species [7]. Comparison of the composition and concentration of various compounds in fruiting bodies and mycelium revealed that mycelium has a higher content of some bioactive compounds than the fruiting body [8].
Mycelium can recently be grown in large quantities using liquid culture approaches [9]. Mycelium liquid culture speeds up the colonization rate, which makes it possible to culture large quantities of mycelium at low cost [10]. Mycelium liquid culture also allows for the effective production of bioactive reagents due to its ability to comply with current good manufacturing practice (cGMP) standards. Therefore, this technology is evaluated as a promising technology that can effectively produce secondary metabolites produced from mycelium.
The genus Polyporus, a type of wood rot mushroom, belongs to the Polyporaceae family [11]. Currently, Polyporous parvovarius was identified to stimulate apoptosis and inhibit cancer cell growth [12]. Despite the fact that this genus contains over 250 species, little research has been done on the anticancer abilities of other Polyporus species. The aim of this study is to examine whether Polyporus ulleungus (P. ulleungus) mycelium culture extract had anticancer activity. Here, we identified that extracts of P. ulleungus mycelium cultured in MEB medium exhibited anticancer properties and examined the underlying mechanism how it effectively kills cancer cells.
Materials and Methods
Procedure to prepare mycelial culture extracts
The National Institute of Biological Resources (NIBR, Incheon, Korea) provided Polyporus ulleungus (Accession number: NIBRFG0000513823). Table 1 lists the four media that were employed in this investigation along with their names and ingredients.
Four media that were employed in this investigation.
| Media name | Ingredients | Company name | Catalogue number | Concentration |
|---|---|---|---|---|
| DYB | • yeast extract | Becton Dickinson, Franklin, NJ, USA | 212750 | 2g/l |
| • dextrose | Becton Dickinson | 215530 | 20 g/l | |
| MYB | • yeast extract | Becton Dickinson | 212750 | 2g/l |
| • malt extract | Becton Dickinson | 218630 | 20 g/l | |
| PDB | • dextrose | Becton Dickinson | 215530 | 20 g/l |
| • potato starch | Sigma, Saint Louis, MO, USA | S2004 | 4 g/l | |
| MEB | • yeast extract | Becton Dickinson | 212750 | 2g/l |
| • malt extract | Becton Dickinson | 218630 | 6 g/l | |
| • dextrose | Becton Dickinson | 215530 | 6 g/l | |
| • maltose | Becton Dickinson | 216830 | 1.8 g/l |
The steps for preparing mycelial culture extracts have been described in detail previously [13]. Briefly, 5 mm pre-cultured mycelial disks were injected into Erlenmeyer flasks containing 700 ml of medium. To test the effect of medium alone, mycelial disks were not injected into Erlenmeyer flasks containing 700 ml of medium. Afterwards, each flask underwent a 60-day incubation period at 25°C with 150 × g shaking. The liquid culture medium containing mycelium and the liquid culture medium without mycelium were passed through Miracloth (475855-1R; Millipore, Burlington, MA, USA). Afterwards, the filtered culture medium was freeze-dried. The dried substance was dissolved using 100% ethanol. The dissolved solution was filtered using a 0.45m membrane filter (HAWP05000, Sigma), and the ethanol was evaporated. The leftover extract was diluted to a 500 mg/ml concentration in dimethyl sulfoxide (DMSO) (41639, Sigma).
Cell Culture
Table 2 provides information on the eight different cell lines utilized. Cells were cultured following procedures described previously [12].
Information on the seven different cell lines utilized.
| Cell line name | Company name | Catalogue number |
|---|---|---|
| HeLa cells | Sigma | 93021013 |
| NCI-H2009 | American Type Culture Collection (ATCC), Manassas, VA, USA | CRL-5911™ |
| PC-9 | Sigma | 90071810 |
| A549 | ATCC | CRL-185™ |
| HCC95 | Sigma | CLL1220 |
| HCC15 | Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, German | ACC 496 |
| NCI-H1299 | ATCC | CRL-5803™ |
| BEAS-2B | ATCC | CRL-9609™ |
Cell proliferation assay
Cell proliferation assay was conducted in 96-well plates with 2,000 cells per well. Each mycelium extract was diluted to a 500 μg/ml concentration. The concentration of cisplatin (PHR1624-200MG; Sigma) was diluted to a final value of 20 μM. Detailed procedures were followed as previously described [13].
Determination of cytotoxicity of pu-MEB
Cytotoxicity assay was conducted in 96-well plates with 2,000 cells per well. pu-MEB was diluted at concentrations of 100-500 μg/ml. Detailed procedures were followed as previously described [13].
Determination of the optimal concentration of pu-MEB
The assay to determine the optimal concentration was carried out in 96-well plates with 2,000 cells per well. Cell proliferation was assessed at 1-4 days at doses of 100-500 μg/ml. Procedures to determine the optimal concentration were followed as previously described [13].
Apoptosis assay
As previously mentioned [12], an analysis for apoptosis was carried out using the Annexin V- FITC Apoptosis Detection Kit (556547; BD Biosciences, Franklin Lakes, NJ, USA).
Cell cycle assay
Procedures to determine cell cycle were followed as previously described [12]. Briefly, centrifugation was used to collect 1 × 106 cells for 2 min at 760 × g. Cells were stained with 50 g/ml propidium iodide (PI, P4170-10MG; Sigma) after being fixed in 70% ethanol.
Information on primary and secondary antibodies used in this study.
| Antibody name | Company name | Catalogue number | Dilution in PBS |
|---|---|---|---|
| caspase 9 antibody | Cell signaling technology, Danvers, MA, USA | 9502s | 1:500 |
| phospho-p53 antibody | Santa Cruz Biotechnology, Dallas, TX, USA | sc-377561 | 1:500 |
| phospho-MEK antibody | Cell signaling technology | 9121s | 1:500 |
| p53 antibody | Abclonal, Woburn, MA, USA | A19585 | 1:500 |
| MEK antibody | Cell signaling technology | 9122s | 1:500 |
| HRP-conjugated β-actin | Santa Cruz Biotechnology | sc-47778 | 1:1,000 |
| anti-mouse HRP-conjugated antibody | Santa Cruz Biotechnology | sc-516102 | 1:1,000 |
| anti-rabbit HRP-conjugated antibody | Santa Cruz Biotechnology | sc-2357 | 1:1,000 |
Western blot analysis
Western blot analysis was followed as previously described [12]. Briefly, utilizing a Chemidoc XRS+ system (1708265; BIO-RAD; Hercules, CA, USA), proteins were detected with SuperSignal™ West Femto chemiluminescent solution (34095; Thermo Fisher Scientific, Waltham, MA, USA). Table 3 provides information on primary and secondary antibodies used in this study.
Statistical analyses
GraphPad Prism 7 (GraphPad Software, Boston, MA, USA) was used for the statistical analysis. To evaluate whether differences were significant, the Student's t-test or two-way ANOVA followed by Bonferroni post hoc test was used.
Results
Chemical screening for anticancer activity in P. ulleungus mycelium culture extracts
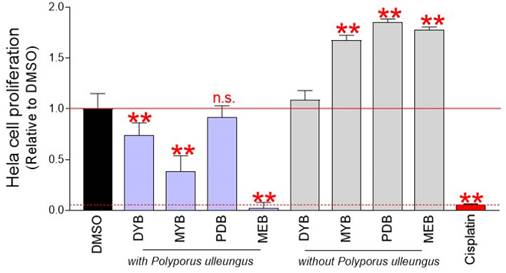
Screening of anticancer properties of P. ulleungus mycelium culture extracts used a technique for counting cells based on DNA amount [14]. Mycelial cultures of P. ulleungus grown on four different media (DYB, MYB, PDB, MEB) were extracted with four different compounds. To determine the impact of the medium on cell growth, four types of extracts were produced in medium without mycelium inoculation. Each extract was diluted to a concentration of 500 μg/ml and treated with cervical cancer-derived Hela cells. On the fourth day, the effect of each extract on cell growth was evaluated. Cisplatin, a first-line chemotherapy drug used for pancreatic, ovarian, esophageal, and cervical cancer, was used as a positive control [15]. Compared with the DMSO control, mycelium culture extracts from DYB, MYB, and MEB media significantly inhibited cell proliferation (Fig. 1). However, mycelium culture extract in PDB medium did not interfere with cell growth (Fig. 1).
Chemical screening for anticancer activity in P. ulleungus mycelium culture extracts. Mycelial cultures of P. ulleungus grown on four different media (DYB, MYB, PDB, MEB) were extracted with four different compounds. To determine the impact of the medium on cell growth, four types of extracts were produced in medium without mycelium inoculation. Each extract was diluted to a concentration of 500 μg/ml and treated with cervical cancer-derived Hela cells. On the fourth day, the effect of each extract on cell growth was evaluated. Cisplatin, a first-line chemotherapy drug used for pancreatic, ovarian, esophageal, and cervical cancer, was used as a positive control. ** P < 0.01 versus control group (DMSO), n.s= not significant, Student's t-test. Mean ± S.D., n = 10.
Determining the cytotoxicity and optimal concentration of pu-MEB. (A) Cell proliferation was measured at pu-MEB concentrations of 100-500 μg/ml. To assess cell viability, proliferation values at each concentration were normalized to those of DMSO. Mean ± S.D., n = 10. (B) Cell proliferation was measured at 1-4 days following treatment at concentration of 100 to 500 μg/ml. **P <0.01 versus control group (DMSO), two-way ANOVA followed by Bonferroni post hoc test. Mean ± S.D., n = 10.
Identification of mycelial culture extracts with anticancer properties raised the possibility that this effect may be due to media components contained in the culture medium. To exclude this possibility, cells were treated with extracts produced in mycelium-free medium. Compared to the DMSO control, culture extracts from mycelium-free MYB, PDB, and MEB media significantly increased cell proliferation (Fig. 1). These results indicate that MYB, PDB, and MEB have nutrient-rich components that play a crucial role in increasing cell proliferation. Moreover, the culture extract of mycelium-free DYB medium was not effective in both inhibiting and inducing proliferation (Fig. 1). These results suggest that the growth inhibitory effect of mycelium extracts cultured in DYB, MYB, and MEB media is due to anticancer substances synthesized during mycelium culture rather than the media itself. Since mycelial culture extract in MEB medium (pu-MEB) inhibited cell proliferation more effectively than cisplatin (a positive control group), pu-MEB were selected for further studies (Fig. 1).
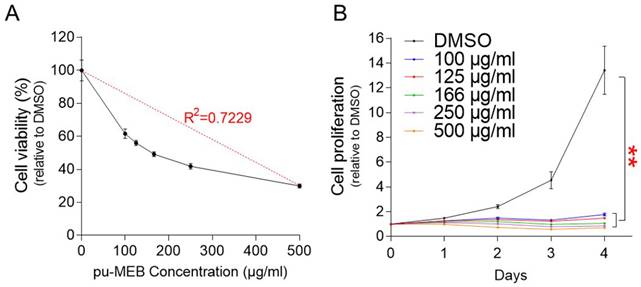
The finding that the antiproliferative effect was caused by the anticancer properties of pu-MEB rather than by the effect of the medium may raise another possibility that the antiproliferative effect may be due to cytotoxicity of pu-MEB. To rule out the possibility, cytotoxicity experiment was carried out using pu-MEB at a concentration of 100-500 μg/ml. R2 value, coefficient of determination, was 0.7229, showing that concentration was a factor in the decreased cell viability (Fig. 2A). These results suggest that pu-MEB's ability to decrease cell growth was not a result of its cytotoxicity.
The optimal concentration of pu-MEB to effectively inhibit cell proliferation was then examined. Cell proliferation was measured at 1-4 days following treatment at concentration of 100 to 500 μg/ml. Each concentration of pu-MEB exhibited a significant decrease in cell proliferation compared to the DMSO control (Fig. 2B). As a result, the lowest concentration of pu-MEB, 100 μg/ml, was chosen for further studies.
pu-MEB induces apoptosis and S phase arrest to reduce cancer cell growth
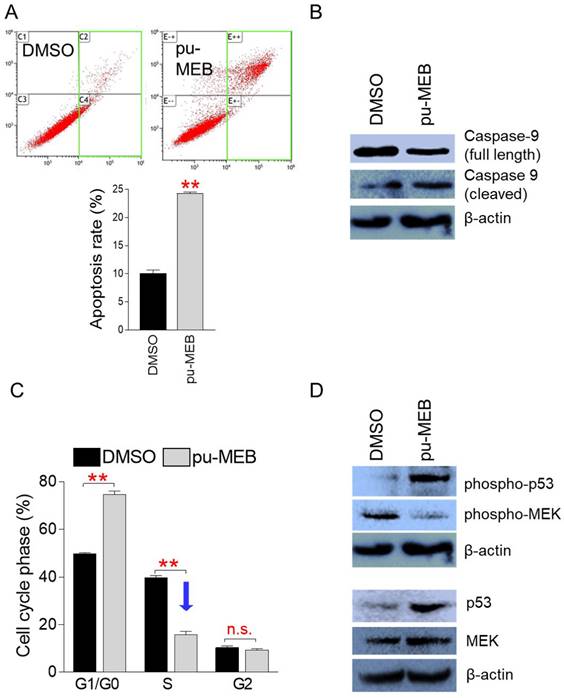
Apoptosis is a programmed cell death process known to be a crucial way to limit the uncontrolled proliferation of cancer cells [16]. Thus, we investigated whether pu-MEB causes apoptosis to stop the proliferation of cancer cells. To perform flow cytometric analysis of apoptotic cells, HeLa cells were treated to pu-MEB at a dose of 100 μg/ml for 2 days. Indeed, compared to controls, pu-MEB treatment significantly increased the apoptosis rate (Fig. 3A). These findings imply that pu-MEB inhibits cancer cell proliferation by triggering programmed cell death.
Caspase 9 exists as an inactive procaspase monomer and is activated by a unique proteolytic process that causes cleavage [17]. Cleaved caspase 9 processes other caspase members to initiate the caspase cascade and induce apoptosis [17]. We examined the cleavage of caspase 9 to provide further evidence for the ability of pu-MEB to induce apoptosis. Compared with the DMSO control group, full-length caspase 9 decreased and cleaved caspase 9 increased in the pu-MEB group, indicating that the pu-MEB group initiated the caspase cascade leading to apoptosis (Fig. 3B).
Cancer is also characterized by unchecked cell cycle progression because of aberrant activation of cell cycle proteins [18]. One of the main indicators to evaluate the anticancer properties of a candidate substance is whether it triggers cell cycle arrest, especially S phase arrest [19, 20]. Therefore, we examined whether pu-MEB induces S phase arrest in cancer cells. To perform flow cytometric analysis of cell cycle, HeLa cells were treated to pu-MEB at a dose of 100 μg/ml for 2 days. Compared with cells treated with DMSO, cells treated with pu-MEB significantly upregulated the proportion of G1/G0 phase from 49.8 to 74.7%, but downregulated the proportion of S phase from 39.9 to 16.0% (Fig. 3C). According to these findings, pu-MEB caused S phase arrest in cancer cells.
Cell cycle arrest is promoted by the oncoprotein p53 (p53), allowing DNA repair [21]. Additionally, the stability of p53, which is required for the initiation of cell cycle arrest, depends on phosphorylation of p53 (phospho-p53) [22]. Therefore, we investigated whether pu-MEB treatment changes the expression levels of p53 and phospho-p53. pu-MEB significantly increased the expression of p53 and phospho-p53, demonstrating the underlying mechanism of pu-MEB-mediated S phase arrest (Fig. 3D).
pu-MEB induces apoptosis and S phase arrest to reduce cancer cell growth. (A) To perform flow cytometric analysis of apoptotic cells, HeLa cells were treated to pu-MEB at a dose of 100 μg/ml for 2 days. Apoptotic cell populations are shown by the green square. **P < 0.01 versus control group (DMSO), student's t-test. Means ± S.D., n = 3. (B) Effect of pu-MEB on the expression levels of proteins that initiate the caspase cascade and induce apoptosis. Compared with the DMSO control group, full-length caspase 9 decreased and cleaved caspase 9 increased in the pu-MEB group. (C) To perform flow cytometric analysis of cell cycle, HeLa cells were treated to pu-MEB at a dose of 100 μg/ml for 2 days. **P < 0.01 versus control group (DMSO), student's t-test. Means ± S.D., n = 3. (D) Effect of pu-MEB on the expression levels of proteins that play important roles in the cell cycle pathway: p53, phospho-p53, MEK, phospho-MEK.
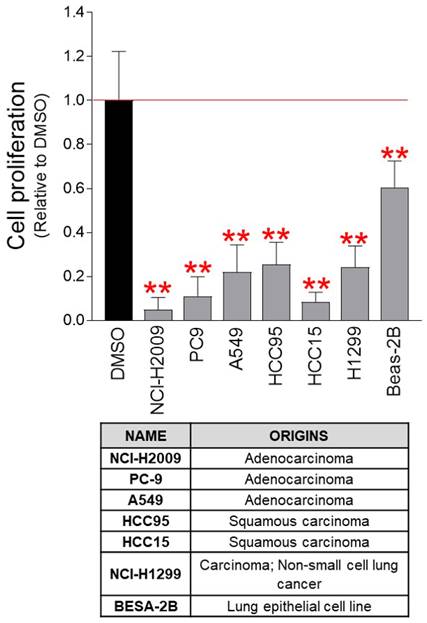
pu-MEB inhibits the proliferation of lung cancer-derived cancer cells. Seven lung cancer-derived cells were treated with pu-MEB at a concentration of 100 μg/ml. On the fourth day, the inhibitory effect on cell proliferation was assessed. **P < 0.01 versus control group (DMSO), student t-test. Means ± S.D., n = 10.
Mitogen-activated protein kinase kinase (MEK) acts as a key component of the MAP kinase signal transduction pathway. Phospho-MEK is an active form of MEK and is a key regulator of S phase entry signals [23, 24]. Therefore, we examined the expression level of MEK and phospho-MEK. pu-MEB did not markedly reduce the expression of MEK, but markedly reduced the expression of phopsho-MEK (Fig. 3D). These results indicate pu-MEB-mediated S phase arrest through reduced expression of phopsho-MEK.
pu-MEB inhibits the proliferation of lung cancer-derived cancer cells
As the anticancer effect of pu-MEB was confirmed in cervical cancer-derived Hela cells, we examined whether the pu-MEB had anticancer properties against various cancer cell lines. Seven lung cancer-derived cancer cell lines (NCI-H2009, PC-9, A549, HCC95, HCC15, NCI-H1299, and BESA-2B) were used to determine the effectiveness of pu-MEB. Seven lung cancer-derived cancer cell lines treated with pu-MEB exhibited significantly less proliferation than cells treated with DMSO (Fig. 4). These findings imply that the anticancer characteristics of pu-MEB are effective not only for cervical cancer-derived Hela cells, but also for lung cancer-derived cancer cells.
Discussion
Mushrooms produce physiologically active secondary metabolites such as polysaccharides, lectins, terpenoids, and alkaloids in response to various external stimuli [25]. Secondary metabolites have low toxicity and no side effects, so they are used for anticancer, antibacterial, and antidiabetic purposes. Numerous clinical trials are being conducted to assess the efficiency and safety of secondary metabolites [4]. In this study, P. ulleungus mycelium was cultured in four media (DYB, MYB, PDB, MEB) and the anticancer ability of mycelium culture extract was investigated. Significant anticancer activity was demonstrated by mycelia grown on DYB, MYB and MEB media. However, extracts grown in PDB did not show anticancer property. Differences in media composition can explain the underlying mechanism for these results. DYB, MYB and MEB media contain yeast extract, but PDB medium does not contain yeast extract. Thus, we propose that yeast extract is essential for P. ulleungus mycelium to produce secondary metabolites that exhibit anticancer activity. Furthermore, among the mycelium culture extracts grown in DYB, MYB, and MEB media, the mycelium culture extract grown in MEB medium showed the most potent anticancer activity. To find the cause of these results, the components of DYB, MYB, and MEB media were analyzed. DYB medium contains yeast extract and dextrose. DYB medium contains two components: yeast extract and glucose. MYB medium contains two components: yeast extract and malt extract. However, MEB medium contains four components: yeast extract, malt extract, dextrose, maltose. Moreover, maltose is an ingredient that is not included in DYB and MYB, but is only included in MEB medium. Therefore, we propose that maltose is essential for P. ulleungus mycelia to synthesize secondary metabolites that exhibit anticancer property. Taken together, this is the first study to demonstrate medium conditions for P. ulleungus mycelium to synthesize metabolites with anticancer property. If the components that make up MEB medium can be improved using methods such as waste medium analysis, P. ulleungus mycelium will be able to synthesize metabolites with more powerful anticancer properties.
Eukaryotic cells have the ability to eliminate their own cells through apoptosis [26]. Because cancer cells have the characteristic of avoiding apoptosis, apoptosis has been used as a successful strategy to eliminate cancer cells [16]. Here, we identified that pu-MEB initiated the caspase cascade by decreasing the inactive caspase 9 form and increasing the active caspase 9 form, ultimately inducing apoptosis. Dysregulation of cell cycle progression is another characteristic of cancer cells [18]. Because cancer cells have the characteristic of uncontrolled cell cycle progression, regulating cell cycle progression have been targeted as strategies to treat the uncontrolled proliferation of malignant tumor cells [18]. Here, we found that pu-MEB regulates the cell cycle progression of cancer cells. pu-MEB activated p53, which controls checkpoints throughout the cell cycle from G1 phase to cytokinesis. In addition, pu-MEB inhibited the activity of MEK, which is activated in S phase and promotes DNA synthesis, thereby inducing S phase arrest. Because apoptosis induction and S phase arrest act as a double-edged sword in effectively eliminating cancer cells, we propose that the use of pu-MEB as a chemotherapy agent should be considered a priority.
Mycelium liquid culture allows mycelium to be grown in liquid without using conventional agar plates [27, 28]. Because mycelium quickly colonizes liquid media, the scale of mycelium liquid culture can be expanded up to 10,000 liters. The expanded culture scale enables the production of large quantities of secondary metabolites at a lower cost [9]. Here, we identified that P. ulleungus mycelium cultured in MEB generated metabolites with anticancer properties. However, because liquid culture was carried out in a laboratory environment, there is a limit to the amount of metabolites produced at one time. If the large-scale mycelium liquid culture method can be improved by altering process variables including dissolved oxygen, temperature, stirring speed, and pH, the amount of metabolites generated per one liquid culture could reach commercial production levels.
Paclitaxel, a well-known anticancer drug, was extracted from the bark of Taxus brevifolia through fractionation and purification [29]. Penicillin, a well-known antibiotic, was also extracted from the mold Penicillium chrysogenum through fractionation and purification [30]. Previous studies found that P. parvovarius mycelium culture extract exhibits anticancer efficacy [12]. Then, active ingredients were obtained through a fractionation/purification process and identified as 3,4-dihydroxybenzaldehyde (Protocatechualdehyde, PCA). PCA showed anticancer activity similar to P. parvovarius mycelium culture extract, confirming PCA as the active ingredient [12]. In this study, we identified that pu-MEB exhibits anticancer efficacy, but did not confirm which active component of pu-MEB exhibits anticancer efficacy. Therefore, future research will need to find active ingredients that exhibit anticancer activity in pu-MEB through separation, purification, and structural analysis. If an active ingredient with anticancer efficacy is discovered through this process, the ingredient can be used as a new anticancer agent to treat cancer.
In summary, we identified that P. ulleungus mycelia cultured in MEB medium (pu-MEB) significantly reduced cancer cell growth by triggering apoptosis and S phase arrest. The efficacy of pu-MEB was not limited to cervical cancer-derived Hela cells, but was also effective against lung cancer-derived cancer cells. This potent anti-cancer candidate can be generated in large quantities at low cost and can be used clinically to treat cancer.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HP23C0024). This study was also supported by the Basic Research Program (NRF-2021R1A2C1004298) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Technology and by the Priority Research Centers Program (2020R1A6A1A0304195411) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education.
Author Contributions
YHL, HWK, JC and JTP conceived of and designed the experiments. YHL, MUK, MKS, HJP, ESS, JP and JHY performed the experiments. YHL and HWK analyzed the data. YHL, HWK, JC and JTP wrote and edited the paper.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Martinez-Medina GA, Chávez-González ML, Verma DK, Prado-Barragán LA, Martínez-Hernández JL, Flores-Gallegos AC. et al. Bio-funcional components in mushrooms, a health opportunity: Ergothionine and huitlacohe as recent trends. Journal of Functional Foods. 2021;77:104326
2. Anke T. Secondary metabolites from mushrooms. The Journal of Antibiotics. 2020;73:655-6
3. Bhambri A, Srivastava M, Mahale VG, Mahale S, Karn SK. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front Microbiol. 2022;13:837266
4. Wasser SP. Medicinal Mushrooms in Human Clinical Studies. Part I. Anticancer, Oncoimmunological, and Immunomodulatory Activities: A Review. International journal of medicinal mushrooms. 2017;19:279-317
5. Liu J, Mao JJ, Li SQ, Lin H. Preliminary Efficacy and Safety of Reishi & Privet Formula on Quality of Life Among Non-Small Cell Lung Cancer Patients Undergoing Chemotherapy: A Randomized Placebo-Controlled Trial. Integrative cancer therapies. 2020;19:1534735420944491
6. Wang X, Ha D, Mori H, Chen S. White button mushroom (Agaricus bisporus) disrupts androgen receptor signaling in human prostate cancer cells and patient-derived xenograft. J Nutr Biochem. 2021;89:108580
7. Berger RG, Bordewick S, Krahe N-K, Ersoy F. Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites. Microorganisms. 2022;10:1379
8. Fijałkowska A, Muszyńska B, Sułkowska-Ziaja K, Kała K, Pawlik A, Stefaniuk D. et al. Medicinal potential of mycelium and fruiting bodies of an arboreal mushroom Fomitopsis officinalis in therapy of lifestyle diseases. Scientific Reports. 2020;10:20081
9. Manan S, Ullah MW, Ul-Islam M, Atta OM, Yang G. Synthesis and applications of fungal mycelium-based advanced functional materials. Journal of Bioresources and Bioproducts. 2021;6:1-10
10. Yang L, Park D, Qin Z. Material Function of Mycelium-Based Bio-Composite: A Review. Frontiers in Materials. 2021;8:737377
11. Cui B-K, Li H-J, Ji X, Zhou J-L, Song J, Si J. et al. Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Diversity. 2019;97:137-392
12. Lee YH, Kim M, Park HJ, Park JY, Song ES, Lee H. et al. Chemical screening identifies the anticancer properties of Polyporous parvovarius. Journal of Cancer. 2023;14:50-60
13. Song ES, So MK, Park HJ, Lee H, Lee YH, Kuk MU. et al. Chemical screening identifies the anticancer properties of Polyporous tuberaster. Journal of Cancer. 2023;14:2075-84
14. Silva LP, Lorenzi PL, Purwaha P, Yong V, Hawke DH, Weinstein JN. Measurement of DNA Concentration as a Normalization Strategy for Metabolomic Data from Adherent Cell Lines. Analytical Chemistry. 2013;85:9536-42
15. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. European journal of pharmacology. 2014;740:364-78
16. Pfeffer CM, Singh ATK. Apoptosis: A Target for Anticancer Therapy. International journal of molecular sciences. 2018;19:448
17. McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656
18. Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nature reviews Cancer. 2017;17:93-115
19. Matthews HK, Bertoli C, de Bruin RAM. Cell cycle control in cancer. Nature Reviews Molecular Cell Biology. 2022;23:74-88
20. Fotedar R, Diederich L, Fotedar A. Apoptosis and the cell cycle. Prog Cell Cycle Res. 1996;2:147-63
21. Chen J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harbor perspectives in medicine. 2016;6:a026104
22. Lazo PA. Reverting p53 activation after recovery of cellular stress to resume with cell cycle progression. Cellular signalling. 2017;33:49-58
23. Wu D, Chen B, Parihar K, He L, Fan C, Zhang J. et al. ERK activity facilitates activation of the S-phase DNA damage checkpoint by modulating ATR function. Oncogene. 2006;25:1153-64
24. Rescan C, Coutant A, Talarmin H, Theret N, Glaise D, Guguen-Guillouzo C. et al. Mechanism in the sequential control of cell morphology and S phase entry by epidermal growth factor involves distinct MEK/ERK activations. Mol Biol Cell. 2001;12:725-38
25. Ziaja-Sołtys M, Kołodziej P, Stefaniuk D, Matuszewska A, Jaszek M, Bogucka-Kocka A. Low-Molecular-Weight Secondary Metabolites from Fungi: Cerrena unicolor as a New Proposal of an Effective Preparation against Rhabditis Nematodes. Molecules. 2022;27:1660
26. D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell biology international. 2019;43:582-92
27. Wang S, Wang C, Cao H, Cui X, Guo H, Zheng W. et al. Comparing the Cosmetic Effects of Liquid-Fermented Culture of Some Medicinal Mushrooms Including Antioxidant, Moisturizing, and Whitening Activities. International journal of medicinal mushrooms. 2020;22:693-703
28. Bakratsas G, Polydera A, Katapodis P, Stamatis H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods. 2021;4:100086
29. Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G. et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxidative medicine and cellular longevity. 2021;2021:3687700
30. Gaynes RP. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerging Infectious Diseases. 2017;23:849-53
Author contact
![]() Corresponding authors: Hyung Wook Kwon, Ph.D., Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, Incheon 22012, Korea. Tel: +82-32-835-8090; E-mail address: hwkwonac.kr. Jaehyuk Choi, Ph.D., Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, Incheon 22012, Korea. Tel: +82-32-835-8242; E-mail address: jaehyukcac.kr. Joon Tae Park, Ph.D., Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, Incheon 22012, Korea. Tel: +82-32-835-8841; E-mail address: joontae.parkac.kr.
Corresponding authors: Hyung Wook Kwon, Ph.D., Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, Incheon 22012, Korea. Tel: +82-32-835-8090; E-mail address: hwkwonac.kr. Jaehyuk Choi, Ph.D., Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, Incheon 22012, Korea. Tel: +82-32-835-8242; E-mail address: jaehyukcac.kr. Joon Tae Park, Ph.D., Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, Incheon 22012, Korea. Tel: +82-32-835-8841; E-mail address: joontae.parkac.kr.

 Global reach, higher impact
Global reach, higher impact