3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2024; 15(4):880-888. doi:10.7150/jca.90494 This issue Cite
Research Paper
Ablation for Single Pulmonary Nodules, Primary or Metastatic. Εndobronchial Ablation Systems or Percutaneous
1. Pulmonary Department, General Clinic Euromedica, Thessaloniki, Greece.
2. Oncology Department, University General Hospital of Larissa, Greece.
3. Oncology Department, General Hospital of Volos, Greece.
4. Oncology Department, General Hospital of Kavala, Greece.
5. Oncology Department, General Hospital of Rhodes, Greece.
6. Surgery Department, Genisis Private Clinic, Thessaloniki, Greece.
7. Med IV IP, Lung Centre Bamberg Clinics aff. Univ.- of Erlangen, Buger Str. 96049 Bamberg Germany.
8. Department of Respiratory and Critical Care Medicine, Changhai Hospital, Navy Military Medical University, Shanghai, 200433, China.
9. Surgery Department, Democritus University of Thrace, Alexandroupolis, Greece.
10. Department of Pathology, University of Cyprus, Cyprus.
11. 3rd University Surgery Department, ``AHEPA`` University Hospital, Thessaloniki, Greece.
12. Third Department of Obstetrics and Gynecology, University General Hospital “ATTIKON”, Medical School of the National and Kapodistrian University of Athens, Athens, Greece.
13. Pulmonary Department, G. Papanikolaou General Hospital, Aristotle University of Thessaloniki, Greece.
Received 2023-11-1; Accepted 2023-11-1; Published 2024-1-1
Abstract
Single pulmonary nodules are a difficult to diagnose imagining artifact. Currently novel diagnostic tools such as Radial-EBUS with or not C-ARM flouroscopy, electromagnetic navigation systems, robotic bronchoscopy and cone beam-compuer tomography (CBCT) can assist in the optimal guidance of biopsy equipment. After diagnosis of lung cancer or metastatic disease as pulmonary nodule, then surgery or ablation methods as local treatment can be applied. The percutaneous ablation systems under computed tomography guidance with radiofrequency, microwave, cryo and thermosphere have been used for several years. In the past 10 years extensive research has been made for endobronchial ablation systems and methods. We will present and comment on the two different ablation methods and present up to date data.
Keywords: Lung Cancer, NSCLC, ablation, radial-ebus, CBCT, Monarch, Archimedes, Covidien, Medronic, biopsy, ROSE, microwave, radiofrequency, thermopshere, cryo, Cone beam computed tomography
Introduction
Lung cancer is usually diagnosed at advanced inoperable stage. A very important reason is the lack of early disease symptoms and lack of blood tests as we have in other cancer types such as breast cancer, prostate cancer and gastrointestinal cancer. Very few patients will present hemoptysis in early stage disease due to endobronchial disease and will search for medical evaluation. Moreover; most patients since they are smokers will attribute their progressive dyspnea to their smoking habit and chronic obstructive disease (COPD) and will seek for medical attention when their clinical status is very severe. There are also cases where pulmonary nodules are observed during re-staging for other cancers types such as; breast cancer, gastrointestinal or prostate cancer. In the past 10 years there was a bloom regarding navigation technologies for single pulmonary nodules. Radial-endobronchial ultrasound has been used with or without C-ARM fluoroscopy for guidance assistance [1]. Electromagnetic technologies with Medronic and virtual bronchoscopy navigation with Archimedes are also in the market in the past 10 years with minor differences between them [2-4]. C-Arm fluoroscopy can be also used when performing electromagnetic guidance. CBCT can be used with another realtime method [5, 6]. Rapid on site evaluation (ROSE) is a method where cytology samples obtained from the biopsy procedure can identify whether there is cancer in the nodule and whether the sample is enough for further investigation for immunohistochemistry and gene expression [7]. Robotic bronchoscopy has been introduced in 2018 with Ion and Monarch platforms [8, 9]. Ablation as a local treatment has been introduced in the past 20 years and different generators and methods are used such as the radiofrequency, microwave, thermosphere and cryo. Percutaneous and surgical probes are also available based on the location of the lesion [10-13]. The usual side effects are pneumothorax, hemoptysis or even hempthorax. A recent meta-analysis presented data where cryo ablation is superior to radiofrequency ablation, but not microwave ablation [12]. In the past 10 years new endobronchial ablation systems have been introduced in the market such as the Bronchus which is a radiofrequency catheter guided by Archimedes electromagnetic navigation systems and NEUWAVETM FLEX Microwave Ablation System guided by the MONARCH® Platform by Ethicon. There are certain advantages for each methodology that will be discussed in the section that follow.
Materials and Methods
We made a thorough search of the literature on pub med for new publications using strictly the following key words: radiofrequency ablation systems for lung cancer, microwave ablation systems for lung cancer, cryo ablation systems for lung cancer, thermosphere ablation systems for lung cancer, endobronchial ablation systems for lung cancer. We identified 35 publications, including case reports and written in all languages and used the data from 28. We focused our manuscript strictly on these data presenting up to date data.
Percutaneous ablation systems
Radiofrequency ablation with percutaneous probes for lung cancer has been well established since 2000 and there are numerous studies with the effectiveness of this technique [14]. The next equipment to be validated for lung cancer was microwave generators [11]. There are differences between the radiofrequency and the microwave effect, and methodology. Microwave technology was observed to be more efficient than radiofrequency [15]. Currently the thermal effect of microwave application has been enhanced by adding transbronchial thermal gel [16]. Moreover; thermosphere technology combined with microwave ablation has increased the efficiency of the method [13]. Cryo ablation is the latest technology on the field of local percutaneous treatment under computed tomography guidance [17]. The effect of the cryo ablation is superior to radiofrequency, however; it is not superior to microwave ablation [12].
Covidien microwave generator (Photo provided by Paul Zarogoulidis).

Navigation for endobronchial ablation systems
The main issue with single pulmonary nodules is the diagnosis. There are 6 distinct reasons to go early for small malignant nodules: a) The lower the T the better the survival (weak relation) acc. to TNM V8 and the lower the probability of N1 and yet the probability of local relapse, b) The smaller the nodule even in the same T-descriptor (e.g. Stage I) the better the long term survival, c) The smaller the nodule the lesser the intratumoral heterogeneity and the mutational probability which is an independent risk factor of relapse, d) No / less reduction of postinterventional lung function loss after minimal invasive approach should pave the way to more options in possible return of cancer and e) The smaller the nodule the better is add-on surgery / SBRT / transthoracic ablation (The KISS) or drug treatment like EPR, ITC and TBNI if not possible ot treat endobronchially completely.
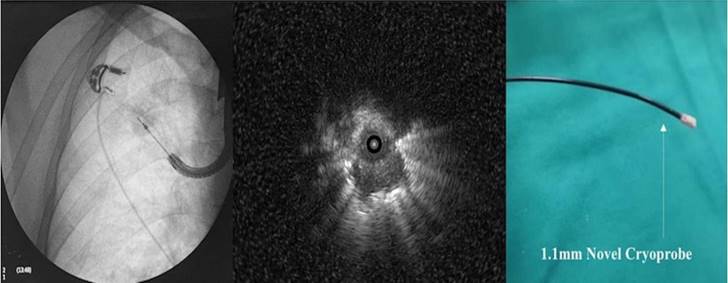
We currently use radial-EBUS with or without C-ARM fluoroscopy or in combination with other navigation systems [1]. The main biopsy tools are cytology brush, mini forceps, fine needle aspiration (22G) and thin cryo probes (Figure 2). We have even the capability of making tunnels through the lung parenchyma with needles and balloon dilation and obtain sample [18, 19]. This method is safe and effective when navigation systems are used such as the Archimedes Virtual Bronchoscopy Navigation (VBN) (Figure 3), IllumisiteTM platform from Medronic [20]. Another novel navigation technique is the robotic assisted navigation with the Monarch® Platform (Auris Health, Inc., Redwood City, CA) [8]. Other combination techniques and equipment is currently available that can be used with radial-ebus such as the Cios Spin® by Siemens Healthineers[21], O-armTM O2 imaging system by Medtronic and Super DimensionTM (Covidien, Plymouth, MN, USA) [22, 23]. Cone-beam CT apparatus (ARTIS zeego; Siemens Healthcare GmbH, Erlangen, Germany) is another equipment-technique that can be also used [24] (Figure 4,5). Several studies have been made with all these equipment, however; higher diagnostic efficiency was observed for those studies with nodules ≥30mm.
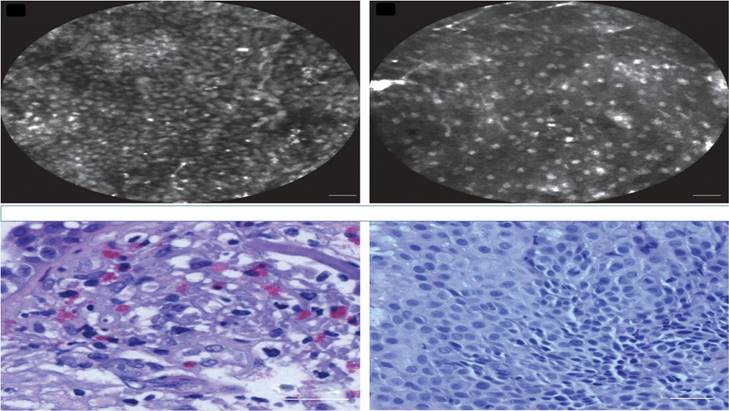
A recent paper has shown in a meta-analysis that CBCT is as stand-alone as well in combination with other technologies superior to all other technologies stand-alone or in combination. It is as well cost-effective in regards to QUALYS in the Dutch Healthcare System versus transthoracical approach [25, 26]. Rapid on-site evaluation was used to increase the diagnostic yield in some studies [27], or confocal laser endomicroscopy [28] (Figure 6,7).
Endobronchial ablation systems
Radiofrequency ablation systems are already in the market by Brochus and there are several studies [29, 30]. A microwave catheter (EmprintTM ablation catheter with the ThermosphereTM technology, Covidien, Plymouth, MN, USA) is already on the market. Flexible water-cooled MWA antenna (Vison-China Medical Devices R&D Center) connected to a microwave platform (Surblate, Vison) and an MWA device for ablation by Nanjing Nisionmedic (Nanjing, China) [31-34]. The first-in-the-world ENB microwave ablation using the IllumisiteTM fluoroscopic navigation platform was successfully performed in mid-2022 [35].
Left; Flouroscopic guidance of cryo probe 1.1mm, Middle; radial ebus image from the pulmonary nodule, right; cryoprobe 1.1mm.
New methods for ablation
Cryo ablation probes are available for percutaneous application but, not for transbronchial application, although one could use in certain cases the available probes from ERBE II system [36]. Robotic assisted guided cryobiopsy has been presented in a previous study and it could be the platform for future application of a cryoprobe system [37]. There have been pilot studies evaluating in vitro a novel transbronchial cryo probe [38, 39]. However; we still need human studies. Bronchoscopic thermal vapor ablation (BTVA) by Bronchus is available since 2021 for emphysema treatment, recently a study demonstrated efficiency when this technique is used as an ablation tool [40, 41]. Further studies are required to explore and improve on it before it can be reliably used for cancer treatment. Until now microwave ablation systems are available for percutaneous usage, however; there are systems being tested for transbronchial treatment in animal models [42]. Pulsed electric field (PEF) is a non-thermal ablative modality that uses a short-living strong electrical field created around a catheter to create microscopic pores in cell membranes (electroporation). Finally, a device with radiofrequency ablation catheter has been investigated which is compatible with endobronchial ultrasound [43].
Archemedes, Bronchus Diagnostic catheter (photo provided by Paul Zarogoulidis).

C-Arm fluoroscopic guidance for radial ebus.
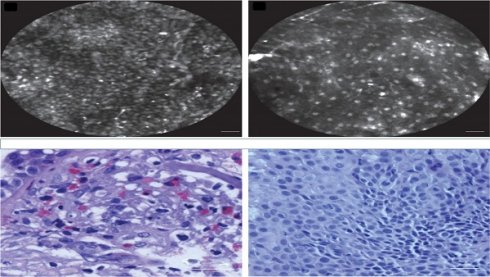
The use of rapid on-site evaluation (ROSE)
Due to the increasing rate of computed tomography scans based on the new screening guidelines for lung cancer, more and more patients are diagnosed with pulmonary nodules. It is absolutely necessary to identify whether we have or not malignancy. We can use positron emission tomography scan (PET-CT) as an initial examination, however, there is still a diagnostic issue correlated with the size of the nodules. In the case of nodules ≤1.2cm and with a low metabolic rate ki-67≤10% the technique cannot provide accurate data even with a second delayed reexamination after 30minutes [44]. Therefore biopsy is absolutely necessary in the case of a PET-CT examination with low metabolic rate ≤3SUV. Novel diagnostic techniques can provide adequate navigation and diagnostic yield for pulmonary nodules ≤1.2cm. However; the sample will mostly be cytologic, since a cytology brush or needle will be used. We can use of course where possible biopsy forceps or small cryo-probes 1.1mm in order to obtain tissue. Again the sample will be small is quantity, but not in quality. We can verify the malignancy with rapid on-site evaluation and the quality of the sample (whether we can perform additional immunohistochemical examinations). After biopsy we need approximately 2-5 minutes to prepare the sample for evaluation in the microscope. We perform evaluation of at least two samples from the same site [45].
Cios Spin Siemens.
Rapid on site evaluation from convex endobronchial ultrasound with 22G fine needle aspiration biopsy (Mediglobe needle).
Confocal laser endomicroscopy, upper row confocal image, lower row cytology image. Left; squamous cell carcinoma, Right; adenocarcinoma. Cellvizio Mauna kea technologies.
Discussion
Currently we propose a screening methodology to smokers, ex-smokers or people of high risk for lung cancer [46]. Therefore, we have more patients diagnosed with pulmonary nodules. In the past ten years we have had both a bloom in novel diagnostic techniques for pulmonary nodules and treatment for advanced stage lung cancer. The radial-ebus along with fluoroscopic techniques such as C-ARM, DYNA-CT, O-ARM and Cios-Spin have achieved increased navigation and diagnostic yield. Electromagnetic platforms such the Archemedes®, IllumisiteTM, and Veran's SPiN Thoracic Navigation System platform have increased even more our navigation and diagnostic yield in pulmonary nodules ≤30mm. We have now the capability to identify the location of vessels during our diagnostic procedure. Robotic assisted bronchoscopy has been also introduced in 2018 with Monarch and Ion platforms, each one with characteristics. Both systems have equal navigation and diagnostic results. Along to all these navigation systems we can use additional methods of rapid on-site evaluation to evaluate our samples and complete a diagnostic sequence earlier. The rapid on-site evaluation has been established more than 5 years and requires a learning curve from the bronchoscopy operator, or a cytologist can be used at the site of diagnosis. Confocal laser endomicroscopy can be also used as a rapid on-site tool, again a learning curve is required, or a cytologist on site. Since we have the ability to diagnose early stage disease, lung cancer or metastatic disease, we can use advanced systems for minimal local therapy. Local treatment for malignant pulmonary nodules either primary lung cancer or metastatic is efficient either percutaneously under computed tomography guidance with radiofrequency, microwave or cryo-ablation systems. Currently there are many studies presenting the efficiency and adverse effects of these systems and methods. The indications are also very specific and well known. There are several other treatment modalities such as stereotactic body radiotherapy or radiotherapy with or without systematic administration of drugs [47] [48]. These treatments have a different application and results from local ablation, it remains for the treating physician to choose the best method of local disease control best on the clinical features of the patient. Radiotherapies are known to have adverse effects such pneumonitis or esophagitis. Moreover; application of this treatment modality is excluded when vessels are near the lesion. Local percutaneous systems have as major adverse effect pneumothorax and hemothorax. The pneumothorax if less than 1lt can be treated on site with a pneumocatheter, if more than 1lt then with a bullau insertion, however; several days of hospitilisation will be necessary. Indeed, due to severe emphysema, the application of percutaneous ablation might not be possible. Transbronchial ablation is an option now more than ever since we have efficient navigation. We have tools to minimize hemorrhage by spraying polymer dust to stop bleeding or in the case of fistulas we can use stents or even emphysema valves to block a sublobar segment. A balloon dilation system can also be applied to block the hemorrhage. The balloon blockers can stay inside a patient for up to 7 days if necessary. Moreover; we can eliminate most malignant pulmonary nodules as a `One Stop Shop`, since we have rapid on-site diagnosis available. Positron emission computed tomography (PET-CT) can provide staging for patients and possible biopsy sites. However; again for small pulmonary nodules ≤1.2cm again for those lesions with low metabolic rate ki-67 ≤10% we need again biopsy.
In the case where a pulmonary nodule is more than ≥3cm then adjuvant systematic therapy should be used. In specific for these patients with non-small cell lung cancer (NSCLC) we have to identify the subtype adenocarcinoma, squamous cell carcinoma or where this is not possible non-other specific (NOS). First line targeted treatment with tyrosine kinase inhibitors (TKIs) are based on the expression of epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) and proto-oncogene 1 (ROS-1) [49, 50]. Regarding EGFR positive patients, mutations were observed such as T790M and therefore second line tyrosine kinase inhibitors (osimertinib) were produced, which are now administered as first line [51]. However; again resistance to osimertinib was observed, and in this case another inhibitor (of the MET pathway) capmatinib was observed to overcome this resistance by decreasing the generation of cancer-associated fibroblasts. Capmatinib suppresses the MET/akt/snail signaling pathway [52]. Moreover; v-Raf-murine-sarcoma-viral-oncogene-homolog B (BRAF V600E), neurotrophic-tyrosine-receptor-kinase (NTRK 1/2/3) gene-fusion, mesenchymal-epithelial-transition exon14 skipping (MET) and re-arranged-during-transfection (RET) rearrangement are also novel gene expressions targeted [53]. Regarding immunotherapy we investigate the programmed death-ligand 1 (PD-L1) expression in order to administer immunotherapy alone (PD-L1≥50%) or in combination with chemotherapy (PD-L1≤49%) [54]. In the recent years based on preclinical and clinical trials other mutations such Kirsten rat sarcoma (KRAS), amplification of human epidermal growth factor receptor-2 (HER2), and other genotypes of the driver genes, have been thought highly targetable [55]. We use next-generation sequencing (NGS) panel to identify gene expressions [49]. Tissue biopsies remain the gold standard for gene expression identification, however; in the case where this is not possible, we can use liquid biopsies which can detect circulating tumor DNA (ctDNA) [56].
We propose that the endobronchial-transbronchial ablation is mainly used inside the blue areas, while the percutaneous in the black areas (yellow arrows). In order to choose the appropriate ablation system the following information should be considered: a) center experience, b) cost-effectiveness, c) site of lesion (surrounding vessels or bronchus), d) size of lesion, e) available navigation equipment, f) ablation effectiveness for lung lesions and g) number of lesions. Also, an additional insight should be the origin of the lesion, kidney cancer needs more ablation time than squamous cell lung carcinoma. Finally, the center should be able to handle all adverse effects, such hemothorax, pneumothorax, fistulas, respiratory distress and must have intensive care unit.
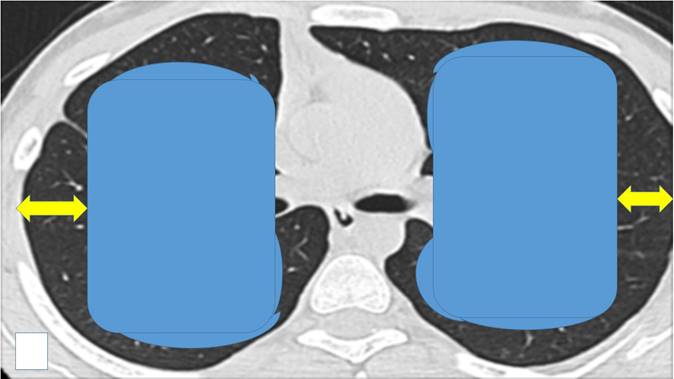
There have been numerous studies where ablation systems have been used along with the administration of local or systematic drugs [57-59]. We already have sufficient data that demonstrate the synergistic effect of ablation systems with the co-administration of drugs and this will be the future direction for research in the field of local treatment [15, 60-62]. Moreover; our group has evaluated a radiofrequency ablation catheter for vessels from Covidien in order to treat small malignant pulmonary nodules [63, 64]. This catheter with minor adjustments such as the addition of a spick tip can be better inserted in pulmonary lesion. The effect of the transbronchial ablation can be identified with radial ebus before and after the ablation effect and possibly by using an additional software such as elastography. Compared to other forms of thermal energy, microwave ablation is the most promising one based on the currently available early to midterm results. We present in our final figure our proposal for transbronchial ablation candidates versus percutaneous ablation based on the site of the pulmonary nodules (Figure 8).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zarogoulidis P, Huang H, Chen W, Petridis D, Matthaios D, Hohenforst-Schmidt W. et al. Radial Endobronchial Ultrasound for Lung Cancer Diagnosis: Tips and Tricks. Journal of Cancer. 2022;13:1307-12
2. Yang H, Turgon E, Pan Y, Wen X, Zhang X, Shen Y. et al. Pilot study of archimedes virtual bronchoscopic navigation system-guided biopsy to diagnose lung nodules in children. Frontiers in pediatrics. 2022;10:1053289
3. Chan JWY, Chang ATC, Siu ICH, Lau RWH, Ng CSH. Electromagnetic navigation bronchoscopy transbronchial lung nodule ablation with Illumisite(TM) platform corrects CT-to-body divergence with tomosynthesis and improves ablation workflow: a case report. AME case reports. 2023;7:13
4. Ho E, Cho RJ, Keenan JC, Murgu S. The Feasibility of Using the "Artery Sign" for Pre-Procedural Planning in Navigational Bronchoscopy for Parenchymal Pulmonary Lesion Sampling. Diagnostics. 2022 12:(12):3059
5. Levine MZ, Goodman S, Lentz RJ, Maldonado F, Rickman OB, Katsis J. Advanced Bronchoscopic Technologies for Biopsy of the Pulmonary Nodule: A 2021 Review. Diagnostics. 2021;11(12):2304
6. Kramer T, Annema JT. Advanced bronchoscopic techniques for the diagnosis and treatment of peripheral lung cancer. Lung cancer. 2021;161:152-62
7. Pradeep I, Rath A, Nigam JS. Adoption of Rapid On-Site Evaluation in the Routine Evaluation of Lung Cytology Specimens. Acta cytologica. 2023;67:451-2
8. Khan F, Seaman J, Hunter TD, Ribeiro D, Laxmanan B, Kalsekar I. et al. Diagnostic outcomes of robotic-assisted bronchoscopy for pulmonary lesions in a real-world multicenter community setting. BMC pulmonary medicine. 2023;23:161
9. Jiang J, Chang SH, Kent AJ, Geraci TC, Cerfolio RJ. Current Novel Advances in Bronchoscopy. Frontiers in surgery. 2020;7:596925
10. Saenger JA, Tahir I, Fodinger M, Cote GM, Muniappan A, Fintelmann FJ. Multimodality local ablative therapy of 23 lung metastases with surgical resection and percutaneous cryoablation in a patient with Li-Fraumeni Syndrome: A case report. Radiology case reports. 2023;18:3586-91
11. Wang J, Li B, Zhang L, Wang Z, Shen J. Safety and local efficacy of computed tomography-guided microwave ablation for treating early-stage non-small cell lung cancer adjacent to bronchovascular bundles. European radiology. 2023 Jul 28. Online ahead of print
12. Xu Z, Wang X, Ke H, Lyu G. Cryoablation is superior to radiofrequency ablation for the treatment of non-small cell lung cancer: A meta-analysis. Cryobiology. 2023;112:104560
13. Reisenauer JS, Eiken PW, Callstrom MR, Johnson GB, Pierson K, Lechtenberg B. et al. A prospective trial of CT-guided percutaneous microwave ablation for lung tumors. Journal of thoracic disease. 2022;14:939-51
14. Botsa E, Thanou I, Tavernaraki K, Thanos L. Ablation techniques in non-small cell lung cancer patients: experience of a single center. Hippokratia. 2022;26:105-9
15. Xu F, Song J, Lu Y, Wang J, Wang J, Xiao H. et al. Clinical efficacy of systemic chemotherapy combined with radiofrequency ablation and microwave ablation for lung cancer: a comparative study. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2021;38:900-6
16. Maxwell AWP, Park WKC, Baird GL, Martin DW, Lombardo KA, Dupuy DE. Effects of a Thermal Accelerant Gel on Microwave Ablation Zone Volumes in Lung: A Porcine Study. Radiology. 2019;291:504-10
17. Mahnken AH, Konig AM, Figiel JH. Current Technique and Application of Percutaneous Cryotherapy. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2018;190:836-46
18. Harzheim D, Sterman D, Shah PL, Eberhardt R, Herth FJ. Bronchoscopic Transparenchymal Nodule Access: Feasibility and Safety in an Endoscopic Unit. Respiration; international review of thoracic diseases. 2016;91:302-6
19. Herth FJ, Eberhardt R, Sterman D, Silvestri GA, Hoffmann H, Shah PL. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax. 2015;70:326-32
20. Chan JWY, Siu ICH, Chang ATC, Li MSC, Lau RWH, Mok TSK. et al. Transbronchial Techniques for Lung Cancer Treatment: Where Are We Now? Cancers. 2023 15
21. Cios Spin®by Siemens Healthineers.; 2022 URL: https://www.siemens-healthineers.com/en-my/news-and-events/events-malaysia/sicot-2022
22. Cho RJ, Senitko M, Wong J, Dincer EH, Khosravi H, Abraham GE 3rd. Feasibility of Using the O-Arm Imaging System During ENB-rEBUS-guided Peripheral Lung Biopsy: A Dual-center Experience. Journal of bronchology & interventional pulmonology. 2021;28:248-54
23. Cicenia J, Avasarala SK, Gildea TR. Navigational bronchoscopy: a guide through history, current use, and developing technology. Journal of thoracic disease. 2020;12:3263-71
24. Moreschini F, Colasanti GB, Cataldi C, Mannelli L, Mondanelli N, Giannotti S. Pre-Operative CT-Based Planning Integrated with Intra-Operative Navigation in Reverse Shoulder Arthroplasty: Data Acquisition and Analysis Protocol, and Preliminary Results of Navigated Versus Conventional Surgery. Dose-response: a publication of International Hormesis Society. 2020;18:1559325820970832
25. Kops SEP, Heus P, Korevaar DA, Damen JAA, Idema DL, Verhoeven RLJ. et al. Diagnostic yield and safety of navigation bronchoscopy: A systematic review and meta-analysis. Lung cancer. 2023;180:107196
26. Gex G, Pralong JA, Combescure C, Seijo L, Rochat T, Soccal PM. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration; international review of thoracic diseases. 2014;87:165-76
27. Balbo PE, Bodini BD, Patrucco F, Della Corte F, Zanaboni S, Bagnati P. et al. Electromagnetic navigation bronchoscopy and rapid on site evaluation added to fluoroscopy-guided assisted bronchoscopy and rapid on site evaluation: improved yield in pulmonary nodules. Minerva chirurgica. 2013;68:579-85
28. Hassan T, Thiberville L, Hermant C, Lachkar S, Piton N, Guisier F. et al. Assessing the feasibility of confocal laser endomicroscopy in solitary pulmonary nodules for different part of the lungs, using either 0.6 or 1.4 mm probes. PloS one. 2017;12:e0189846
29. Tanabe T, Koizumi T, Tsushima K, Ito M, Kanda S, Kobayashi T. et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest. 2010;137:890-7
30. Koizumi T, Tsushima K, Tanabe T, Agatsuma T, Yokoyama T, Ito M. et al. Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration; international review of thoracic diseases. 2015;90:47-55
31. Mak KL, Chan JWY, Lau RWH, Ng CSH. Management of bronchopleural fistula with endobronchial valve in hybrid operating room following transbronchial microwave ablation. Interactive cardiovascular and thoracic surgery. 2021;33:992-4
32. Xie F, Chen J, Jiang Y, Sun J, Hogarth DK, Herth FJF. Microwave ablation via a flexible catheter for the treatment of nonsurgical peripheral lung cancer: A pilot study. Thoracic cancer. 2022;13:1014-20
33. Bao F, Yu F, Wang R, Chen C, Zhang Y, Lin B. et al. Electromagnetic bronchoscopy guided microwave ablation for early stage lung cancer presenting as ground glass nodule. Translational lung cancer research. 2021;10:3759-70
34. Zeng C, Fu X, Yuan Z, Hu S, Wang X, Ping W. et al. Application of electromagnetic navigation bronchoscopy-guided microwave ablation in multiple pulmonary nodules: a single-centre study. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2022;62(4):ezac071
35. Medtronic News—Business & Regional News. 2022: URL: https://news.medtronic.com/Business-News
36. Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. Journal of vascular and interventional radiology: JVIR. 2010;21:S187-91
37. Oberg CL, Lau RP, Folch EE, He T, Ronaghi R, Susanto I. et al. Novel Robotic-Assisted Cryobiopsy for Peripheral Pulmonary Lesions. Lung. 2022;200:737-45
38. Hammer D, Budi L, Nagy A, Varga R, Horvath P. Evaluation of a transbronchial cryoprobe for the ablation of pulmonary nodules: an in vitro pilot study. BMC pulmonary medicine. 2023;23:71
39. Hetzel J, Linzenbold W, Boesmueller H, Enderle M, Poletti V. Evaluation of Efficacy of a New Cryoprobe for Transbronchial Cryobiopsy: A Randomized, Controlled in vivo Animal Study. Respiration; international review of thoracic diseases. 2020;99:248-56
40. Harris K, Puchalski J, Sterman D. Recent Advances in Bronchoscopic Treatment of Peripheral Lung Cancers. Chest. 2017;151:674-85
41. Steinfort DP, Christie M, Antippa P, Rangamuwa K, Padera R, Muller MR. et al. Bronchoscopic Thermal Vapour Ablation for Localized Cancer Lesions of the Lung: A Clinical Feasibility Treat-and-Resect Study. Respiration; international review of thoracic diseases. 2021;100:432-42
42. Sebek J, Kramer S, Rocha R, Yu KC, Bortel R, Beard WL. et al. Bronchoscopically delivered microwave ablation in an in vivo porcine lung model. ERJ open research. 2020 6
43. Gonzalez X. et al. Evaluation of a Novel Ablation Device Compatible with Endobronchial Ultrasoundbronchoscopy. Chest. 2019: 4: Suppl A197-A198.
44. Khodabakhshi Z, Amini M, Hajianfar G, Oveisi M, Shiri I, Zaidi H. Dual-Centre Harmonised Multimodal Positron Emission Tomography/Computed Tomography Image Radiomic Features and Machine Learning Algorithms for Non-small Cell Lung Cancer Histopathological Subtype Phenotype Decoding. Clinical oncology. 2023Nov;35(11):713-725
45. Lozano MD, Benito A, Labiano T, Pijuan L, Tejerina E, Torres H. et al. Recommendations for optimizing the use of cytology in the diagnosis and management of patients with lung cancer. Revista espanola de patologia: publicacion oficial de la Sociedad Espanola de Anatomia Patologica y de la Sociedad Espanola de Citologia. 2023;56:58-68
46. Long JP, Shen Y. Detection method has independent prognostic significance in the PLCO lung screening trial. Scientific reports. 2023;13:13382
47. Zhou X, Zhou L, Yao Z, Huang M, Gong Y, Zou B. et al. Safety and Tolerability of Low-Dose Radiation and Stereotactic Body Radiotherapy + Sintilimab for Treatment-Naive Stage IV PD-L1+ Non-Small-Cell Lung Cancer Patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2023Oct13;29(20):4098-4108
48. Khalifa J. [Impact of immunotherapy on the therapeutic strategy for the management of stage I non-small cell lung cancer: The radiation oncologist's point of view]. Cancer radiotherapie: journal de la Societe francaise de radiotherapie oncologique. 2023Sep;27(6-7):653-658
49. Tsoulos N, Papadopoulou E, Metaxa-Mariatou V, Tsaousis G, Efstathiadou C, Tounta G. et al. Tumor molecular profiling of NSCLC patients using next generation sequencing. Oncology reports. 2017;38:3419-29
50. Gendarme S, Bylicki O, Chouaid C, Guisier F. ROS-1 Fusions in Non-Small-Cell Lung Cancer: Evidence to Date. Current oncology. 2022;29:641-58
51. Di Noia V, D'Aveni A, D'Argento E, Rossi S, Ghirardelli P, Bortolotti L. et al. Treating disease progression with osimertinib in EGFR-mutated non-small-cell lung cancer: novel targeted agents and combination strategies. ESMO open. 2021;6:100280
52. Zhu K, Lv Z, Xiong J, Zheng H, Zhang S, Jin H. et al. MET inhibitor, capmatinib overcomes osimertinib resistance via suppression of MET/Akt/snail signaling in non-small cell lung cancer and decreased generation of cancer-associated fibroblasts. Aging. 2021;13:6890-903
53. Cheng Y, Zhang T, Xu Q. Therapeutic advances in non-small cell lung cancer: Focus on clinical development of targeted therapy and immunotherapy. MedComm. 2021;2:692-729
54. Mielgo-Rubio X, Uribelarrea EA, Cortes LQ, Moyano MS. Immunotherapy in non-small cell lung cancer: Update and new insights. Journal of clinical and translational research. 2021;7:1-21
55. Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543-50
56. Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, Barlesi F. et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement from the International Association for the Study of Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2021;16:1647-62
57. Kosmidis C, Sardeli C, Zarogoulidis P, Hohenforst-Schmidt W, Vagionas A, Ioannis-Katsios N. et al. Producing the appropriate model and drug for intratumoural ablation. British journal of cancer. 2020;123:335-6
58. Hohenforst-Schmidt W, Zarogoulidis P, Stopek J, Kosmidis E, Vogl T, Linsmeier B. et al. Enhancement of Intratumoral Chemotherapy with Cisplatin with or without Microwave Ablation and Lipiodol. Future Concept for Local Treatment in Lung Cancer. Journal of Cancer. 2015;6:218-26
59. Hohenforst-Schmidt W, Zarogoulidis P. Time to get started with endobronchial microwave ablation-chances, pitfalls and limits for interventional pulmonologists. Translational lung cancer research. 2020;9:163-7
60. Zhu H, Huang C, Di J, Chang Z, Li K, Zhang S. et al. Doxorubicin-Fe(III)-Gossypol Infinite Coordination Polymer@PDA:CuO(2) Composite Nanoparticles for Cost-Effective Programmed Photothermal-Chemodynamic-Coordinated Dual Drug Chemotherapy Trimodal Synergistic Tumor Therapy. ACS nano. 2023;17:12544-62
61. Zhang W, Zhou H, Gong D, Wu H, Huang X, Miao Z. et al. AIPH-Encapsulated Thermo-Sensitive Liposomes for Synergistic Microwave Ablation and Oxygen-Independent Dynamic Therapy. Advanced healthcare materials. 2023;12:e2202947
62. Meng L, Wei Y, Xiao Y. Chemo-immunoablation of solid tumors: A new concept in tumor ablation. Frontiers in immunology. 2022;13:1057535
63. Zarogoulidis P, Hohenforst-Schmidt W, Papadopoulos V, Perdikouri EI, Courcoutsakis N, Porpodis K. et al. Case Report: Endoscopic radiofrequency ablation with radial-EBUS and ROSE. Frontiers in medical technology. 2023;5:1022220
64. Zarogoulidis P, Hohenforst-Schmidt W, Chen W, Porpodis K, Kosmidis C, Kotsakis A. et al. Endobronchial Radiofrequency Ablation for pulmonary nodules with Radial-Ebus and Navigation: Pros and Cons. Journal of Cancer. 2023;14:1562-70
Author contact
![]() Corresponding author: Paul Zarogoulidis, M.D, Ph.D, Pulmonary Department, General Clinic Euromedica, Thessaloniki, Greece. Tel: 00306977271974. E-mail: pzarogcom.
Corresponding author: Paul Zarogoulidis, M.D, Ph.D, Pulmonary Department, General Clinic Euromedica, Thessaloniki, Greece. Tel: 00306977271974. E-mail: pzarogcom.

 Global reach, higher impact
Global reach, higher impact