3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(2):508-525. doi:10.7150/jca.85446 This issue Cite
Research Paper
A Comprehensive Pan-cancer Analysis of the Biological Immunomodulatory Function and Clinical Value of CD27
1. The First Clinical Medical College of Lanzhou University, Lanzhou, Gansu, 730000, China.
2. General Surgery Clinical Medical Center, Gansu Provincial Hospital, Lanzhou, Gansu, 730000, China.
3. Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province, Gansu Provincial Hospital, Gansu 730000, China.
4. NHC Key Laboratory of Diagnosis and Therapy of Gastrointestinal Tumor, Gansu Provincial Hospital, Lanzhou, 730000, China.
5. School of Stomatology, Lanzhou University, Lanzhou, Gansu, 730000, China.
6. College of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China.
Received 2023-4-20; Accepted 2023-9-26; Published 2024-1-1
Abstract
Background: CD27 is an immunological checkpoint gene, plays a critical function inInhibition or activation of cancer immunity. The CD27/CD27L axis is its pathway of action. Therefore, our goal was to examine the predictive role of CD27 in the clinical prognosis of 33 cancer types and its functions in cancer progression, as well as explore the link between pan-cancer CD27 gene expression and immune infiltration.
Methods: By comprehensive use of datasets and methods from TCGA, cBioPortal, GTEx, HPA, KM-plotter, Spearman, CellMinerTM, R packages and RT-qPCR, we delved deeper into the potential impact of the CD27 on cancer development. These include expression differences, immune infiltration, matrix infiltration, gene mutations, DNA methylation, signaling pathways, TMB, MSI, and prognosis. Also, we explored CD27 interactions with different drugs.
Results: The results showed that, mutated CD27 was highly expressed in most cancers. The CD27 showed strong diagnostic value in 4 cancers and marked a positive prognosis for CESC, intracervical adenocarcinoma, HNSC, and endometrial cancer, and a poor prognosis for UVM. In addition, CD27 affects multiple immune and inflammatory signaling pathways and is positively correlated with immune cell infiltration, T cell differentiation, macrophage M1 polarization, stromal infiltration, and drug sensitivity. DNA methylation is involved in CD27 expression in cancer.
Conclusion: CD27, which is mutated in cancers and appears widely highly expressed and altered tumor immune invasion and stromal invasion by affecting multiple immune-related and inflammation signaling pathways, plays a significant role in CESC, HNSC, UCEC and UVM, and may be used as a therapeutic target for related cancers.
Keywords: CD27, pan-cancer, immune, prognosis.
Introduction
Cancer has had a significant negative impact on worldwide public health, and its morbidity and death rate have quickly jeopardized human health, and most of the treatments that are currently available have a poor level of efficacy [1-3]. Studies of cancer can assist us find distinctive aspects of malignant tumors, as well as provide brand new ideas for the treatment of human cancers [4]. Nowadays, the adhibition of biomarkers in cancer has aroused lots of attention, this suggests that the study of new biological markers of cancer is necessary [5-7]. In order to do this, pan-cancer analysis can be adopted to locate useful prognostic biomarkers. Establishing more efficient molecular targets for cancer therapy and discovering new diagnostic and predictive markers [8-11].
The CD27, as known as TNFRSF7[12]. It is essentially a type-I transmembrane glycoprotein that binds to its ligand CD27L (CD70) and initiates a series of signal transduction pathways to regulate cellular function [13, 14]. CD27 is widely expressed in immune cells [15], and it performs a lot of biological functions. What is most noteworthy is that as a co-stimulated T, B cell receptors initiate functional immune responses and promote the proliferation and differentiation of T cells [16].
The autoimmune system is considered to be the most important means of fighting tumors, directly killing and removing tumor cells. Tumor-specific immunity relies on CD8+T cell-mediated cellular immunity [17-19]. The activation of T cell is a complex biological process that relies on the first signal provided by the TCR with the MHCI and the second signal provided by the T cell co-receptor [20, 21]. Deficiency of stimulatory T cell co-receptors or activation of inhibitory co-receptors is common in the TME and is one of the pathways by which tumors initiate immune escape [22, 23]. For example, activation of the PD-1/PD-L1 axis inhibits tumor-specific immunity and causes adverse outcomes [24].
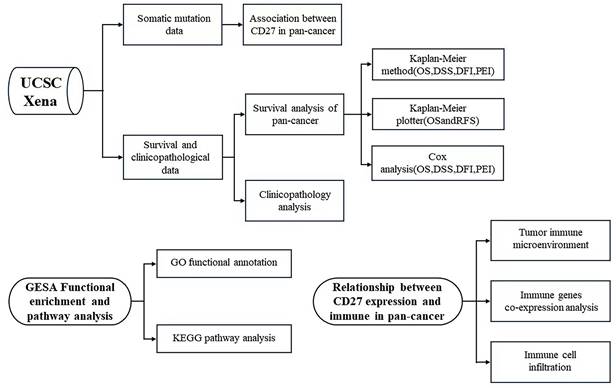
As a T cell co-receptor that has not been fully studied, CD27 has a great possibility to play a regulatory and therapeutic role in tumor progression. In this work, we studied the prognostic relevance of the expression of CD27 in 33 different cancer types. Further, we investigated if there was a correlation between CD27's expression, TMB, MSI, and the levels of immune infiltration. We also analyzed the co-expression of CD27 and the correct route with other immune-related genes. Based on these findings, it appears that CD27 may influence cancer patients' prognoses by interacting with invading immune cells. We used a flow chart to show the design and analysis of this study in Figure 1.
Methods
Sample Information
The expression data of CD27, survival data and clinicopathological of the 33 cancers were obtained from the TCGA database. We included normal samples and cancer tissue samples from the TCGA and GTEx databases to compare their CD27 expression levels. Besides, the HPA was utilized to present the human's protein expression models in both normal and tumor tissues [25].
Expression Profiling of CD27
CD27 is multi-expressed in lymphocytes and APCs and CD27L binds to CD27 expressed on T cells to promote T cell signal transduction. The TCGA and GTEx databases and Perl software were used to analyze CD27 expression in 33 cancers.
The flow chart of the study design and analysis.
Analyses of CD27 Expression Levels in Human Cancer for Prognosis and Clinicopathological Association
To further investigate the link between CD27 expression and clinical outcome, we gathered the survival data from the TCGA database. All the important metrics were calculated. And we evaluated the survival analysis using the Kaplan-Meier method and log-rank test.
For the human cancer dichotomy, the cut-off value was determined by using the median CD27 expression level as the basis. This help classify patients into high-risk and low-risk categories. Besides, we conducted a COX analysis in order to evaluate the relationship between CD27 expression and the prognosis of pan-cancer.
We used KM-plotter to investigate the link between CD27 expression and the survival of pan-cancer.
Indicators of gene mutation and DNA Methyltransferase Analysis
TMB is an indicator of cancer gene mutations and is associated with the effectiveness of immune checkpoint therapy. TMB can be assessed by MSI and MMR [26, 27]. The TCGA database was used to determine the mutation levels of 5 MMR genes (EPCAM, MLH1, MSH6, MSH2, and PMS2). DNMTs also have a significant impact on how chromatin structure and gene expression are altered. This study employed Spearman's Pearson analysis to evaluate the connection between CD27 expression and 5 MMR genes, as well as the 4 methyltransferases (DNMT3B, DNMT3A, DNMT2, and DNMT1) by using the R-packages “reshape2” and “RColorBrewer”.
Correlations Between CD27 Expression and Immune
The TIMER and CIBERSORT databases were used to obtain immune invasion data in 33 cancers, the scores of 6 kinds of TIICs (CD4+T cells, CD8+T cells, macrophages, B cells, dendritic cells, and neutrophils) in 33 cancers were obtained. Additionally, using Spearman correlation analyses, we assessed the associations between CD27 expression and TIICs, immunological checkpoint marker expression levels as well as immune/stromal scores. An estimation algorithm in R-package “estimation” and “limma” was applied to assess the matrix score and immune score of stromal cells and immune cells (P < 0.001 as a cut-off value). And the co-expression analysis of CD27 with other immune-related genes was conducted to further investigate the relations between CD27 expression and immune.
Pathway Analysis of CD27
Specifically, the study used gene sets downloaded from the GSEA website. GO and KEGG were implemented for CD27 annotation by R-package"org.Hs.eg.db", "clusterProfiler", and "enrichplot".
Drug Sensitivity of CD27
To analyze CD27 chemosensitivity in tumors, we applied CallMiner-TM to download NCI-60 compound activity data and RNA-seq expression files. Some drugs which have been approved by FDA were chosen for analysis.
Cell Culture
Human hepatocyte line L-O2, human hepatoma cell line HUH-7 HepG2 and SMMC-7721; Human colonic epithelial cell line NCM460, human colorectal cancer cell line SW480, HCT116 and RKO; Human renal proximal convoluted tubular epithelial cell line HK-2, human renal cancer cell line Caki-2; Human gastric mucosal cell line GES-1, human gastric cancer cell line AGS, MKN-45 and MGC-803. These cell lines were from our research group and preserved in Gansu Provincial People's Hospital Central Laboratory. They were all incubated in RPMI-1640 supplemented with 10 % FBS (Hy clone) plus 1% antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) (MA0110, MEILUNE, China), and maintained at 37℃ in a 5% CO2 incubator (HF90-HT, Heal Force, China).
RNA isolation and Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
Follow the reagent provider's instructions for total RNA extraction, cDNA transcription, and RT-qPCR using the RNA Generic Kit, RNA Reverse Transcription Kit. CD27 primers were designed by website Primer, and were synthesized by Bioengineering (Shanghai) Co. The primer sequences are as follows, forward: (5'-3'): TGCAGAGCCTTGTCGTTACAG, reverse (5'-3'): GCTCCGGTTTTCGGTAATCCT.
Statistical analysis
Standard tests include the student t-test, the Kluscal-Walli's test, the Wilcoxon rank sum test, and the chi-square test. The Wilcox test could be used to examine the gene expression data. In survival analysis, univariate Cox regression analysis was used to get the HRs and P values. Using Spearman correlation analysis to assess the relationship between CD27 expression and immune scores. The R package is used for all calculations. P <0.05 was judged significant.
Results
mRNA and protein expression of CD27 in pan-cancer
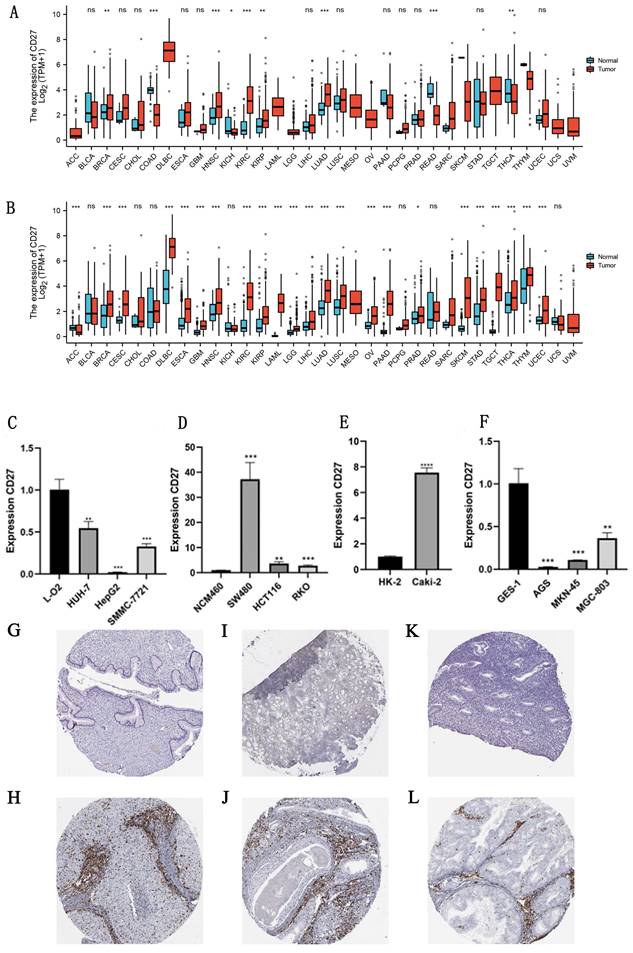
The TCGA and GTEx databases were used to analyze differences of CD27 expression in 33 cancers, the data from these two databases formed a mutually validated comparison. And the absolute expression levels between normal and cancer tissues in TCGA, and TCGA combined with GTEx were displayed separately in Table S1 and Table S2. And the detailed information of the sample was summarized in Table S3. The results showed that CD27 have a significant high expression profile in the vast majority of cancer samples. Such as GBM, HNSC, KIRC, KIRP, BRCA, CESC, DLBC, ESCA, PRAD, SKCM, STAD, TGCT, THYM, LAML, LGG, LIHC, LUSC, LUAD, OV, PAAD, and UCEC. THCA was excluded due to inconsistent expression in both databases. Uniquely, CD27 was significantly low expressed in ACC (Figure 2A, 2B).
Furthermore, we experimentally verified the difference in the mRNA expression of CD27 in some cancer cell lines and normal cell lines. Which showed that CD27 expression was significantly reduced in human liver cancer cell line (HUH-7, HepG2, and SMMC-7721) compared to human normal liver cells line (L-O2) (Figure 2C). Compared with normal colon epithelial cells (NCM460), CD27 was highly expressed in three colon cancer cells lines, SW480, HCT116 and RKO (Figure 2D). And the expression of CD27 in the human renal clear cell cancer cell line (Caki-2) was significantly increased compared with the Human Kidney-2 cell line (HK-2) (Figure 2E). Compared with normal human stomach epithelial cells, CD27 was highly expressed in three stomach cancer cells lines, AGS, MGC-803, and MKN-45 (Figure 2F).
The results of immunohistochemistry showed that CD27 was highly expressed in most cancers compared to normal tissues, which was consistent with mRNA expression profiles in TCGA and GTEx databases. Such as CESC, HNSC and UCEC (Figure 2 G-L). In addition, in the mRNA expression profile, we found a significantly higher expression of CD27 in DLBC that is different from other cancers. This suggests that CD27 may serve as a marker for DLBC.
Multifaceted Prognostic Value of CD27
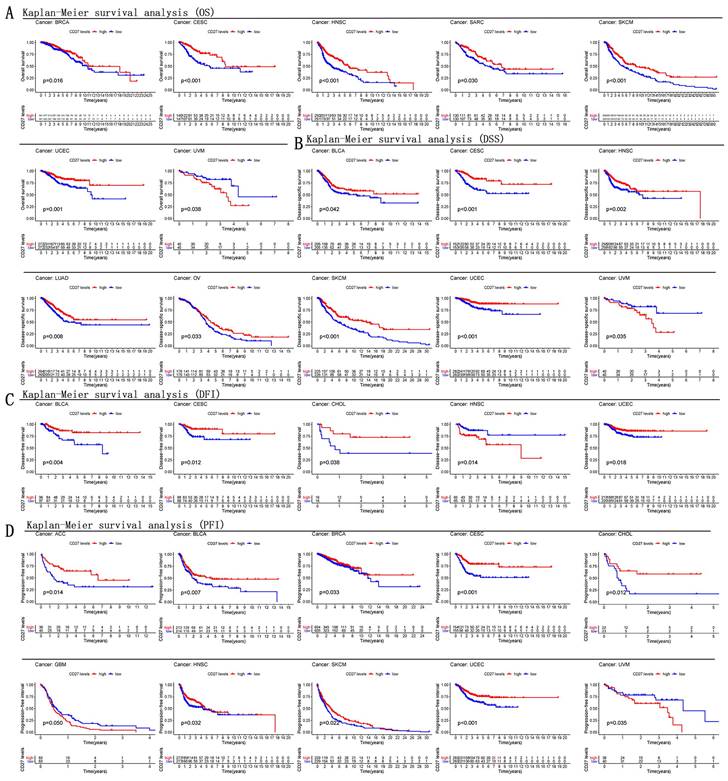
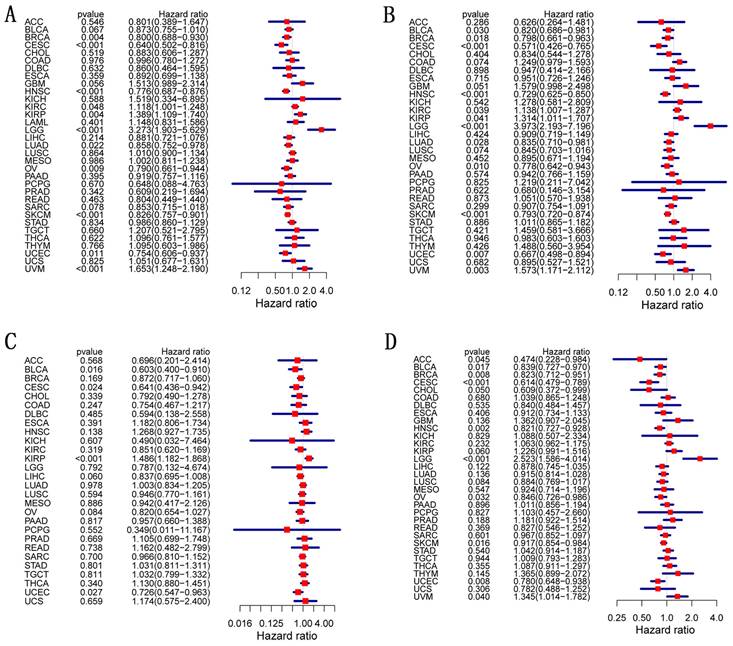
The predictive significance of CD27 for pan-cancer was investigated utilizing several databases. Notably, CD27 expression was linked with prognosis in a several of Kaplan-Meier cumulative curves for cancers in the TCGA database. CD27 was found to play a aggressive role against the prognosis of eleven distinct cancers, which included CESC, HNSC, SKCM, BLCA, CHOL, OV and ACC. Patients with increased CD27 expression fared better than those with low CD27 expression in this study. Contrarily, CD27 played a detrimental role in other cancers, including UVM and GBM, in which patients whose CD27 expression was high had a lower chance of survival than those whose CD27 mRNA levels were low (Figure 3A-D).
We analyzed OS, DSS, DFI, and PFI in pan-cancer to determine the roles CD27 played in the prognosis, furtherly. Using the cox proportional hazards model, the results showed that CD27 expression levels were linked with OS in BRCA, CESC, HNSC, OV, SKCM, UCEC, KIRP, LGG, and UVM. Further, CD27 was a high-risk gene in LGG, KIRP, and UVM (Figure 4A). The analysis of DSS revealed associations between low CD27 expression and poor prognosis in BLCA, BRCA, CESC, LUAD, HNSC, OV, SKCM, and UCEC. however, in patients with KIRC, KIRP, LGG and LIHC, CD27 expression displayed the inverse connection that one would expect with prognosis (Figure 4B). In addition, an examination of the DFI data (Figure 4C) uncovered links between low CD27 expression and a poor prognosis in patients who had BLCA and CESC. However, in patients with KIRP, CD27 expression displayed the inverse connection that one would expect with prognosis (Figure 4C). As for PFI and CD27 expression, findings from forest plots demonstrated relationships between high expression and poor PFI in LGG and UVM (Figure 4D).
Correlation of CD27 Expression and Clinical pathology
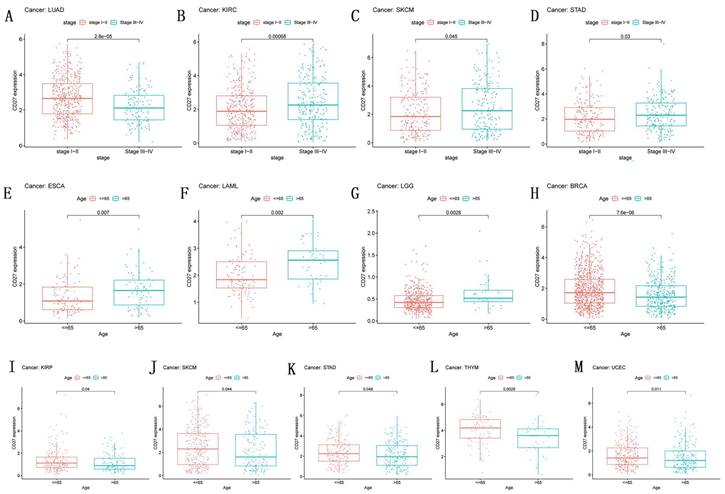
Numerous clinicopathological features of malignancies have been connected to CD27 expression (Figure 5). Next, we looked at how age affected the expression of CD27 in individuals with each type of tumor. We discovered that people under the age of 65 with BRCA, KIRP, SKCM, STAD, THYM, and UCEC (Figure 6H-M) had higher expression levels, while patients after the age of 65 had higher CD27 expression levels with ESCA, LAML, and LGG (Figure 5E-G). Moreover, CD27 was strongly expressed in LUAD patients at stages I and II, but in later stages, its expression was attenuated. Expression of CD27 was greatest in patients with stages III and IV of KIRC, SKCM, and STAD (Figure 5B-D), and least in those with stages I and II.
mRNA and protein expression of CD27. (A) CD27 expression data from TCGA. (B) CD27 expression data from TCGA and GTEx. (C) mRNA expression of CD27 in liver cell lines. (D) mRNA expression of CD27 in colon cell lines. (E) mRNA expression of CD27 in kidney cell lines. (F) mRNA expression of CD27 in gastric cell lines. (G) Immunohistochemical of normal cervix uterus. (H) Immunohistochemical of CESC. (I) Immunohistochemical of normal nasopharynx epithelial tissue. (J) Immunohistochemical of HNSC. (K) Immunohistochemical of normal endometrium. (L) Immunohistochemical of UCEC. *P<0.05, ** P<0.01, and *** P<0.001.
Kaplan-Meier survival curves comparison of high and low expression of CD27 gene for different cancer types. (A) OS of BRCA, CESC, HNSC, SARC, SKCM, UCEC, UVM. (B) DSS of BLCA, CESC, HNSC, LUAD, OV, SKCM, UCEC, UVM. (C) DFI of BLCA, CESC, CHOL, HNSC, UCEC. (D) PFI of ACC, BLCA, BRCA, CESC, CHOL, GBM, HNSC, SKCM, UCEC, UVM.
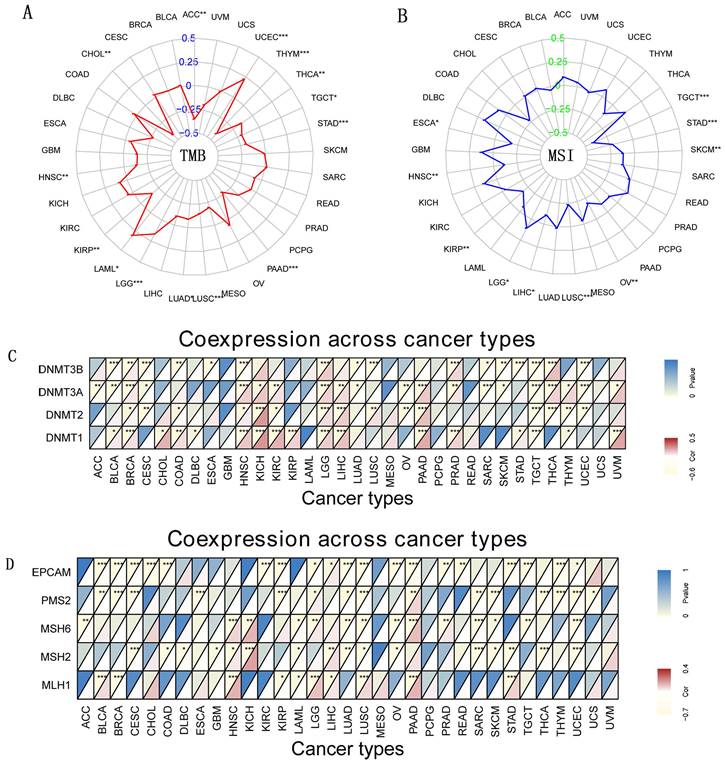
Indicators of gene mutation and DNA Methyltransferase with CD27 Expression
TMB and MSI are newly discovered biomarkers linked to immune checkpoint inhibitor sensitivity [26, 27]. Therefore, it is necessary to look at how TMB or MSI and CD27 expression are related to various cancer types. Our data showed TMB was favorably linked with CD27 expression in three different cancer types, including LAML, LGG, and UCEC. In contrast, in eleven additional cancer types, CD27 expression was inversely related to TMB, which included ACC, CHOL, HNSC, KIRP, LUSC, LUAD, PAAD, STAD, TGCT, THCA, and THYM (Figure 6A). We further examined the possibility of an association between CD27 expression and MSI and we found that MSI was substantially linked with CD27 expression in 10 different kinds of cancer. CD27's expression was correlated favorably with MSI in two subtypes of LGG cancer. CD27 expression was positively correlated with MSI in two cancers, LGG, but was negatively correlated with MSI in the other nine cancers, including ESCA, HNSC, KIRP, LUSC, LIHC, OV, SKCM, STAD, and TGCT (Figure 6B). At least one MMR-related gene was shown to be linked with CD27 expression in 27 of the 33 cancer types studied (Figure 6C). In most malignant tumors, CD27 expression was associated with MLH1 expression (Figure 6D).
Correlation of TME and CD27 Expression
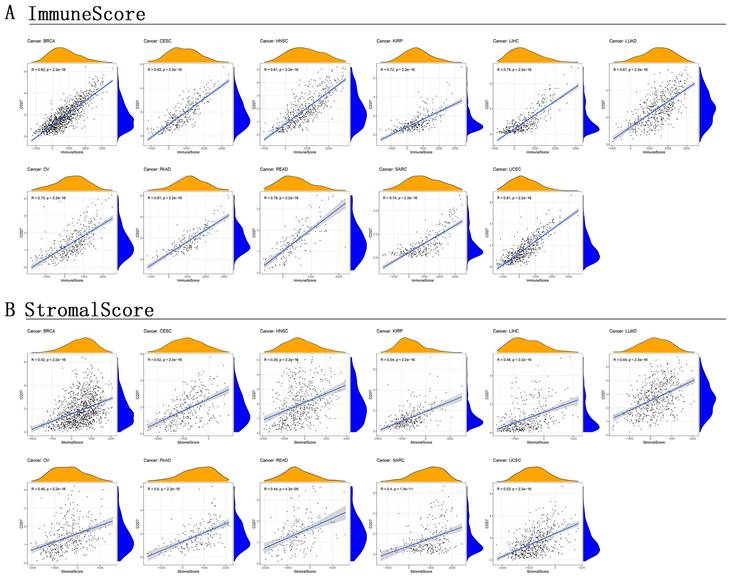
TME is a microenvironment that contains not only tumor cells, immune cells, stromal cells, and other types are all present in TME [28, 29]. It is crucial in promoting cancer cell heterogeneity, which raises treatment resistance and promotes the spread of cancer cells. Further investigation into the link between TME and CD27 expression in various cancer types would be extremely appropriate given that our findings have verified the predictive significance of CD27 in pan-cancer. This evaluation would be particularly important for BRCA, CSEC, KIRP, LUAD, LIHC, OV, PAAD, READ, HNSC, SARC, and UCEC. This allowed us to generate the stromal score and the immune score. In these cancers, CD27 expression was positively correlated with immune (Figure 7A) and stromal (Figure 7B) scores, and this correlation is statistically significant (Figure 7).
Correlation of CD27 mRNA expression with survival in TCGA. (A) OS. (B) DSS. (C) DFI. (D) PFI.
Immune correlations
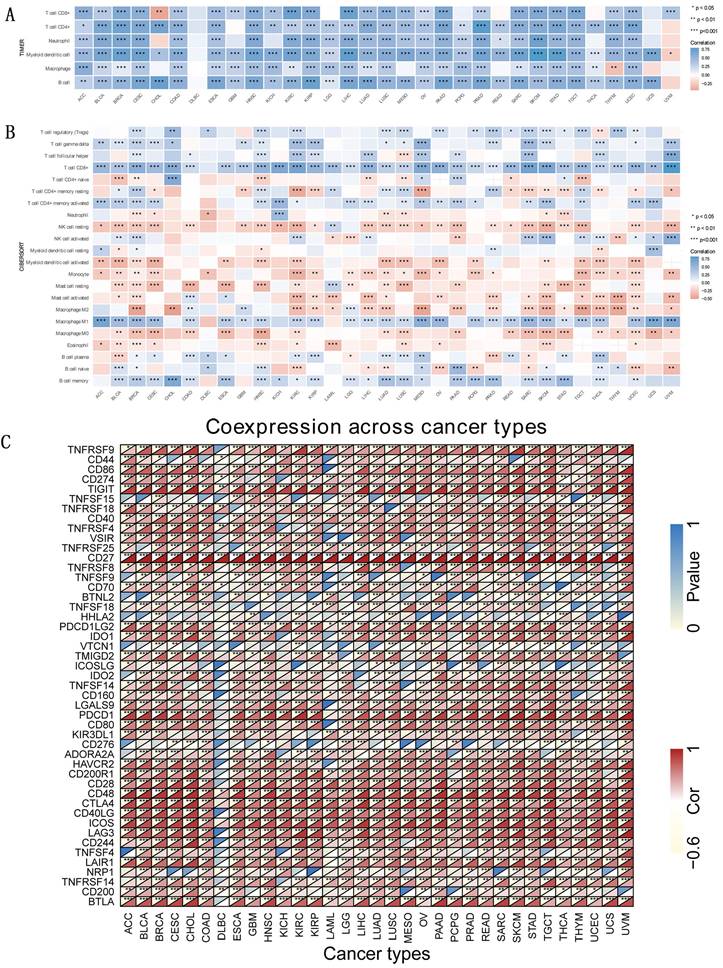
TIICs and their quantity correlated with CD27 expression. As the parameter option for graphing, we went with the “log2” transformation of the expression data (Figure 8A). There are 22 classes of immune infiltrating cells (Figure 8B). To gain a deeper understanding of the underlying mechanism behind the immune suppression of CD27 signaling, we investigated the association between CD27 expression and a variety of immunological checkpoint markers in 33 different cancer types (Figure 8C). CD8+T cells have been shown to be positively correlated with CD27 expression in most tumors. In addition, the high expression of CD27 also had a significant effect on macrophage polarization, mainly in inhibiting macrophage M2 polarization and promoting its M1 polarization. This promotes the presentation of tumor-associated antigens and immune activation. This suggests that the upregulation of CD27 can promote tumor-specific cellular immunity. The positive association between CD27 and TIGIT, CD48 in the majority of cancers suggested a complete co-expressing landscape, and our data demonstrated that CD27 expression was strongly connected with various immunological checkpoints in varied immunocytes and unique T cells.
Drug Sensitivity Analysis of CD27
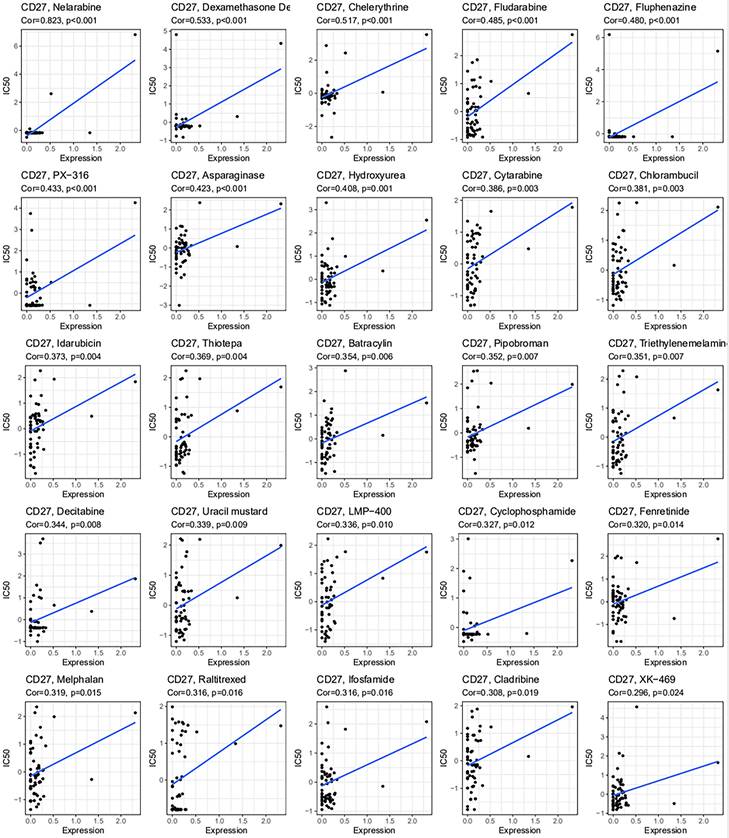
Using the CellMiner-TM database, we looked at the link between CD27 expression and drug sensitivity (Figure 9). Notably, the drug sensitivity was positively linked with CD27 expression.
Functional Analysis by Gene Set Enrichment Analysis
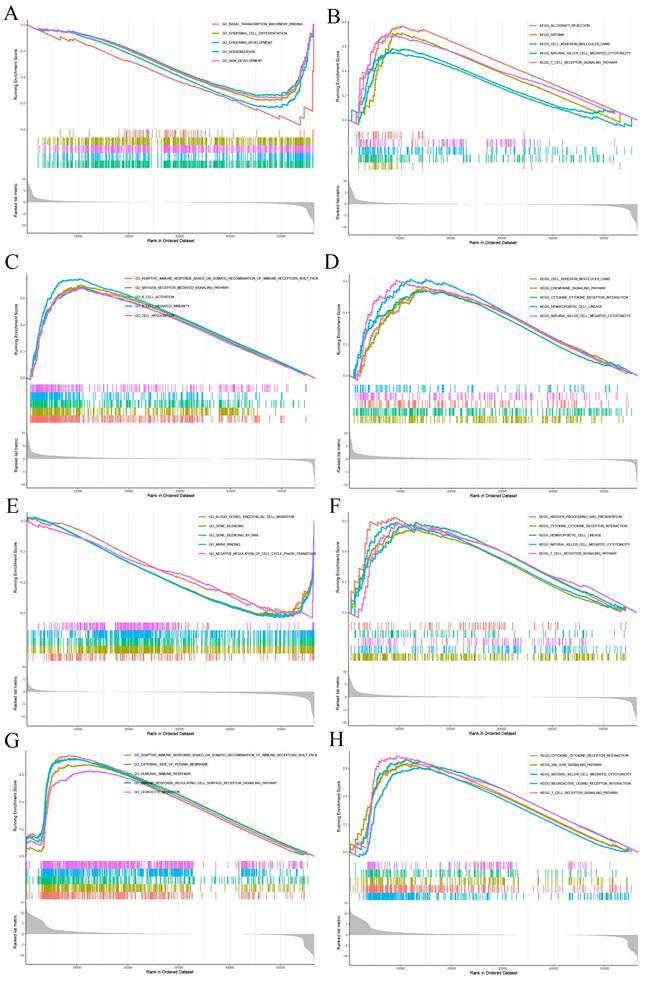
GSEA was applied to study the primary biological function of CD27 in CESC, HNSC, UCEC and UVM. In CESC, high expression of CD27 corresponds to low levels of basal transcription, epithelial development, and epithelial differentiation (Figure 10A). This corresponds to elevated levels of cell adhesion, NK cytotoxicity, and TCR signaling pathways (Figure 10B). In HNSC, highly expressed CD27 corresponds to elevated levels of adaptive immune response pathways based on somatic recombination, antigen receptor-mediated signaling pathways, B cell activation, chemokines, and NK cytotoxicity (Figure 10C-D). In UCEC, high expression of CD27 corresponds to lower vascular endothelial migration, gene silencing, and cell cycle regulation (Figure 10E). However, it corresponds to a higher TCR pathway and NK cytotoxicity (Figure 10F). In UVM, high expression of CD27 corresponds to higher humoral immunity, NK cytotoxicity, JAK-STAT inflammatory signaling pathway, and TCR signaling pathway (Figure 10G-H).
Relationship between CD27 expression and prognosis of SKCM and UVM
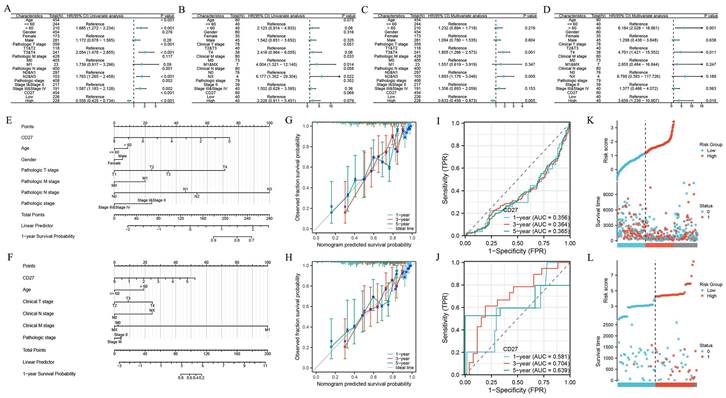
By Cox regression analyses, we analyzed the predictors (the parameters include age, gender, clinical stage, pathologic stage, CD27 expression level and so on). The univariate analysis showed that the factors we included were significantly associated with the OS of SKCM (Figure 11A) and UVM patients (Figure 11B). And these risk factors were further included in multivariate Cox regression (Figure 11C-D). Results showed that CD27 was an independent prognostic factor of SKCM and UVM patients. The clinical features of SKCM (Figure 11E) and UVM (Figure 11F) were integrated into the nomogram mode. We developed time-dependent ROC curves and calibration plots to predicate the probability of 1-year, 3-year, and 5-year OS rate. The AUCs of 1-year, 3-year, and 5-year were 0.356, 0.364, and 0.365 of SKCM patients respectively (Figure 11I). And for UVM patients, the statistics are 0.581, 0.704, 0.639 respectively (Figure 11J). The predicted probability of calibration plots of SKCM (Figure 11G). and UVM (Figure 11H) was consistent with the results observed. We also analysis the correlation between risk score, survival time, and CD27 expression of SKCM (Figure 11K) and UVM (Figure 11L) patients.
Discussion
Cellular immunity against tumors is mainly achieved by initiating the activation of CD8+T cells. Specific receptors that TCR present on the surface of T cells recognize tumor antigens presented on the surface of tumor cells or APC, which require the assistance of MHC I [20, 21]. In addition, the second necessary pathway to activate T cells is co-stimulation of receptors [30], which can result in cellular immune tolerance when in the state of deficiency [31, 32]. Common T cell co-stimulation pathways such as CD28/CD28L axis, PD-1/PD-L1 axis, etc. These co-stimulatory pathways, called immune checkpoints, can be divided into stimulatory and inhibitory [24, 33]. For example, the PD-1/PD-L1 axis is a common inhibitory immune checkpoint pathway. PD-1 or PD-L1 monoclonal antibodies have been used clinically to block inhibitory immune checkpoints to modulate the tumor immune environment [34, 35]. Our research focuses on the CD27, which belongs to the tumor necrosis factor receptor family and regulates TCR signaling as a T cell co-receptor expressed on lymphocytes [12]. And CD70 is the only ligand for CD27[36]. The CD27/CD70 axis is an immune checkpoint pathway that has not been fully studied and there is evidence that the CD27 plays an important role as a stimulatory immune checkpoint in tumor progression and cellular immunity. In a B16 melanoma model, targeting the CD27 with an agonistic antibody resulted in the reduction of growth in lung metastases and subcutaneous tumors [37]. And in preclinical models of several cancers such as LAML and GBM, CD27 targeting antibodies showed great efficacy [38-41]. Therefore, the effects of the CD27 in cancer were comprehensively evaluated by pan-cancer analysis, including regulation of immune invasion, TME, signal transduction, and prognosis. This helps us screen for high-value cancers targeted by the CD27 and provide constructive advice on their treatment.
First, we evaluated the CD27 expression differences in 33 cancers and came to meaningful conclusions. CD27 showed significantly high expression in 21 cancers, the high expression and good prognosis of CD27 in these cancers prove the theory that CD27 acts as a co-stimulatory immune checkpoint in tumor cell immunity. Moreover, our study found that CD27 expression exhibits consistency, which is manifested in common high expression [14]. Therefore, we can infer that the CD27 is initiated in tumor progression and have a positive inhibitory effect on the development of tumors. There are exceptions, the high expression of CD27 in UVM always corresponds to a poor prognosis. We also did not detect significant CD27 changes in UVM, suggesting that the CD27 may be inhibited by certain pathways in UVM and alter its co-stimulatory immune checkpoint role. These show that the CD27 has a dual role in the progression of certain cancers [42]. The study of Ortiz-Cuaran et al. demonstrated that CD27L is highly expressed in non-small cell lung cancer and predicts reduced infiltration of CD8+T cells as well as immune depletion [43, 44]. At the same time, CAR-T therapy targeting CD27L has also become a new role in cancer treatment [45, 46]. A plausible explanation for this is that the CD27/CD27L axis activates the expression of other inhibitory immune checkpoints in long-term co-stimulation and ultimately depletes cellular immunity.
Correlation of CD27 Expression and Clinical pathology. CD27 expression related with the stage in LUAD (A), KIRC (B), SKCM (C), and STAD (D).CD27 expression associated with age in ESCA (E), LAML (F), LGG (G), BRCA (H), KIRP (I), SKCM (J), STAD (K), THYM (L) and UCEC (M).
Indicators of gene mutation and DNA Methyltransferase. (A) The radar chart of the association between TMB and CD27 gene expression. (B) The radar chart of the relationship between MSI and CD27 gene expression (C) DNA methyltransferase. (D) Immune genes. *P < 0.05, **P < 0.01, and ***P < 0.001.
To further explore the role of the CD27 in regulating cancer progression, we analyzed its effect on TIICs. Tumor infiltration of immune cells plays an important regulatory role in tumor progression. APC, mainly DC cells and macrophages. APC recognize tumor-specific receptors and stimulate T cell differentiation and proliferation [47-49]. Our study found that the CD27 is highly correlated with macrophage infiltration and differentiation, which is mainly manifested in the high expression of the axis enhancing the infiltration of M1-polarized macrophages and reducing the infiltration of M2-polarized macrophages. M1 polarization of macrophages significantly inhibits tumor growth, while M2 polarization is often accompanied by immune escape [50-52]. This can reflect the positive effect of the CD27 on tumor antigen presentation. CD8+T cells, as effector cells, are directly involved in the killing of tumor cells. Our study confirms that activation of the CD27 is positively correlated with CD8+T cells in almost all cancers. However, CD27 showed a negatively correlated co-expression landscape with NK cells. In UVM, we found that the expression of CD27 initiates the NK cell activation pathway, which is inconsistent with the landscape in other cancers. This indicates that there may be other pathways in UVM that affect the effect of NK cells [53], resulting in abnormal landscape of TIICs, and corresponding to the poor prognosis reflected by the CD27.
Tumor-specific immunity is not only related to tumor infiltration of immune cells, but also to stromal cells. Stromal cells contain a variety of components and constitute tumor heterogeneity and even immunosuppression [54, 55]. We use immunological scoring to gauge the quantity of invading CD3+/CD45RO+, CD3+/CD8+, or CD8+/CD45RO+ lymphocytes at the tumor's center and borders. A higher Immune Score or Stromal Score represents that the TME has more immune or matrix components. Our findings showed a substantial positive connection between CD27 expression and stromal and immunological scores in these malignancies, showing that the quantity of stromal or immune cells rises concurrently with an increase in CD27 expression levels. Additionally, we can see that immunological scores in the six cancers have a favorable connection with CD27 expression. Additionally, the relationship between immunological check-point markers and CD27 expression suggests that CD27 has a function in controlling tumor immunology in malignancies, particularly in BRCA, PRAD, LUAD, BLCA, and OV. These data collectively point to a potential connection between abnormal CD27 expression and immune infiltration of tumor cells.
Correlation of CD27 expression with stromal score and immune score. (A) Immune score. (B) Stromal score.
Immune correlation. TIICs analysis and correlation analysis of CD27 expression with immune checkpoints in tumors. (A) CD27 expression and immune-associated cell infiltration in the TIMER database. (B) CD27 expression and immune-associated cell infiltration using the CIBERSORT algorithm. (C) The heatmap of the association between CD27 and immunosuppressive genes in TCGA.
Drug sensitivity analysis of CD27. CD27 expression was associated with the sensitivity of nelarabine, dexamethasone, chelerythrine, fludarabine, fluphenazine, PX-316, asparaginase, hydroxyurea, cytarabine, chlorambucil, idarubicin, thiotepa, batracylin, pipobroman, triethylenemelamine, decitabine, uracil mustard, LMP-400, cyclophosphamide, fenretinide, melphalan, raltitrexed, ifosfamide, cladribine, XK-469.
GSEA for samples with high CD27 expression and low expression. (A, C, E and G) GO functional annotation of CD27 in CESC, HNSC, UCEC and UVM. (B, D, F and H) KEGG pathway analysis of CD27 in CESC, HNSC, UCEC and UVM.
Univariate Cox regression analysis of CD27 and other clinicopathological variables in SKCM (A), UVM (B). Multivariate Cox analysis of CD27 and other clinicopathological variables in SKCM (C), UVM (D). Nomogram for 1-year, 3-year and 5-year OS of SKCM (E), UVM (F). Time-dependent ROC curves and AUC values for 1-year, 3-year and 5-year OS prediction of SKCM (G) and UVM (H) patients. Calibration plots for 1-year, 3-year and 5-year OS prediction of SKCM (I) and UVM (J). CD27 expression, risk score and survival time distribution of SKCM (K) and UVM (L) patients.
CD27 regulates multiple signaling pathways to influence cancer progression. We found that CD27 was inversely correlated with multiple tumor-associated signaling pathways in four cancers, CESC, HNSC, UCEC and UVM, which were highly associated with the CD27. These include epidermal structure and differentiation, gene silencing, and cell cycle regulation. These pathways are associated with proto-oncogene activation, tumor heterogeneity, and abnormal cell cycle regulation [56-58]. Therefore, it can be inferred that CD27 can directly inhibit tumor progression by downregulating the relevant pathway. In addition, CD27 synergistically upregulated several immune-related signaling pathways in four cancers, including antigen receptor-mediated signaling pathways, TCR signaling pathways, and chemokine signaling pathways. On the other hand, this proves that the CD27 activates CD8+T cell-mediated cellular immunity and improves cancer prognosis [59, 60]. In particular, CD27 upregulates the JAK-STAT inflammatory pathway in UVM, activating NK cell-mediated cytotoxic pathway [61]. This is related to previous findings that up-regulation to NK cell infiltration in UVM and may lead to UVM-specific adverse prognosis.
The CD27 is expressed in cancer, which is related to different mutation patterns. The DNA damage repair mechanism known as MMRs is made up of several heterodimers [62]. The accumulation of DNA replication mistakes caused by the functional loss of important genes in this pathway increases somatic mutation rates, MSI, and cancer [63, 64]. Through correlation analysis, we discovered in this study that CD27 expression was strongly correlated with the mutation levels of 5 MMR genes in human pan-cancer. Changes in DNA methylation status also play a role in the growth of cancer. According to research, cancer frequently exhibits hypermethylation of the gene promoter. Additionally, we found a significant link between CD27 expression and four methylation-related genes, particularly in the cases of BRCA, HNSC, KICH, LIHC, LGG, PAAD, and TGCT. However, this evidence does not prove whether differential expression of CD27 is related to genetic mutations in MMR. MMR mutations in cancer cells may be the direct cause of CD27 expression and function abnormalities.
Patients with different forms of cancer who were treated with immune checkpoint inhibitors had a higher chance of surviving if they had TMB [65]. In three cancers, including LAML, LGG, and UCEC, CD27 expression was linked favorably with TMB. TMB was negatively correlated with CD27 expression in eleven different cancers. Moreover, CD27 expression was negatively correlated with MSI. And in most tumors the correlation between CD27 expression and immune checkpoint biomarkers was incredibly significant, especially in BRCA, COAD, HNSC, KIRC, KICH, LIHC, PAAD, TGCT, and LUSC. We found that CD27 expression had a positive correlation with the amount of MLH1 genes in most malignancies. Besides, the results showed the prognostic impact of CD27 across different cancers.
However, there are still caveats to our study that need to be addressed, despite the extensive investigation of pan-cancer using numerous databases. As a first step, a large amount of microarray and sequencing data was gathered through the examination of cancer tissue material. Additional research, including utilizing single-cell RNA sequencing data, is needed to resolve this issue. Second, in vivo and in vitro investigations were not included due to the study's emphasis on bioinformatic analysis of CD27 expression and patient survival using multiple databases. To further understand CD27 function in various cancers, more research is needed into its mechanism at the cellular and molecular levels. More mechanistic insights may be gleaned from prospective studies of CD27 expression and immune cell infiltration in different cancer populations in the future.
Conclusion
The CD27 mutated in cancer and appears widely highly expressed and altered tumor immune invasion and stromal invasion by affecting multiple immune-related and inflammation signaling pathways, and upregulated macrophage M1 polarization and CD8+T cell activation and predicted a positive prognosis of cancer. The CD27 plays a significant role in CESC, HNSC, UCEC and UVM, and may be used as a therapeutic target for related cancers.
Abbreviations
K-M: The Kaplan-Meier; HPA: Human Protein Atlas; MSI: microsatellite instability; TMB: tumor mutational burden; DNMTs: DNA methyltransferases; MMR: the associated genes of mismatch repair; MSI: microsatellite instability; TIME: tumor immune microenvironment; ICIs: immune checkpoint inhibitors; TCGA: the Cancer Genome Atlas; CCLE: the Cancer Cell Line Encyclopedia; TCR: T cell reporter; MHCI: major histocompatibility complex class-I; TME: tumor microenvironment; APCs: antigen-presenting cells; GTEx: the Genotype-Tissue Expression; TIICs: tumor-infiltrating immune cells; GSEA: the Gene Set Enrichment Analysis; GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes; FDA: Food and Durg Administration; HNSC: head and neck squamous cell carcinoma; STAD: stomach adenocarcinoma; ESCA: esophageal carcinoma; COAD: colon adenocarcinoma; KIRC: kidney renal clear cell carcinoma; UCEC: uterine corpus endometrial carcinoma; KIRP: kidney renal papillary cell carcinoma; PAAD: pancreatic adenocarcinoma; LUSC: lung squamous cell carcinoma; LUAD: lung adenocarcinoma; LIHC: liver hepatocellular carcinoma; PRAD: prostate adenocarcinoma; BRCA: breast invasive carcinoma; DLBC: lymphoid neoplasm diffuse large B cell lymphoma; THCA: thyroid carcinoma; CESC: cervical squamous cell carcinoma; TGCT: testicular germ cell tumors; SKCM: skin cutaneous melanoma; READ: rectum adenocarcinoma; OV: ovarian serous cystadenocarcinoma; ACC: adrenocortical carcinoma; UCS: uterine carcinosarcoma; CLL: chronic lymphocytic leukemia; SARC: sarcoma; LGG: brain lower grade glioma; THYM: thymoma; BLCA: bladder urothelial carcinoma; DSS: disease-specific survival; PFI: progression-free interval; GBM: glioblastoma multiforme; LAML: acute myeloid leukemia; CHOL: cholangiocarcinoma; PCPG: pheochromocytoma and paraganglioma; TGCT: uveal melanoma; ECM: extracellular matrix.
Supplementary Material
Supplementary tables.
Acknowledgements
The authors thank the Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province and the DaVinci Surgery System Database (DSSD, www.davincisurgerydatabase.com) for their help and support in the methodology and pan-cancer analysis process.
Funding
This work was funded by the School/Hospital of Stomatology Lanzhou University (lzukqky-2022-t09).
Author contributions
Yongfeng Wang, Ling Guan, Yanzong Zhao were the co-first authors. YFW, LG, YZZ, and YLY conceived and designed the study, LG, and YTW revised the manuscript. YFW, YZZ, and SJF conducted relevant experiments and organized experimental data. AQZ, YWL, BTZ and DZZ conducted all data collection and analysis and compiled charts. FYL and WQC provided funding support for this study. All authors read and approved the final manuscript.
Availability of data and materials
The raw data of this study are freely available from the website TCGA ResearchNetwork (https://portal.gdc.cancer.gov/), GTEx (http://commonfund.nih.gov/GTEx/), TIMER database (https://cistrome.shinyapps.io/timer/), Kaplan Meier plotter portal (https://kmplot.com/analysis/), cBioPortal database (http://cbioportal.org), the Gene Set Enrichment Analysis (GSEA) website (https://www.gsea-msigdb.org/gsea/downloads.jsp), the CellMinerTM database (https://discover.nci.nih.gov/cellminer/home.do). and HPA (https://www.proteinatlas.org/). All the analyzed data are included in the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424
2. Tang J, Pearce L, O'Donnell-Tormey J, Hubbard-Lucey VM. Trends in the global immuno-oncology landscape. Nat Rev Drug Discov. 2018;17:922
3. Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M. et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356-87
4. Zhang K, Wang H. [Cancer Genome Atlas Pan-cancer Analysis Project]. Zhongguo Fei Ai Za Zhi. 2015;18:219-23
5. Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20:209-15
6. Jin P, Xiang B, Lin J, Huang G, Zhou WD, Zheng C. et al. [Association of CTLA-4 + 49A/G and CT60 gene polymorphism with type 1 diabetes and thyroid autoimmunity]. Zhonghua Yi Xue Za Zhi. 2009;89:1246-9
7. Soussi T, Wiman KG. TP53: an oncogene in disguise. Cell Death Differ. 2015;22:1239-49
8. Izzi V, Davis MN, Naba A. Pan-Cancer Analysis of the Genomic Alterations and Mutations of the Matrisome. Cancers (Basel). 2020;12:125-32
9. Cui Y, Guo W, Li Y, Shi J, Ma S, Guan F. Pan-cancer analysis identifies ESM1 as a novel oncogene for esophageal cancer. Esophagus. 2021;18:326-38
10. Zhu L, Wu W, Jiang S, Yu S, Yan Y, Wang K. et al. Pan-Cancer Analysis of the Mitophagy-Related Protein PINK1 as a Biomarker for the Immunological and Prognostic Role. Front Oncol. 2020;10:569887
11. Ge Q, Li G, Chen J, Song J, Cai G, He Y. et al. Immunological Role and Prognostic Value of APBB1IP in Pan-Cancer Analysis. J Cancer. 2021;12:595-610
12. Starzer AM, Berghoff AS. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open. 2020;4:e000629
13. van de Ven K, Borst J. Targeting the T-cell co-stimulatory CD27/CD70 pathway in cancer immunotherapy: rationale and potential. Immunotherapy. 2015;7:655-67
14. Wajant H. Therapeutic targeting of CD70 and CD27. Expert Opin Ther Targets. 2016;20:959-73
15. Grant EJ, Nussing S, Sant S, Clemens EB, Kedzierska K. The role of CD27 in anti-viral T-cell immunity. Curr Opin Virol. 2017;22:77-88
16. Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275-81
17. Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557-69
18. Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21:298-312
19. Wik JA, Skalhegg BS. T Cell Metabolism in Infection. Front Immunol. 2022;13:840610
20. Konig R. Interactions between MHC molecules and co-receptors of the TCR. Curr Opin Immunol. 2002;14:75-83
21. Dong D, Zheng L, Lin J, Zhang B, Zhu Y, Li N. et al. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature. 2019;573:546-52
22. Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-42
23. Lucca LE, Dominguez-Villar M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat Rev Immunol. 2020;20:680-93
24. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704
25. Chen G, Luo D, Zhong N, Li D, Zheng J, Liao H. et al. GPC2 Is a Potential Diagnostic, Immunological, and Prognostic Biomarker in Pan-Cancer. Front Immunol. 2022;13:857308
26. Rizzo A, Ricci AD, Brandi G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers (Basel). 2021;13:72-9
27. Zhang L, Li B, Peng Y, Wu F, Li Q, Lin Z. et al. The prognostic value of TMB and the relationship between TMB and immune infiltration in head and neck squamous cell carcinoma: A gene expression-based study. Oral Oncol. 2020;110:104943
28. Bejarano L, Jordao MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021;11:933-59
29. Tiwari A, Trivedi R, Lin SY. Tumor microenvironment: barrier or opportunity towards effective cancer therapy. J Biomed Sci. 2022;29:83
30. Guerder S, Flavell RA. T-cell activation. Two for T. Curr Biol. 1995;5:866-8
31. Tang R, Rangachari M, Kuchroo VK. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin Immunol. 2019;42:101302
32. Perez-Gracia JL, Labiano S, Rodriguez-Ruiz ME, Sanmamed MF, Melero I. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Curr Opin Immunol. 2014;27:89-97
33. Kaempfer R, Arad G, Levy R, Hillman D, Nasie I, Rotfogel Z. CD28: direct and critical receptor for superantigen toxins. Toxins (Basel). 2013;5:1531-42
34. Upadhaya S, Neftelinov ST, Hodge J, Campbell J. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov. 2022;21:482-3
35. Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. 2022;19:37-50
36. Flieswasser T, Van den Eynde A, Van Audenaerde J, De Waele J, Lardon F, Riether C. et al. The CD70-CD27 axis in oncology: the new kids on the block. J Exp Clin Cancer Res. 2022;41:12
37. Pich C, Sarrabayrouse G, Teiti I, Mariame B, Rochaix P, Lamant L. et al. Melanoma-expressed CD70 is involved in invasion and metastasis. Br J Cancer. 2016;114:63-70
38. Riether C, Schurch CM, Buhrer ED, Hinterbrandner M, Huguenin AL, Hoepner S. et al. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J Exp Med. 2017;214:359-80
39. Riether C, Pabst T, Hopner S, Bacher U, Hinterbrandner M, Banz Y. et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat Med. 2020;26:1459-67
40. Ji F, Zhang F, Zhang M, Long K, Xia M, Lu F. et al. Targeting the DNA damage response enhances CD70 CAR-T cell therapy for renal carcinoma by activating the cGAS-STING pathway. J Hematol Oncol. 2021;14:152
41. Jin L, Ge H, Long Y, Yang C, Chang YE, Mu L. et al. CD70, a novel target of CAR T-cell therapy for gliomas. Neuro Oncol. 2018;20:55-65
42. Nie M, Ren W, Ye X, Berglund M, Wang X, Fjorden K. et al. The dual role of CD70 in B-cell lymphomagenesis. Clin Transl Med. 2022;12:e1118
43. Tang XY, Luo ZL, Xiong YL, Yang J, Shi AP, Zheng KF. et al. The Proliferative Role of Immune Checkpoints in Tumors: Double Regulation. Cancers (Basel). 2022;14:703918
44. Ortiz-Cuaran S, Swalduz A, Foy JP, Marteau S, Morel AP, Fauvet F. et al. Epithelial-to-mesenchymal transition promotes immune escape by inducing CD70 in non-small cell lung cancer. Eur J Cancer. 2022;169:106-22
45. Panowski SH, Srinivasan S, Tan N, Tacheva-Grigorova SK, Smith B, Mak YSL. et al. Preclinical Development and Evaluation of Allogeneic CAR T Cells Targeting CD70 for the Treatment of Renal Cell Carcinoma. Cancer Res. 2022;82:2610-24
46. Sauer T, Parikh K, Sharma S, Omer B, Sedloev D, Chen Q. et al. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood. 2021;138:318-30
47. Waisman A, Lukas D, Clausen BE, Yogev N. Dendritic cells as gatekeepers of tolerance. Semin Immunopathol. 2017;39:153-63
48. Verneau J, Sautes-Fridman C, Sun CM. Dendritic cells in the tumor microenvironment: prognostic and theranostic impact. Semin Immunol. 2020;48:101410
49. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369-82
50. Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090
51. Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv Mater. 2020;32:e2002054
52. Mills CD, Lenz LL, Harris RA. A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Res. 2016;76:513-6
53. Sadegh L, Chen PW, Brown JR, Han Z, Niederkorn JY. NKT cells act through third party bone marrow-derived cells to suppress NK cell activity in the liver and exacerbate hepatic melanoma metastases. Int J Cancer. 2015;137:1085-94
54. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-37
55. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174-86
56. Voldborg BR, Damstrup L, Spang-Thomsen M, Poulsen HS. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol. 1997;8:1197-206
57. Lee J, You JH, Kim MS, Roh JL. Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020;37:101697
58. Recasens A, Munoz L. Targeting Cancer Cell Dormancy. Trends Pharmacol Sci. 2019;40:128-41
59. Hwang JR, Byeon Y, Kim D, Park SG. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp Mol Med. 2020;52:750-61
60. Singh A, Ranjan A. Adrenergic receptor signaling regulates the CD40-receptor mediated anti-tumor immunity. Front Immunol. 2023;14:1141712
61. Meng S, Zhu T, Fan Z, Cheng Y, Dong Y, Wang F. et al. Integrated single-cell and transcriptome sequencing analyses develops a metastasis-based risk score system for prognosis and immunotherapy response in uveal melanoma. Front Pharmacol. 2023;14:1138452
62. Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D. et al. Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. J Clin Oncol. 2019;37:286-95
63. Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther. 2018;189:45-62
64. Yamamoto H, Imai K. Microsatellite instability: an update. Arch Toxicol. 2015;89:899-921
65. Dudnik E, Peled N, Nechushtan H, Wollner M, Onn A, Agbarya A. et al. BRAF Mutant Lung Cancer: Programmed Death Ligand 1 Expression, Tumor Mutational Burden, Microsatellite Instability Status, and Response to Immune Check-Point Inhibitors. J Thorac Oncol. 2018;13:1128-37
Author contact
![]() Corresponding authors: 204 Donggang West Road, Lanzhou, Gansu, 730000, China. Institutional email address: caialonteamcom. E-mail addresses: FYLiu0204com (FY. Liu) and chewq20edu.cn (WQ. Che); ORCID: 0000-0001-5857-1744; Fax: 0931-8266957; Tel: 0931-8281975; Postal address: 204 Donggang West Road, Lanzhou, Gansu, 730000, China.
Corresponding authors: 204 Donggang West Road, Lanzhou, Gansu, 730000, China. Institutional email address: caialonteamcom. E-mail addresses: FYLiu0204com (FY. Liu) and chewq20edu.cn (WQ. Che); ORCID: 0000-0001-5857-1744; Fax: 0931-8266957; Tel: 0931-8281975; Postal address: 204 Donggang West Road, Lanzhou, Gansu, 730000, China.

 Global reach, higher impact
Global reach, higher impact